Abstract
Background
Chronic humoral rejection (CHR) is a major complication after kidney transplantation. The cause of CHR is currently unknown. Autoantibodies have often been reported in kidney transplant recipients alongside anti-donor human leukocyte antigen antibodies. Yet, the lack of comprehensive studies has limited our understanding of this autoimmune component in the pathophysiology of CHR.
Methods
By using a series of ELISA and immunocytochemistry assays, we assessed the development of autoantibodies in 25 kidney transplant recipients with CHR and 25 patients with stable graft function. We also compared the reactivity of five CHR and five non-CHR patient sera with 8027 recombinant human proteins using protein microarrays.
Results
We observed that a majority of CHR patients, but not non-CHR control patients, had developed antibody responses to one or several autoantigens at the time of rejection. Protein microarray assays revealed a burst of autoimmunity at the time of CHR. Remarkably, microarray analysis showed minimal overlap between profiles, indicating that each CHR patient had developed autoantibodies to a unique set of antigenic targets.
Conclusion
The breadth of autoantibody responses, together with the absence of consensual targets, suggests that these antibody responses result from systemic B-cell deregulation.
Keywords: Chronic humoral rejection, Autoantibodies, Human
Chronic humoral rejection (CHR) is currently recognized as one of the most serious complications after kidney transplantation. The reason why some transplant recipients develop CHR and others do not is still unknown. CHR is primarily characterized by the development of de novo donor-specific antibodies (DSA). These alloantibodies have been directly associated with late graft failure (1–4). In 2005, a series of criteria were adopted to define CHR beyond the development of DSA (5). Because this classification was accepted, a growing number of cases have been diagnosed in different institutions, revealing a higher incidence of CHR than initially presumed. Retrospective studies looking at the deposition of the complement molecule C4d and the development of DSA as markers of antibody-mediated rejection estimated that up to 61% of all chronic rejection cases involved a humoral component (3, 6, 7). In addition, Lee et al. (8) demonstrated that virtually all cases of late graft loss were accompanied by some levels of antibody responses to the allografts. Collectively, these findings suggest that CHR is responsible for a majority of late graft loss cases.
In addition to DSA, a number of studies have demonstrated the development of de novo autoantibodies after organ transplantation. Anti-endothelial cell antibodies (AECAs) have been observed for a number of years (9, 10). Their detection in the serum or directly in the graft of kidney transplant recipients has been associated with tissue damage and subsequent graft rejection (11). Although recognized targets of AECAs are often alloantigens, it has been shown that these antibodies can also react to self-proteins (12). Additional studies have reported the development of antibodies to specific self-antigens after transplantation. Autoantigenic targets include angiotensin II type 1 receptor (13), agrin (14), tubulin-α, heat shock protein 60 (15), vimentin (15, 16), cardiac myosin (17), cardiolipin (18), and ribosomal protein L7 (19). The detection of these antibodies was associated with graft rejection episodes or hypertension. Increased serum reactivity to nonprotein structures such as phospholipids and oxidized low-density lipoprotein have also been observed in kidney transplant recipients (20–23). These antibodies were associated with atherosclerosis, a common feature among late transplant recipients and patients with CHR.
Overall, these studies demonstrated the presence of various types of autoantibodies in transplant recipients. We hypothesized that such autoantibodies develop more frequently after organ transplantation than initial studies indicated. We also surmised that this humoral autoimmunity could reveal an underlying context of immunologic imbalance that may also have facilitated the generation of DSA. The aim of the present study was to investigate the development and specificity of autoantibody responses in patients with biopsy-proven CHR.
MATERIALS AND METHODS
Sample Collection
Serial serum samples were collected from kidney transplant recipients as a part of their standard clinical care. The use of those samples in our study was approved by the Massachusetts General Hospital internal review board. The CHR group consisted of 25 kidney transplant recipients whose serum was taken before transplantation, after transplantation, and at the time of a graft biopsy documenting CHR. CHR was diagnosed histologically by the presence of at least two of the following features in the graft tissue: arterial intimal fibrosis, duplication of glomerular basement membrane, multilaminated peritubular capillary basement membrane, and interstitial fibrosis (4). Along with these pathologic findings, evidence of C4d deposition in the tissues and the presence of antidonor antibodies in the serum contributed to the final diagnosis of CHR (4). According to these criteria, all 25 CHR patients in our study had intragraft deposition of C4d, circulating DSA (see Table, Supplemental Digital Content 8, http://links.lww.com/TP/A186), and two of the aforementioned histologic features of CHR. The non-CHR control group consisted of 25 kidney transplant recipients with stable allograft function at the time of sample collection. Biopsies are not performed on transplant recipients with stable graft function at our institution. Therefore, we do not know whether some “non-CHR” patients had renal lesions at the time of serum sampling. Only patients who received single organ primary transplants were included in this study. None of the 50 patients included in the study had autoimmune disease before transplant. A summary of patient characteristics is provided in Table 1.
TABLE 1.
Summary of patient characteristics
| Non-CHR | CHR | P | |
|---|---|---|---|
| N | 25 | 25 | |
| Median age in yr (range) | 44 (23–82) | 37 (15–65) | 0.050 |
| Female, n (%) | 6 (24) | 7 (28) | 0.808 |
| DSA, n (%)a | 1 (4) | 25 (100) | <0.001 |
| Class I | 1 (4) | 17 (68) | |
| Class II | 1 (4) | 22 (88) | |
| Donation, n (%) | |||
| Deceased | 13 (52) | 12 (48) | |
| Living related | 8 (32) | 6 (24) | 0.658 |
| Living unrelated | 4 (16) | 7 (28) | |
| Median sampling time (years posttransplantation) | 2.7 | 6.3 | 0.001 |
| Induction therapy, n (%) | 13 (52) | 12 (48) | 1 |
| Antithymocyte globulin | 13 (100) | 9 (75) | |
| Other | 0 (0) | 3 (25) | |
| Immunosuppression regimen, n (%) | |||
| Four-drug regimen | 0 (0) | 2 (8) | |
| Three-drug regimen | 15 (60) | 15 (60) | 0.447 |
| Two-drug regimen | 10 (40) | 8 (32) | |
| Tacrolimus or sirolimus | 24 | 15 | 0.005 |
| Cyclosporine A or azathioprine | 2 | 13 | 0.001 |
Detailed patient DSA data shown in Supplemental Table 3 (see Supplemental Digital Content 8, http://links.lww.com/TP/A186).
CHR, chronic humoral rejection; DSA, donor specific antibodies.
ELISA
Concentration of total serum IgG was measured using an IgG ELISA quantification kit (Bethyl Laboratories, Montgomery, TX) according to the manufacturer’s instructions. Serum samples were diluted 1:200,000 in phosphate-buffered saline (PBS).
Antinuclear antibodies (ANA) and antibodies to cardiolipin were quantified using ANA screen IgG ELISA II and cardiolipin IgG ELISA II kits, respectively (Wampole Laboratories, Princeton, NJ). Assays were carried out according to the manufacturer’s instructions.
ELISA for the detection of antibodies to double-stranded DNA (dsDNA) and insulin were performed as described previously (24). Briefly, maxisorp plates (Nunc, Thermo Fisher Scientific, Rochester, NY) were coated over-night at 4°C with 10 µg/mL dsDNA or recombinant human insulin (Sigma-Aldrich, St. Louis, MO). After five washes in PBS with 0.05% Tween 20, serum samples diluted at 1:50 in PBS were added and incubated for 3 hr at room temperature. Plates were then washed in PBS with 0.05% tween 20, incubated for 1 hr with alkaline phosphatase (AP)–conjugated goat antihuman IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), washed again, and developed using AP substrate colorimetric reagents (Sigma-Aldrich). Optical density was read at 405 nm. For the detection of dsDNA- and Insulin-reactive IgG subclasses, ELISA was carried out using horseradish peroxidase (HRP)- conjugated anti-IgG1, IgG2, IgG4 (Invitrogen, Carlsbad, CA), and AP-conjugated IgG3 (Invitrogen) secondary antibodies. ELISA revealed with HRP-conjugated secondary antibodies were developed using 3, 3′, 5, 5′-tetramethylbenzidine (Sigma-Aldrich). Optical density was read at 450 nm for IgG1, IgG2, IgG4 (HRP) and at 405 nm for IgG3 (AP).
ELISA to whole protein extract from human embryonic kidney cell line (HEK-293) was adapted from a previously described protocol (25). Briefly, proteins were extracted in radioimmunoprecipitation assay buffer (Boston Bioproducts Inc, Worcester, MA) supplemented with protease inhibitor (Roche Diagnostics, Indianapolis, IN) and phenylmethanesulfonyl fluoride (Sigma-Aldrich). The protein extract was used to coat maxisorp microplates (Nunc, Thermo Fisher Scientific) at the equivalent of 10,000 cells per well. The rest of the procedure is identical to the one described for dsDNA and insulin. Sera were diluted 1:50 in PBS.
ELISA protocol for the detection of total lipid rat brain extract was adapted from Pierangeli and Harris (26). Ninety-six flat bottom culture plates (BD Biosciences, San Jose, CA) were coated with 30 µl of total brain extract (Avanti Polar Lipids Inc., Alabaster, AL) at a concentration of 50 µg/mL in ethanol, incubated overnight at 4°C, and uncovered to allow ethanol evaporation. After three washes, plates were blocked in PBS supplemented with 10% fetal calf serum for 1 hr at room temperature. The rest of the protocol is identical to the one described for dsDNA and insulin. Sera were diluted 1:50 in PBS.
Immunofluorescence Assays
Hep-2 cell-coated slides (Bion Enterprises Ltd, Des Plaines, IL) were incubated at room temperature with diluted serum samples (1:50 in PBS) for 30 min, washed in PBS, stained with fluorescein isothiocyanate-conjugated anti-human IgG (Invitrogen), washed with PBS, and visualized by fluorescence microscopy.
Immunoprofiling Using Protein Microarrays
Antibody profiling of selected serum samples was performed using high-density protein microarrays. We used V4.0 protoarrays (Invitrogen), consisting of 8027 recombinant proteins spotted in duplicate on a glass slide. All proteins were produced in insect cells through a baculovirus expression system. Arrays were first saturated in blocking buffer (50 mM HEPES, pH 7.5; 200mMNaCl; 0.08% Triton X-100; 25% glycerol; 20mMreduced glutathione; 1 mM dithiothreitol (DTT); 4 mM NaOH; 1% bovine serum albumin) for 1 hr at 4°C on an orbital shaker (50 rpm). Arrays were then probed with serum diluted 1:500 in probing buffer (PBS,1%bovine serum albumin, 0.1% Tween 20) for 90 min at 4°C, 50 rpm. After five washes in probing buffer, arrays were incubated (90 min, 4°C, 50rpm) with 1 µg/mL of secondary antibody (goat anti-human IgG Alexa fluor 647 [molecular probes, Carlsbad, CA]) diluted in probing buffer. Arrays were then washed five times, dried, and scanned on a GenePix 4000B scanner (Molecular Devices, Union City, CA). Fluorescence was measured with the GenePix Pro version 3.0 software (Molecular Devices) and quantified with the Prospector version 4 software (Invitrogen) using the protein-protein interaction algorithm. Signal intensity obtained for each protein reflected the concentration of antibody specific to that protein in the serum sample. All signals were first normalized based on the IgG concentration in each sample to ensure that the difference in signal intensity to array proteins was not because of the IgG level in the serum. Signals obtained with serum samples collected at two different time points posttransplant were then compared with one another to examine the changes in IgG reactivity. Similar two-dimensional scatterplot analysis using Invitrogen protoarrays have already been reported (27). Variations in protein signals were then evaluated by calculating the ratio between signals obtained before and at the time of DSA. Because ratio can be artificially increased with values close to background level, we only considered values more than 50 arbitrary units. The reproducibility of the Invitrogen protoarray platform was tested by comparing signals obtained from two arrays probed with the same serum sample collected from a kidney transplant recipient experiencing rejection. Results are presented in Supplemental Figure (see Supplemental Digital Content 1, http://links.lww.com/TP/A179). In addition, to further minimize the variability, we compared only arrays from the same lot that were probed and scanned in the same experiment, that is, the same day.
Statistical Methods
Comparisons between the CHR and non-CHR groups were based on Fisher’s exact test for categorical variables, whereas Wilcoxon rank-sum test was applied to continuous data. One-sided P values are reported for comparing serum reactivity to autoantigens as the alternative hypothesis is defined intrinsically by an increased concentration, whereas other P values are based on a two-sided hypothesis. General data analysis was conducted using SAS version 9.1 (SAS Institute, Cary, NC). The overall change in serum reactivity between pretransplant and posttransplant samples was analyzed using a permutation test of Hotelling’s T2 statistic (28). The computation was based on 10,000 random resamplings using the ICSNP package (29) in R version 2.5.1 (www.R-project.org).
Multivariate analysis of covariance was used to compare the overall change in serum reactivity between pretransplant and posttransplant samples between the patient groups while adjusting for IgG increase, sampling time, and use of tacrolimus- or sirolimus-based immunosuppression. As the CHR group was younger on average, age was initially included as a covariate but was excluded in the final model because of a lack of significance.
RESULTS
Increased Concentration of Autoreactive IgG in CHR Patients’ Sera at Time of Diagnosis
A retrospective cross-sectional study was conducted to examine the presence of autoreactive IgG in sera collected from 25 CHR patients at the time of diagnosis and sera from 25 non-CHR control kidney transplant recipients with stable graft function. Patients were chosen based on sample availability. The median time between transplantation and sampling was significantly shorter for the non-CHR group compared with the CHR group (2.7 and 6.3 years, respectively, P=0.001). All transplant recipients received a comparable number of immunosuppressive drugs. However, CHR patients were more likely to have received cyclosporine A or azathioprine whereas patients without this complication were more likely to have received tacrolimus or sirolimus (Table 1). Allegedly, the choice of immunosuppressive agents reflects a shift toward tacrolimus and sirolimus in more recent years.
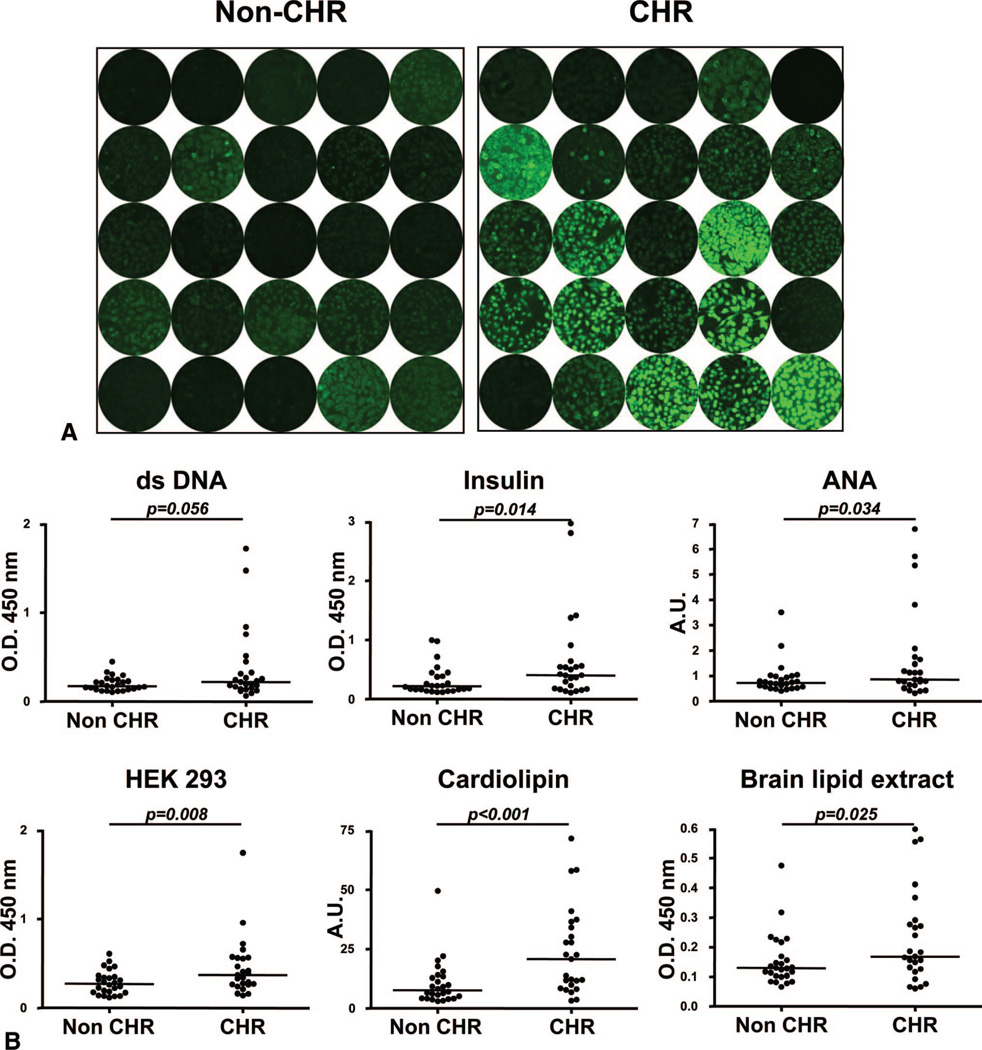
Indirect immunofluorescence staining of the standard carcinoma cell line Hep-2 revealed weak reactivity for 8 and strong reactivity for 9 of the 25 CHR patients. In comparison, only 3 of the 25 stable patients displayed a weak signal (Fig. 1A). The reactivity patterns of positive CHR patient sera indicated the presence of ANA and anti-cytoplasmic antibodies.
FIGURE 1.
Serum reactivity to autoantigens. (A) Serum reactivity to Hep-2 cells. Staining of Hep-2 cells was carried out with serum samples collected from 25 chronic humoral rejection (CHR) patients at the time of rejection and from 25 non-CHR control patients. Staining was revealed using an anti-IgG secondary antibody conjugated to fluorescein isothiocyanate. Patients’ serum reactivity was assessed at 1:50. All pictures were acquired using the same settings for consistency. (B) Serum reactivity to protein antigens, whole-cell extract, and lipids. Samples collected from 25 CHR patients at the time of rejection and from 25 non-CHR patients were tested for IgG reactivity to double-stranded DNA, insulin, antinuclear antigens, HEK-293 cell line whole protein extract, cardiolipin, and whole rat brain extract by ELISA.
The reactivity of the same serum samples to a panel of classical self-antigens was then assessed by ELISA. Antigenic targets included nucleic acids (dsDNA), individual protein (insulin), protein bulk (nuclear antigens, kidney cell line HEK293 lysate), and lipids (rat brain lipid extract, cardio-lipin). These assays revealed a higher concentration of auto-reactive IgG in sera from CHR patients compared with sera of control non-CHR transplant recipients for all six autoantigenic structures (Fig. 1B). The difference in serum reactivity for the two patient groups was statistically significant for five of six antigens (P<0.05). A substantially more spread-out distribution was also noticeable for the CHR group with some individual patients displaying a strong autoreactivity. These high responders were different patients in each assay.
Autoantibodies Develop Posttransplant in CHR Patients
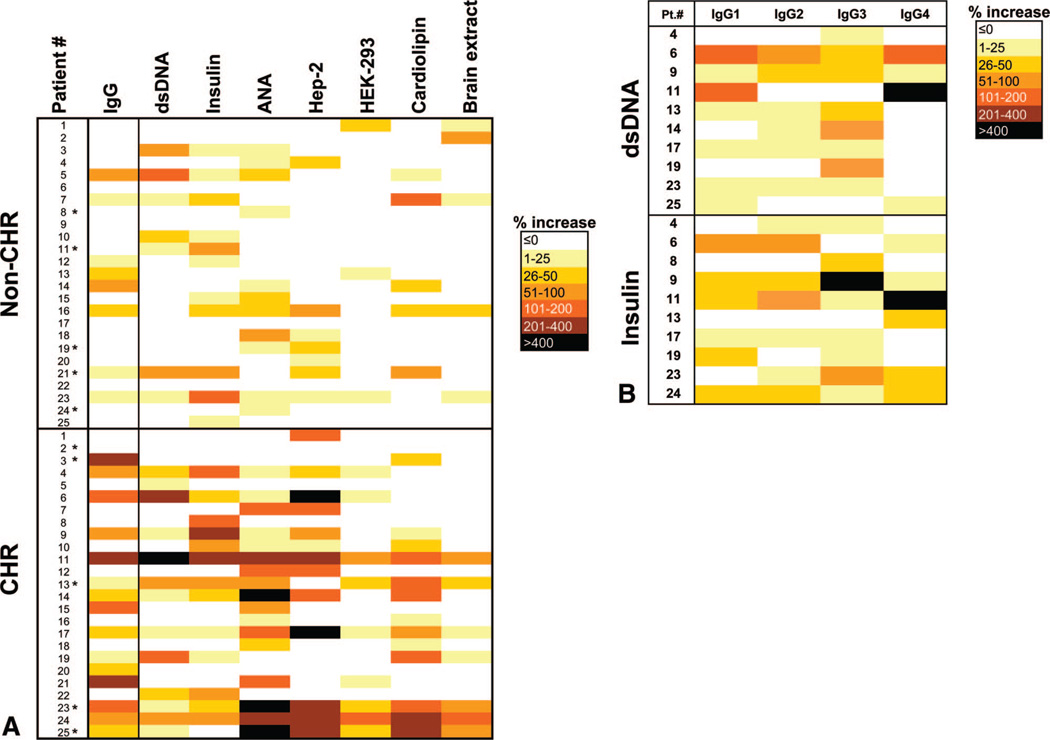
To determine whether these autoantibodies had developed after transplantation or existed at the same level before transplantation, we compared pretransplant and posttransplant serum reactivity with the same autoantigens for our series of patients. Results were expressed as percentage increase between signals obtained with pretransplant and posttransplant samples. The data are summarized in Figure 2(A) and show that the sera of the CHR patients had significant increases in reactivity to self-antigens when compared with those of non-CHR patients (P=0.006). Remarkably, profiles seemed unique for each patient. Moreover, serum reactivity increase did not seem to correlate with time post-transplant (see Figure, Supplemental Digital Content 2, http://links.lww.com/TP/A180). An increase in serum IgG levels was also observed in significantly more CHR patients than patients without this complication (Fig. 2A, left column; P=0.045). This increase in IgG levels may account for some of the observed reactivity to the series of autoantigens tested. Yet, several patients showed increased reactivity to some autoantigens without increased IgG levels and vice versa. A multivariate analysis showed that post-transplant increase in serum reactivity to various autoantigens among the CHR group is still significant (P=0.056) after adjusting for the differences in total serum IgG levels, sampling time, or immunosuppressive therapies. To determine whether this increase of reactivity corresponded to a particular IgG subclass, we carried out dsDNA and insulin ELISA using anti-IgG1, IgG2, IgG3, or IgG4 secondary antibody. Sera from 10 patients showing increase in reactivity toward dsDNA or insulin were tested for each IgG subclass. As depicted in Figure 2(B), no preferential IgG subclass seemed to be responsible for the increased autoreactivity at time of CHR.
FIGURE 2.
Development of autoantibodies between pre-transplant and posttransplant. (A) “Heat map” recapitulating increases in patient serum reactivity to a broad range of autoantigens and increases in total serum IgG concentrations (left column) between samples collected before transplant and at the time of chronic humoral rejection (CHR). Samples collected pretransplant and posttransplant from kidney transplant recipients with stable allograft function were used as controls. Each color code corresponds to a level of increase. Patients marked with an asterisk were selected for protein microarray analysis. (B) “Heat map” recapitulating IgG1, IgG2, IgG3, and IgG4 increases in CHR patient serum reactivity to double-stranded DNA (dsDNA) and insulin. Sera were selected for each autoantigen based on their total IgG reactivity to dsDNA and insulin (A). Each color code corresponds to a level of increase.
Development of Autoantibodies to a Broad Range of Proteins at Time of CHR
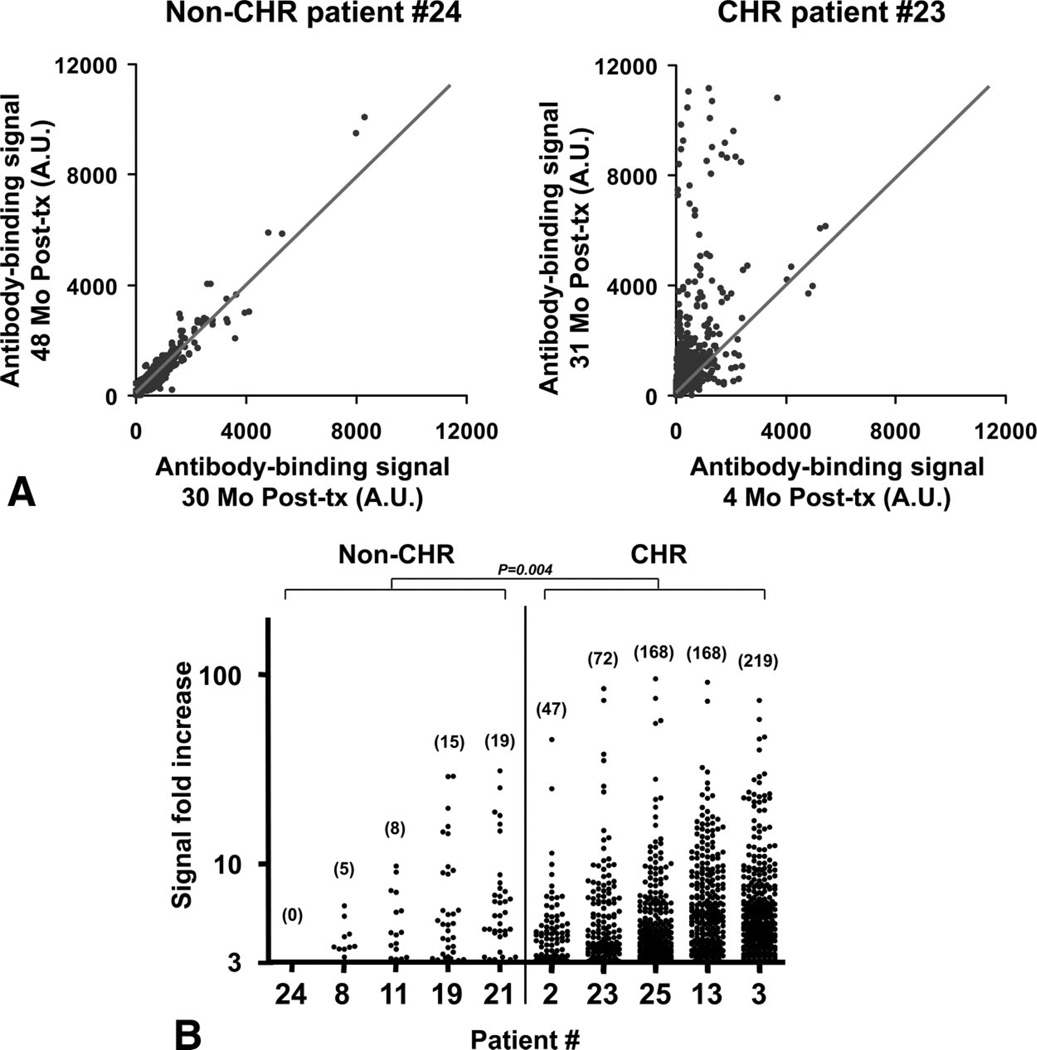
By using a protein microarray platform, we assessed the development of antibodies to 8027 proteins posttransplant for five non-CHR patients with stable allograft function and five patients with CHR. These 10 patients (marked with an asterisk in Fig. 2A) were selected solely based on sample availability. All IgG containing human sera display some level of reactivity to many self-proteins with a great degree of individual variability. For this reason, we decided to focus on variations in serum reactivity between two time points for the same patients rather than to compare absolute reactivity with array proteins between subjects. We compared signals obtained from two identical arrays probed with serum samples collected months before and at the time of rejection for each CHR patient. Similar samples from two different time points posttransplant were selected from control non-CHR patients to match those of the CHR patients. The timetable for sample collections is summarized in supplemental Table 1 (see Supplemental Digital Content 3, http://links.lww.com/TP/A181). Importantly, time intervals between the two time points were matched for CHR and non-CHR patients. Array results were first normalized based on the IgG concentration in each sample to exclude any difference in signal intensity to array proteins because of the IgG level in the serum. The ratio of signals obtained between the two time points was then calculated for each protein on the arrays to evaluate the global responses in CHR and non-CHR transplant recipients. Figure 3(A) shows an example of the array results for a non-CHR and a CHR patient. A linear regression analysis performed with values obtained for internal control proteins in the two arrays was used to establish a “trend line” shown in the figure. Signal values falling within range of this trend line correspond to proteins for which serum reactivity did not change between the two time points. Conversely, signal values distant from this line correspond to proteins for which serum reactivity increased or decreased. Additional dot plots depicting variations in serum reactivity for the four CHR and four non-CHR remaining patients are shown in Supplemental Figure 2 (see Supplemental Digital Content 4, http://links.lww.com/TP/A182).
FIGURE 3.
Protein array profiling of auto-antibody responses. (A) Two-dimensional scatterplot of IgG binding values obtained for all 8027 proteins on the array using serum samples collected at two different time points posttransplant for a kidney transplant recipient with stable graft function (left) and a chronic humoral rejection (CHR) patient (right). The second time point (31 months posttransplant) for the CHR patient is the time of CHR diagnosis. All proteins are spotted in duplicate. Signals are expressed as arbitrary units (AU). The depicted line was established using values obtained for array internal control proteins. Scale was arbitrarily set at 12,000. (B) Protein array signal fold increase between the two time points for five non-CHR and five CHR patients. The patient numbers correspond to Figure 2. Only duplicate signals above a cutoff of threefold increase are depicted. The number of proteins for which the signal increased more than threefold is reported for each patient. Tx, transplant.
Figure 3(B) presents the number of target antigens toward which the serum reactivity increased more than three-fold. As shown in this figure, CHR patients developed auto-antibodies to a wide range of proteins (47–219), whereas an increase in serum reactivity to only a few targets (0 –19) was detected in patients with stable graft function. A Wilcoxon rank-sum test was carried out on the number of proteins toward which serum reactivity increased more than threefold. The number of targeted proteins was significantly higher among the CHR group compared with the patients with stable graft function (P=0.004).
CHR Patients Develop Unique Antibody Signatures
To further examine whether some autoantibody specificities were shared among CHR patients, we compared the panels of target proteins (signal fold increase ≥3) obtained for each individual serum. As showed in Table 2, only 34 proteins were shared among the CHR patient antibody signatures. None of these 34 antigens were recognized by more than two sera (Table 3). The identity of these 34 shared proteins is displayed in Supplemental Table 2 (see Supplemental Digital Content 5, http://links.lww.com/TP/A183).
TABLE 2.
Number of autoantigen targets in common between CHR patients
| CHR patient | 2 | 23 | 25 | 13 | 3 |
|---|---|---|---|---|---|
| 2 | 0 | 1 | 2 | 2 | |
| 23 | 2 | 1 | 5 | ||
| 25 | 11 | 6 | |||
| 13 | 4 | ||||
| 3 |
CHR, chronic humoral rejection.
TABLE 3.
Overlap between serologic response profiles
| No. autoantigens shared | |
|---|---|
| 2 CHR patients | 34 |
| 3 CHR patients | 0 |
| 4 CHR patients | 0 |
| 5 CHR patients | 0 |
CHR, chronic humoral rejection.
To validate the protein microarrays, we selected three proteins toward which the serum reactivity increased more than threefold between the two time points. These target proteins were selected based on the high level of increase in serum reactivity between the two time points (Cytokeratin 8) or because they were targeted by two patients (Fragile X Mental Retardation 1 [FMR1] and Phosphoribosyl Pyrophosphate Synthetase 1[PRPS1]). ELISA confirmed a substantial increase in serum reactivity for all three proteins (see Figure, Supplemental Digital Content 6, http://links.lww.com/TP/A184).
DISCUSSION
This study provides an in-depth analysis of the nature and specificities of arising autoantibodies in kidney transplant recipients with antibody-mediated chronic rejection. Our data showed the development of IgG reactive to a set of self-antigens including nucleic acids, proteins, and lipids in a series of 25 CHR patients who also had circulating DSA. In contrast, control non-CHR transplant recipients with stable allograft function demonstrated minimal IgG reactivity to autoantigens. To widen our screening strategy, we also used a recently developed protein microarray platform and assessed IgG specificity to 8027 human recombinant proteins. A similar approach was recently undertaken in pediatric kidney transplant recipients using a previous version of the same technology (ProtoArray V3.0) (30). Both this study and our own study examined the variations in serum reactivity to array proteins between samples collected at two different time points. Although Li et al. (30) measured serologic changes between pretransplant and posttransplant samples, we assessed changes between two posttransplant samples, that is, before and at the time of CHR. We reasoned that the induction therapy and other immunomodulating intervention at the time of transplant may have substantially modified the patients’ serologic profiles. Assessing two posttransplant samples collected within months of each other would, therefore, more likely reflect antibody variations associated with this complication.
Several observations can be made from our set of data. (1) All but 2 of the 25 CHR patients demonstrated some increase in IgG levels to one or multiple autoantigens in ELISA or Hep-2 assay. This consistency underscores the homogeneity among CHR patients who were characterized following the criteria defined at the BANFF ’05 workshop (5). (2) A significant proportion of the non-CHR control patients also had circulating autoantibodies. Biopsies are not performed on transplant recipients with stable graft function at our institution. Therefore, it is possible that some of these non-CHR patients had renal lesions and were at an early stage of developing CHR at the time of sampling. In that respect, it is noteworthy that samples from non-CHR patients were collected on average at an earlier time point posttransplant than those from CHR patients. In our limited series of patients, however, we did not find any significant correlation between autoantibody generation and time posttransplant (see Figures, Supplemental Digital Content 2, http://links.lww.com/TP/A180 and Supplemental Digital Content 7, http://links.lww.com/TP/A185). Further longitudinal studies on a larger group of patients matched for sampling time posttransplant will be required to corroborate our findings. (3) Recognition patterns of serum reactivity to self-antigens are exquisitely specific to each CHR patient. Our array analyses indicate that none of the 8027 protein tested seems to be recognized by more than two patients. Such specificity does not support the view that serologic responses from CHR patients are directed toward a restricted set of autoantigens. (4) Autoantibody responses did not seem to be associated with any preferential IgG subtype in CHR patients. Such absence of distinctive pattern further supports the hypothesis of immune deregulation rather than the development of conventional antigen driven responses.
The prevalence of self-reactive IgG in patients experiencing CHR raises two important questions. First, are some of these antibodies pathogenic, that is, are they involved in allograft tissue destruction alongside with DSA? All CHR patients in our study had biopsy-documented staining for C4d in the graft at time of diagnosis, implicating the binding of immunoglobulins. However, we cannot establish whether C4d molecules were deposited in the kidney as the result of alloautoantibodies or autoantibodies. A number of studies have previously associated the presence of autoantibodies with graft rejection (reviewed in Ref. 31–33). Yet, the formal demonstration that autoreactive IgG are involved in the pathophysiology of rejection is lacking. A single study by Dragun et al. (13) attests to the role of antiangiotensin II type 1 receptor IgG in symptoms of hypertension in kidney graft recipients. Our analysis on CHR autoantibody specificities revealed that a noticeable proportion of self-reactive IgG were directed at membrane-bound proteins (see Table, Supplemental Digital Content 5, http://links.lww.com/TP/A183). Binding of these antibodies directly to the graft tissue could result in complement-mediated cytotoxicity. Sera from CHR patients also contained IgG specific to cytosolic proteins. These latter antibodies could form immune complexes with cytosolic antigens released during tissue damage and activate additional components of the immune system.
Conversely, it is also possible that these autoantibodies do not have any definite role in the pathogenicity of CHR. Remarkably, we could not associate the presence of autoreactive IgG in our patient cohort with any clinical manifestations of autoimmunity. The fact that autoantibody profiles were unique for each CHR patient does not suggest a common function. Further studies are now warranted to investigate the contribution of these autoantibodies to the deteriorating graft function during CHR.
The second key question relates to the cause of such broad humoral autoimmunity. Self-reactive antibodies could result from inflammation processes associated with tissue destruction during graft rejection. This possibility, however, suggests that autoantibodies would react primarily to common renal antigens exposed locally. This prospect is difficult to reconcile with the frequency and wide specificity of auto-antibodies in CHR patients, which resemble those of systemic autoimmune diseases. As an alternate hypothesis, we propose that the development of broadly specific, yet unique, autoantibody signatures for each patient is a hallmark of a wide B-cell deregulation. B-cell deregulation and autoantibody production have been observed in situations of regulatory T-cell (Treg) insufficiency (34, 35). It is conceivable that kidney transplant recipients may also experience reduced Treg functions because of long-term immunosuppressive therapy. In that regard, increase of total serum IgG levels in CHR patients could reflect Treg dysfunction as this has been observed in animals and humans (34, 35). In support of this view, Louis et al. (36) observed a decrease in both CD4+CD25+ T-cell numbers and FOXP3 expression in patients with chronic rejection compared with kidney transplant recipients with stable allograft function. Remarkably, CHR and non-CHR patients had received significantly different immunosuppressive medication in our study (Table 1). These various treatments may have had different impact on the Treg function and the global regulation of the immune system. Likewise, differences in the treatments may have contributed to the generation of autoantibodies and alloantibodies and the development of CHR. Studies on a larger number of patients are warranted to single out the exact influence of each type of immunosuppressive maintenance therapy on this complication. Overall, we believe that our study contributes to elucidating the complexity of humoral immunity in kidney transplant recipients developing CHR.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Fahd and Nadia Alireza’s Research Fund.
The authors thank Dr. Christian Leguern and Dr. Christene Huang for their thoughtful review of the article.
Footnotes
F.P. and J.D. performed experiments; E.Z. designed the study; F.P. and E.Z. wrote the article; B.Y.Y. performed statistical analysis; L.X., I.D., R.P., and T.C.G. contributed new reagents or analytic tools; and S.L.S., R.B.C., and W.W. participated in data analysis.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Terasaki PI, Cai J. Humoral theory of transplantation: Further evidence. Curr Opin Immunol. 2005;17:541. doi: 10.1016/j.coi.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3:665. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 3.Racusen LC, Haas M. Antibody-mediated rejection in renal allografts: Lessons from pathology. Clin J Am Soc Nephrol. 2006;1:415. doi: 10.2215/CJN.01881105. [DOI] [PubMed] [Google Scholar]
- 4.Colvin RB. Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 5.Solez K, Colvin RB, Racusen LC, et al. Banff ’05 meeting report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN′) Am J Transplant. 2007;7:518. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 6.Regele H, Bohmig GA, Habicht A, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- 7.Mauiyyedi S, Pelle PD, Saidman S, et al. Chronic humoral rejection: Identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12:574. doi: 10.1681/ASN.V123574. [DOI] [PubMed] [Google Scholar]
- 8.Lee PC, Terasaki PI, Takemoto SK, et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74:1192. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 9.Glotz D, Lucchiari N, Pegaz-Fiornet B, et al. Endothelial cells as targets of allograft rejection. Transplantation. 2006;82(1 suppl):S19. doi: 10.1097/01.tp.0000231348.55262.5a. [DOI] [PubMed] [Google Scholar]
- 10.Lucchiari N, Panajotopoulos N, Xu C, et al. Antibodies eluted from acutely rejected renal allografts bind to and activate human endothelial cells. Hum Immunol. 2000;61:518. doi: 10.1016/s0198-8859(00)00109-9. [DOI] [PubMed] [Google Scholar]
- 11.Le Bas-Bernardet S, Hourmant M, Coupel S, et al. Non-HLA-type endothelial cell reactive alloantibodies in pre-transplant sera of kidney recipients trigger apoptosis. Am J Transplant. 2003;3:167. doi: 10.1034/j.1600-6143.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 12.Kalil J, Guilherme L, Neumann J, et al. Humoral rejection in two HLA identical living related donor kidney transplants. Transplant Proc. 1989;21(1 pt 1):711. [PubMed] [Google Scholar]
- 13.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 14.Joosten SA, Sijpkens YW, van Ham V, et al. Antibody response against the glomerular basement membrane protein agrin in patients with transplant glomerulopathy. Am J Transplant. 2005;5:383. doi: 10.1111/j.1600-6143.2005.00690.x. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler CH, Collins A, Dunn MJ, et al. Characterization of endothelial antigens associated with transplant-associated coronary artery disease. J Heart Lung Transplant. 1995;14(6 pt 2):S188. [PubMed] [Google Scholar]
- 16.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 17.Warraich RS, Pomerance A, Stanley A, et al. Cardiac myosin autoantibodies and acute rejection after heart transplantation in patients with dilated cardiomyopathy. Transplantation. 2000;69:1609. doi: 10.1097/00007890-200004270-00015. [DOI] [PubMed] [Google Scholar]
- 18.Forman JP, Lin J, Pascual M, et al. Significance of anticardiolipin antibodies on short and long term allograft survival and function following kidney transplantation. Am J Transplant. 2004;4:1786. doi: 10.1046/j.1600-6143.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 19.Linke AT, Marchant B, Marsh P, et al. Screening of a HUVEC cDNA library with transplant-associated coronary artery disease sera identifies RPL7 as a candidate autoantigen associated with this disease. Clin Exp Immunol. 2001;126:173. doi: 10.1046/j.1365-2249.2001.01654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ducloux D, Bourrinet E, Motte G, et al. Antiphospholipid antibodies as a risk factor for atherosclerotic events in renal transplant recipients. Kidney Int. 2003;64:1065. doi: 10.1046/j.1523-1755.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 21.Ducloux D, Carron P, Racadot E, et al. T-cell immune defect and B-cell activation in renal transplant recipients with monoclonal gammopathies. Transpl Int. 1999;12:250. doi: 10.1007/s001470050218. [DOI] [PubMed] [Google Scholar]
- 22.Fang JC, Kinlay S, Behrendt D, et al. Circulating autoantibodies to oxidized LDL correlate with impaired coronary endothelial function after cardiac transplantation. Arterioscler Thromb Vasc Biol. 2002;22:2044. doi: 10.1161/01.atv.0000040854.47020.44. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Fresnedo G, Lopez-Hoyos M, Segundo DS, et al. Clinical significance of antiphospholipid antibodies on allograft and patient outcome after kidney transplantation. Transplant Proc. 2005;37:3710. doi: 10.1016/j.transproceed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 24.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 25.Chatlynne LG, Lapps W, Handy M, et al. Detection and titration of human herpesvirus-8-specific antibodies in sera from blood donors, acquired immunodeficiency syndrome patients, and Kaposi’s sarcoma patients using a whole virus enzyme-linked immunosorbent assay. Blood. 1998;92:53. [PubMed] [Google Scholar]
- 26.Pierangeli SS, Harris EN. A protocol for determination of anticardiolipin antibodies by ELISA. Nat Protoc. 2008;3:840. doi: 10.1038/nprot.2008.48. [DOI] [PubMed] [Google Scholar]
- 27.Marina O, Biernacki MA, Brusic V, et al. A concentration-dependent analysis method for high density protein microarrays. J Proteome Res. 2008;7:2059. doi: 10.1021/pr700892h. [DOI] [PubMed] [Google Scholar]
- 28.Good P. Permutation tests: A practical guide to resampling methods for testing hypothesis [ed. 2] New York: Springer; 2000. [Google Scholar]
- 29.Nordhausen K, Sirkiä S, Oja H, et al. ICSNP: Tools for multivariate nonparametrics. R Package Version. 2007 [Google Scholar]
- 30.Li L, Wadia P, Chen R, et al. Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and “antibodyome” measures. Proc Natl Acad Sci USA. 2009;106:4148. doi: 10.1073/pnas.0900563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dragun D. Humoral responses directed against non-human leukocyte antigens in solid-organ transplantation. Transplantation. 2008;86:1019. doi: 10.1097/TP.0b013e3181889748. [DOI] [PubMed] [Google Scholar]
- 32.Boros P, Bromberg JS. De novo autoimmunity after organ transplantation: Targets and possible pathways. Hum Immunol. 2008;69:383. doi: 10.1016/j.humimm.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Rose ML. Activation of autoimmune B cells and chronic rejection. Transplantation. 2005;79(3 suppl):S22. doi: 10.1097/01.tp.0000153294.38939.5f. [DOI] [PubMed] [Google Scholar]
- 34.Baud O, Goulet O, Canioni D, et al. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) by allogeneic bone marrow transplantation. N Engl J Med. 2001;344:1758. doi: 10.1056/NEJM200106073442304. [DOI] [PubMed] [Google Scholar]
- 35.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151. [PubMed] [Google Scholar]
- 36.Louis S, Braudeau C, Giral M, et al. Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation. 2006;81:398. doi: 10.1097/01.tp.0000203166.44968.86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.