Abstract
Fibrin is the primary extracellular constituent of blood clots, and plays an important role as a provisional matrix during wound healing and tissue remodeling. Fibrin-based biomaterials have proven their utility as hemostatic therapies, scaffolds for tissue engineering, vehicles for controlled release, and as platforms for culturing and studying cells in three dimensions. Nevertheless, fibrin presents a complex milieu of signals to embedded cells, many of which are not well understood. Synthetic ECMs provide a blank slate that can ostensibly be populated with specific bioactive cues, including growth factors, growth factor binding motifs, adhesive peptides, and peptide cross-links susceptible to proteases, thereby enabling a degree of customization for specific applications. However, the continued evolution and improvement of synthetic ECMs requires parallel efforts to deconstruct native ECMs and decipher the cues they provide to constituent cells. The objective of this review is to reintroduce fibrin, a protein with a well-characterized structure and biochemistry, and its ability to support angiogenesis specifically. Although fibrin’s structure-function relationships have been studied for decades, opportunities to engineer new and improved synthetic hydrogels can be realized by further exploiting fibrin’s inspiring design.
Keywords: fibrin, biomaterial, extracellular matrix, angiogenesis
1. Introduction
Fibrin is a natural biomaterial and the primary extracellular constituent of blood clots, halting the flow of the blood from open wounds. Given fibrin’s role in the earliest stages of wound healing, it is not a surprise that it has been explored for use in regenerative therapies. Fibrin’s structure and bioactive properties encourage sprouting of new blood vessels (angiogenesis) into the wound, and cells readily degrade it during healing. Biodegradable matrices such as fibrin are ideal for tissue engineering applications because they potentially allow for complete tissue regeneration, leaving no trace of the original scaffolding material. Although fibrin can support many complex morphogenetic processes, this review will highlight its strength as a material capable of supporting angiogenesis. Coupled with the fact that it has been extensively characterized and studied as a biomaterial, fibrin’s permissiveness of vascular morphogenesis makes it useful for studying the engineering principles governing cellular behavior in native three-dimensional (3D) extracellular matrices (ECMs) [1]. Furthermore, fibrin structure-function relationships are currently being exploited in two primary contexts: modifying native fibrin to achieve specific functionalities, and guiding synthetic biomaterial engineering to more closely mimic native tissue.
2. Structure and Degradation of Native Fibrin
Fibrin’s complexity confers many functions, which are primarily dictated by the protein’s structure. Although this acknowledgement appears elementary, understanding structure-function relationships of well-characterized extracellular proteins, such as fibrin, is fundamentally essential to dictate the design of novel biomaterials. To act reproducibly and controllably, engineered biomaterials must possess similar structure-function properties as the proteins they wish to emulate. In a decade of research in our own laboratory, we’ve come to appreciate that fibrin’s structure is not an unnecessary clutter that can be swept away by clean synthetic biomaterials, but rather that it is a blueprint for superior biomaterial design.
2.1 Fibrinogen and Fibrin Polymerization
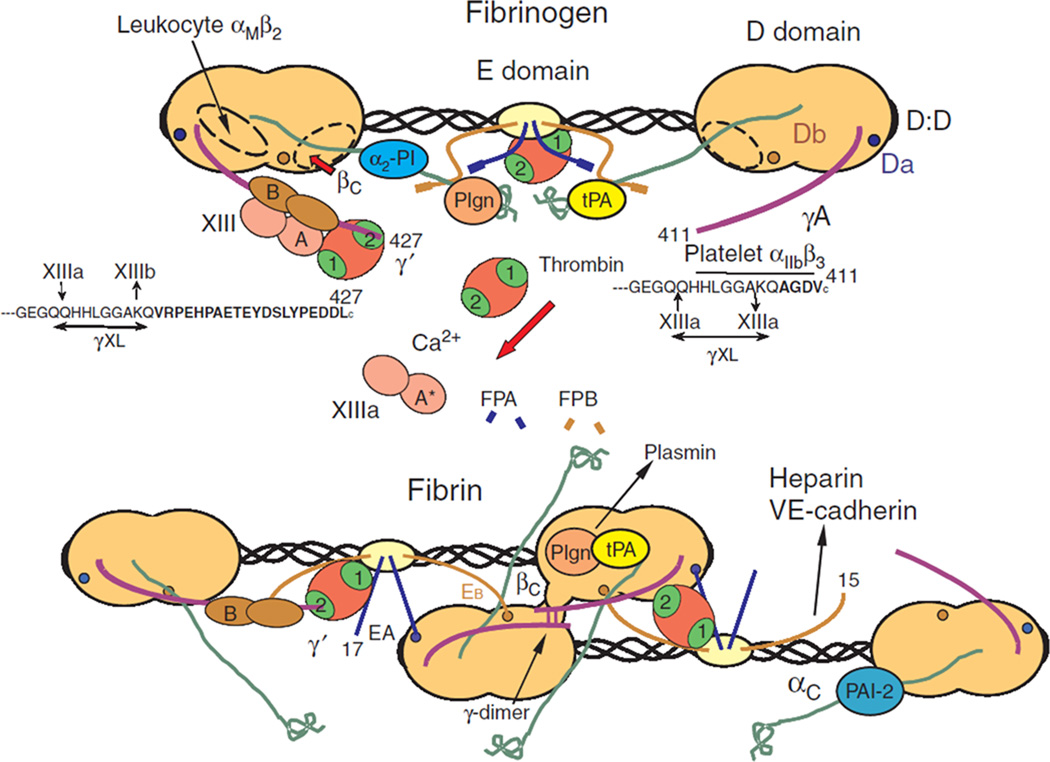
The fibrin matrix is a web of cross-linked fibrils generated by cleavage of fibrinogen, a soluble plasma protein which circulates in the body at a concentration of approximately 3 mg/ml [2]. Fibrinogen is a hexameric protein containing two identical sets of three disulfide-linked peptides, termed the Aα, Bβ(3, and γ chains. These peptides form a symmetric, extended protein composed of two flanking D-domains and a central E-domain linked by two coiled-coil regions [3]. This dumbbell-shaped structure is central to the polymerization of fibrin, formed from fibrinogen when fibrinopeptides A and B are cleaved by thrombin during the clotting cascade [4]. These cleavages expose the knob-”A” and knob- “B” regions, and their complementary binding sites, holes “a” and “b” [5]. Fibrinopeptides A and B, and therefore knobs “A” and “B,” are located in the E-domain, at the molecule’s center, while holes “a” and “b” are located in the D-domains. In addition, two αC regions are liberated upon cleavage of fibrinopeptide A. These regions are long and thin with a globular head, which can interact with other αC regions to allow for lateral aggregation of fibrils [6]. Studies of the knob:hole interactions support John Ferry’s 1952 hypothesis of fibrin fiber formation [3, 7, 8], which suggests that cleavage of the fibrinopeptides leads to aggregation of fibrinogen monomers through binding of the D and E domains, with lateral aggregation through the αC regions promoting aggregation into protofibrils and larger fibers [9]. This is illustrated in Figure 1, which depicts fibrinogen structure and the early polymerization events of fibrin. Upon assembly, fibrin protofibrils exist in a twisted conformation, reflected in the full fibrin fiber, causing it to twist as well. As additional protofibrils bind the fiber and increase its radius, the fibers undergo ever greater stretching. Once the energy required to stretch a protofibril exceeds the energy of binding, thickening of fibers ceases, limiting fibrin fiber diameter [10].
Figure 1. Schematic of fibrinogen and its assembly into fibrin.
This cartoon representation shows the central E domain of fibrinogen flanked by the two globular D domains linked together by coiled-coil regions. Thrombin cleavage of fibrinopeptides A and B from the E domain leads to dimerization of soluble fibrin molecules into fibrin dimers, the first step of fibrin polymerization. Additionally, thrombin cleavage of factor XIII produces factor XIIIa, which crosslinks adjacent γ chains, linking the D domains in the growing fibrin polymer. Figure adapted from [6] with permission from John Wiley and Sons.
The transglutaminase factor XIII is critical for improving the stiffness and stability of the fibrin matrix. Factor XIII is carried by fibrinogen in the blood, bound to fibrinogen’s γ-domain [11], ensuring the presence of ample factor XIII during clotting (Fig. 1). Like fibrinogen, factor XIII is cleaved by thrombin to generate its active form, factor XIIIa. Factor XIIIa reinforces the fibrin matrix by cross-linking laterally-adjacent γ chains, creating γ-γ dimers in protofibrils and accelerating fibrillogenesis. These Factor XIIIa-mediated cross-links are believed to contribute to the extensibility of fibrin fibers [12]. Factor XIII also creates αC-αC cross-links in the fibrin matrix to further stabilize laterally-aggregated protofibrils, increase the elastic modulus of the matrix, and decrease its susceptibility to proteolysis [9, 13, 14].
2.2 Fibrinolysis
Within the lumens of blood vessels, vascular patency is chiefly regulated by the plasmin/plasminogen system, which serves to dissolve clots and prevent fibrin from depositing in various organs. Urokinase-type plasminogen activator (uPa) and tissue-type plasminogen activator (tPa) are capable of cleaving plasminogen and converting it to its active form, plasmin, the protein most responsible for fibrin proteolysis and maintenance of vascular patency in vivo. Plasminogen is a soluble, inactive pro-peptide found in serum that also binds to the αC region of fibrin, at residues Aα392–610. Plasminogen is converted to plasmin via cleavage of Arg561-Val562 [15] in the presence of a ternary complex in which plasminogen and tPa are both bound to fibrin, localizing proteolysis to the clot [16]. Plasmin cleaves fibrin in a series of steps resulting in fragments of various sizes being solubilized and freed from the clot; the size of the final degradation products is believed to be dependent on the presence of blood flow, which can carry away larger fragments [17].
Additionally, cell-surface receptors for plasminogen are hypothesized to be of use for localizing fibrin proteolysis during various cell processes [18]. For example, ECs have been shown to express the cell membrane protein urokinase plasminogen activator receptor (uPaR), which binds uPa, and in certain contexts is required for tube formation [19]. However, as we will discuss later in this review, it is now pretty clear that an alternate fibrinolytic mechanism that depends on matrix metalloproteineases (MMPs) is responsible for EC-mediated degradation of the provisional fibrin matrix during sprouting angiogenesis.
2.3 Cell Binding to Fibrin
Cells can adhere to fibrin directly via multiple motifs, and indirectly via interactions between fibrin and other ECM proteins. Discussing all of the possible mechanisms by which cells can bind to fibrin is beyond the scope of this review, but we would like to highlight a few that are particularly important in how endothelial cells (ECs) interact with fibrin. Perhaps the most significant adhesive interaction between ECs and fibrin occurs indirectly via fibronectin, which has the capacity to bind directly to fibrin. Like fibrinogen itself, fibronectin is present in blood serum, which ensures it is readily available to bind to fibrin during clot formation in vivo. Multiple adhesive domains within fibronectin are then available for binding of ECs and other cells. Furthermore, ECs [20, 21] and fibroblasts [22] can also both bind to Arg-Gly-Asp (RGD) sequences in fibrin using the αvβ3 integrin receptor, although there is evidence that this binding mechanism is not required for endothelial tube formation [23]. Alternatively, the β15–42 segment of fibrin, a heparin binding domain exposed upon cleavage of fibrinopeptide B [24, 25], can also play a role in EC attachment to fibrin via their VE-cadherin receptors [26–28], and exposure of this sequence may be critical for EC spreading and capillary formation in fibrin gels [23, 25]. Additional peptide sequences accommodate leukocyte [29] and platelet [21] binding.
3. Using fibrin to study angiogenesis
3.1 Conventional angiogenesis assays
Fibrin supports robust angiogenesis in a variety of assays including 2D and 3D environments, mono- and co-culture systems, and other systems capable of examining the influences of mechanical stimuli and microfluidic gradients. The simplest 2D assay performed on a fibrin gel is a cord formation assay, where endothelial cells (ECs) are seeded atop the gel and induced to form cords [30]. This assay typically requires only 18–24 hours to generate cord structures, making it a popular assay. Although many molecules regulating angiogenesis have been discovered via this type of assay, a major weakness is that non-EC types have also been shown to form cords [30].
By contrast, many truly 3D assays have been developed in fibrin and other natural hydrogels. One of the most convenient and widely-used 3D assays was originally developed by Nehls and Drenckhahn, who coated calf pulmonary artery ECs onto microcarrier beads, embedded them in a fibrin gel, and showed they migrate off the beads and undergo angiogenesis when stimulated by certain soluble factors [31]. An advantage of this type of technique is that the microcarrier provides a reference point for easy quantification of vessel length and direction. The microcarrier-based technique has been expanded upon to include various EC types, but modification of the assay was required for translation to human cells. Human umbilical vein endothelial cells (HUVECs) were shown to form capillaries only when co-cultured with a supporting cell type. Human dermal and lung fibroblasts cultured atop the gel stimulated the formation of robust, lumen-bearing capillaries (Figure 2) [32, 33]. Attenuation of angiogenesis was achieved by increasing the distance between the two cell types [34] and by increasing matrix density [32, 35]. Distributing either mesenchymal stem cells (MSCs) [32] or lung fibroblasts [35] throughout the matrix resulted in a nearly complete rescue of capillary network length, demonstrating that this system can be used to study more intimate interactions between different cell types and their effects on angiogenic sprouting.
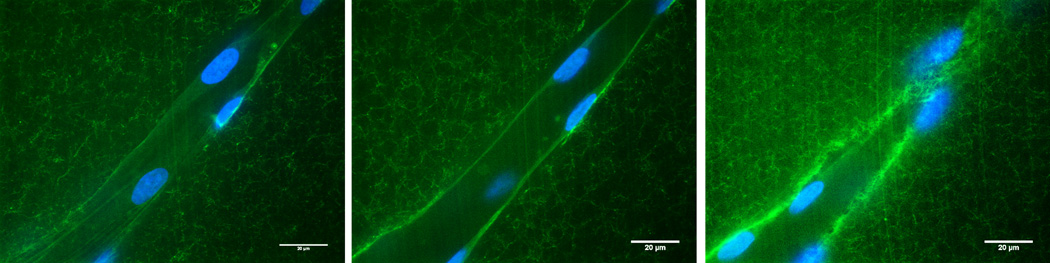
Figure 2. Fibrin hydrogels are useful as 3D model systems of angiogenic sprouting.
High magnification confocal images of a sprouting capillary in a 3D fibrin matrix containing FITC-conjugated fibrinogen. HUVECs cultured in fibrin gels form capillaries with hollow lumens as demonstrated by confocal sections obtained at the bottom (left), center (middle), and top (right) z-planes of a capillary. HUVECs were stained with Oregon Green 488 phalloidin and nuclei were counterstained with DAPI. The HUVECs create a 3D channel in the fibrin matrix via proteolysis.
A variation of the microcarrier model uses EC spheroids, generated by culturing ECs in non-adherent dishes, causing them to aggregate. When these spheroids are placed into fibrin and collagen gels, they are capable of forming capillaries containing hollow lumens [36]. This assay was originally performed as a mono-culture but has also been adapted for co-culture. Microcarrier and spheroid assays have two additional advantages: first, ECs begin the assay very close together and thus have a large “reserve” of cells to from which to form extensive capillary networks; second, the assay is more representative of an in vivo angiogenic program because the cells begin as a confluent monolayer.
Another simpler 3D assay is more representative of vasculogenesis. ECs randomly distributed in a hydrogel are induced to form a de novo capillary network. The advantage of this system is that the homogeneous cell distribution is amenable to in vivo, microfluidic, and multi-well plate formats. Systems with homogeneously distributed ECs have been used to examine integrin attachments during EC vacuolation, a result that would be difficult to observe in 2D culture [37]. Fibrin/collagen composite matrices containing a co-culture of ECs and MSCs have also been created to probe the role of matrix composition on vascular network formation. Notably, increasing the fibrin content improved vasculogenesis in these gels [38]. Although more difficult to quantify than microcarrier-based angiogenesis assays, vasculogenesis assays replicate many of the same features and may also be more representative of the process by which a bolus of cells injected within a supporting ECM in vivo form new vasculature.
3.2 Microfluidic Systems
Studying angiogenesis, and specifically the perivascular niche, is simplified in microfluidic systems [39, 40]. Our laboratory has used one such device to image logenesis in fibrin gels, and discovered that MSC recruitment to perivascular locations is α6β1 integrin-dependent [39]. Recently, systems with perfusable vasculature have been developed to improve the physiological relevance of these in vitro microfluidic assays. In one study, human endothelial colony forming cell-derived Ecs (ECFC-ECs) and normal human lung fibroblasts were co-cultured in a microfluidic device containing daisy-chained tissue chambers and shown to produce vasculature that inosculates with a perfusable flow chamber [41]. Another device used fibrin-filled parallel chambers that allowed invading ECs to form perfusable lumens spanning the length of the chamber [42].
3.3 Structure-Function Relationships in Angiogenesis
Fibrin provides a permissive matrix for angiogenesis, circumventing the need to engineer a new material to study cell behavior in this context. As a result, fibrin-based systems remain useful tools in which to study morphogenesis, but understanding the precise signals fibrin provides to the constituent cells remains an ongoing challenge.
There are several unanswered questions about fibrin’s ability to support angiogenesis. Few studies directly compare performance between ECM materials, and those that do use widely differing protocols and materials [38, 43, 44], making it difficult to compare across studies. Additionally, the differences between the cues fibrin presents to constituent cells and the cues other ECM materials present are not well understood. Parsing out how the structural properties of fibrin differentiate it from other materials, with respect to how it presents morphogenetic cues to constituent cells, can guide the development of future biomaterials capable of more intricate control of cell behavior.
4. Protease-mediated migration and angiogenesis in fibrin
The present and the future of biomaterial design lies in the concept of biomimicry. Generating materials that interact with living tissues in predictable, therapeutically-useful ways is dependent on understanding how cells interact with native tissues in vivo, especially in both regenerative and pathologic microenvironments. The presence of fibrin-rich matrices in both of these microenvironments has led to investigation of cell migration and signaling in fibrin gels.
Damage to existing vessels as a consequence of wounding results in fibrinogen leaking from existing vessels and forming a provisional clot upon enzymatic cleavage by thrombin. This clot serves as an interstitial matrix that must be actively remodeled by resident cells for a wounded tissue bed to be revascularized. Furthermore, during sprouting angiogenesis in vivo, fibrinogen may leak from budding neovessels and generate a fibrin-rich matrix that must be negotiated by invading cells. The MMP family of zinc-dependent endopetidases break down a variety of matrix proteins, and are now known to complement, and sometimes replace, the plasmin/plasminogen system during angiogenesis. Membrane type-1 matrix metalloproteinase (MT1-MMP) was first implicated in angiogenesis when it was observed that plasmin deficiency did not inhibit revascularization of mouse skin wounds [45] or fibrin neovascularization [46], leading to the discovery that MT1-MMP is a critical, and perhaps required, fibrinolytic enzyme during angiogenesis [46]. Our own lab has expanded upon this work with in vitro studies using primary human cells, where we have shown that both MMPs and plasmin can be employed for neovascularization in fibrin gels. Which proteases are involved depends in part on the choice of supporting stromal cells used to support angiogenesis in our fibrin-based co-culture system [47, 48]. In addition, silencing MMP-2 and MMP-9 exhibited no effect on angiogenesis in our co-culture model, implying that MT1-MMP is the most important, and possibly only, MMP used by endothelial cells in this system [49]. When plasmin is employed, MT1-MMP is still active and may play a role in regulating vessel width [48]. This illustrates that in addition to understanding how particular cell types interact with the ECM in isolation, it is critical to understand how paracrine cross-talk affects cell-matrix interactions.
An evolving mechanistic understanding of these and other types of cell-matrix interactions continues to enhance efforts to engineer ECM mimetics tailored for specific microenvironments. Although fibrin can be degraded by multiple proteases, polyethylene glycol (PEG) based blank-slate materials can be generated with proteolytic susceptibility that is more restricted in a customized manner [50, 51], facilitating mechanistic studies of proteolysis [52, 53]. Building on these concepts, several promising in vitro models of neovascularization have been created in the context of synthetic hydrogels containing adhesive and proteolytically-sensitive peptides [54–57]. These studies are particularly remarkable considering that these gels present a fairly minimal set of instructive cues, relative to a natural material like fibrin. Tethering or sequestering angiogenic growth factors adds an additional element of biomimicy to protease-susceptible synthetic hydrogels, and such systems have shown potential to enhance angiogenesis in vivo [56, 58, 59]. Although it cannot be said that these materials are directly “fibrin-inspired,” there is at least one strategy that claims inspiration directly from fibrin: factor XIIIa has been used to create hydrogels of PEG-peptide conjugates [60]. An enzymatic cross-linking step, which is completed under more physiological conditions than other chemical cross-linking strategies, represents a step toward bridging the gap between natural and synthetic materials. We believe that synthetic materials can be further improved by mimicking additional, perhaps undiscovered, features present within natural hydrogels like fibrin.
5. Studying mechanotransduction with fibrin
As the provisional matrix in wound healing, fibrin’s main function is to plug wounds and stop blood loss, resisting the blood pressure and shear stress to stay in place. Fibrin fibers and clots exhibit unique mechanical properties that are likely to assist in its primary function, although the details of how are as yet incompletely known. Understanding the mechanical design principles behind fibrin clots is already facilitating strategies to engineer new biomaterials, but also led to efforts to understand how cells embedded within fibrin sense and respond to mechanical cues.
Fibrin is one of the most extensible proteins known, exceeding even elastin [61]. Individual fibers can be strained to approximately 330% [62] and whole clots to at least 150% [63, 64], and still recover elastically. Fibrin also exhibits strain stiffening, like most biological matrix proteins. These characteristics are believed to be caused by the unique unfolding of the individual fibrin monomers under strain, maintaining gel integrity and extensibility while possessing a low volume of protein [61–64]; these effects are typically not present in synthetic gels. Furthermore, fibrin’s nonlinear elasticity enables cell-cell communication through mechanical means across relatively long distances. For example, fibroblasts sparsely seeded on (2D) and within (3D) fibrin matrices were able to communicate and form patterns at distances of approximately 0.5 mm, or about 5 cell-lengths away. This effect was not present when linearly elastic polyacrylamide gels were used [65].
One potential mechanism by which both externally applied and cell-generated forces can potentially alter cell responses in fibrin is via force-induced alignment of the matrix. However, there are discrepant views about fibrin’s potential for contact guidance. Korff and Augustin reported that sprouts from EC-containing spheroids cultured close together grew toward each other in 3D collagen gels [36]. They also cultured EC spheroids atop collagen and fibrin gels, and observed directional migration only on collagen. They concluded that alignment of the large (several microns long) collagen fibers was responsible for the directional migration, while the fibrin fibers were too short to align and thus unable to provide as a contact-guidance cue. This study was a key step in the examination of how fibrillar structure can be exploited to guide cell growth, but the 2D nature of the comparison between collagen and fibrin limited its utility. Later work has shown that fibrin can play a role in contact guidance.
Recent studies in fibrin have shown the importance of mechanical effects such as fiber alignment and gradients in fiber stiffness for directing cellular alignment, migration, and angiogenesis. Fibrin clots undergo pronounced fiber alignment at only 25% strain, which induces alignment of smooth muscle and endothelial cells cultured within the gels [66], and EC sprouts have been shown to align parallel to fibers aligned magnetically and by cell traction forces [67]. Fiber alignment also affects ECM deposition by embedded cells; however it is difficult to separate the effects of fiber alignment from those of matrix stress generated by cell traction forces [68].
Cell alignment in fibrin gels also appears to be dependent on local anisotropies in matrix stiffness. Using a device capable of applying small strains to fibrin gels, combined with laser tweezers for measuring local matrix stiffness, it has been demonstrated that applied strains generate stiffness gradients in fibrin matrices without detectable changes in fiber architecture. Smooth muscle cells cultured within these strained gels aligned perpendicular to the gradient (or parallel to the applied strain), showing that cells respond to gradients of matrix stiffness within natural biomaterials [69]. Such matrix anisotropies may also be important in angiogenesis. For example, we have shown that the matrix surrounding growing capillaries is 2X stiffer than the matrix elsewhere [70], which complements other results showing that branching morphogenesis occurs in mammary epithelium at regions of high strain [71]. Matrix anisotropies may thus be a general mechanism by which cells orient themselves and communicate with each other in 3D environments, and natural biomaterials are especially conducive to studying these types of phenomena. However, quantification of stress-strain relationships is difficult in natural materials because they are often inhomogeneous. This complication highlights a potential advantage of synthetic biomaterials in the context of better understanding mechanical influences on cell behavior in 3D. However, the dynamic, strain-stiffening properties of natural materials provide a means for long range cell-cell communication, and ways to replicate this type of behavior in synthetic biomaterials likely need to be incorporated into synthetic biomaterials if they are to support robust morphogenetic programs.
The vast majority of mechanobiology studies of ECs have focused on their response to shear stresses induced by blood flow [72], but ECs are also sensitive to cyclic strain. ECs cultured in vitro have shown increased expression and secretion of tPA [73, 74] and various MMPs [75] when strained, implying a role for cyclic strain in vascular remodeling. Work from our own lab has used a modified version of the microcarrier bead assay described earlier to show that HUVECs will form capillaries parallel to the direction of applied uniaxial strain without affecting total vascular network length [76]. Fibrin enables these experiments because it robustly supports angiogenesis, but improvements in synthetic gel preparation, inspired by structure-function relationships in fibrin, may allow them to support these experiments as well.
6. Clinical Applications of Fibrin
Unmodified fibrin has also been extensively used as a vehicle for cell delivery in many tissue engineering applications, and a broad review can be found in [1]. Notably, Fibrin glues and constructs more closely representing physiological fibrin concentrations have been prepared, and cells from virtually all tissues have been implanted into animals using fibrin scaffolds. Cell transplantation is much more effective when using a scaffold material to contain the implanted cells [77, 78], and fibrin is an ideal scaffold for transplantation because precursor solutions containing fibrinogen, thrombin, and cells can be injected in a minimally-invasive manner without the need for open surgery.
The most notable achievement of fibrin research is the use of fibrin glue as a surgical sealant. Tisseel™, cleared for use by the Food and Drug Administration in 1998, was the first commercial fibrin sealant in the United States. Surgeons had earlier been using fibrin glue from noncommercial sources [79]. Fibrin sealants have displayed biocompatibility and performance in surgical hemostatic applications. Although all naturally derived, non-recombinant biomaterials carry a risk of viral or prion infection, fibrin sealants have benefitted from excellent screening procedures and have not been linked to transmission of any viral pathogen [80]. The sealants are a combination of high-concentration, frozen or lyophilized fibrinogen and thrombin packaged in a double-barreled syringe so that the components mix as the sealant is dispensed [81]. These glues typically contain factor XIII and aprotinin to improve clot mechanics and prevent sealant degradation. Concentrations of fibrinogen and thrombin vary from 60–120 mg/ml and 250–1000 IU/ml, respectively, representing concentrations several orders of magnitude above physiological. These features decrease clotting time and improve clot rigidity [82].
Fibrin matrices have been used to study vascularization strategies for tissue engineering and treatment of ischemic disease. The most prominent of these is ischemia due to myocardial infarction (MI), a leading cause of death in the United States. The first attempt to treat this in mice using fibrin glue showed that the injection of a Tisseel™ fibrin matrix improved cardiac function and neovascularization in the infarct site with and without the inclusion of cardiomyocytes in the gel [83, 84]. Additionally, the fibrin glue supported cardiomyocyte survival in vitro for seven days, demonstrating that high concentrations of fibrin are capable of supporting cells.
Another strategy for treating ischemic conditions is the use of pre-vascularized, implantable fibrin tissues. HUVECs and lung fibroblasts have been used to generate such tissues prior to subcutaneous implantation into immune-deficient mice, which led to faster inosculation with the host vasculature than cell-laden, non-vascularized tissues [85]. Pre-vascularization is a promising and potentially translational strategy that may prove even more useful once tissue engineering has matured and generates large tissues and even whole organs. However, whole tissue implantation is invasive and therefore not ideal when an organ may be saved through alternative means. One non-invasive option is to inject a fibrin precursor solution containing appropriate cells to an injury site. We have used subcutaneous injection of cell-laden fibrin precursor solutions to study how co-transplantation of HUVECs with various supporting cell types affects the subsequently formed vasculature. The stromal cells supported vasculature of varying quality, with bMSCs and adipose-derived stem cells (ADSCs) supporting slower vessel growth but more mature vasculature that displayed little leakage, possibly indicative of enhanced wound healing with normal vessel ingrowth. By contrast, NHLFs supported vasculature that grew quickly but remained immature and leaky, characteristic of a pathological wound healing environment or tumor vasculature [86]. The 2.5 mg/ml fibrin gels used persisted up to 2 weeks in vivo, demonstrating that supraphysiological fibrin concentrations are not required in such applications. The versatility of supporting various angiogenic programs and co-culture systems makes fibrin an ideal matrix to study angiogenesis in vivo.
7. Re-engineering fibrin for specific applications
7.1 Modifying Fibrin for Controlled Growth Factor Presentation
Fibrin can be used as a substrate for studying cell behavior in vitro and as a cell-delivery vehicle in vivo, but a fundamental understanding of fibrin’s biochemistry has also led to the ability to augment its capabilities and turn it into a “smart” material. A demonstration of this concept pioneered by Jeffrey Hubbell and colleagues exploits the ability of Factor XIIIa to incorporate bifunctional peptides into the fibrin matrix [87] for novel growth factor presentation and drug delivery applications (Fig. 3). In one example, the extracellular domain of chick ephrin-B2 was attached to fibrin via factor XIII and was shown to increase angiogenesis over control fibrin gels [88] in the absence of cells. Other factors, including β-nerve growth factor (β-NGF) and vascular endothelial growth factor (VEGF), have also been tethered to fibrin matrices [89, 90] via an additional plasmin-sensitive linker, which allows the factors to be released from the matrix upon enzymatic digestion. Similar strategies have been used to engineer increased heparin affinity of fibrin by incorporating a heparin-binding domain at the biologically active site at a ratio of up to 8 moles of peptide per mole of fibrinogen [91]. This approach enabled fibrin to efficiently sequester a variety of heparin-binding growth factors, including acidic and basic fibroblast growth factors (aFGF, bFGF) [92], neurotrophin-3 (NT-3), and platelet-derived growth factor-AA (PDGF-AA) [93].
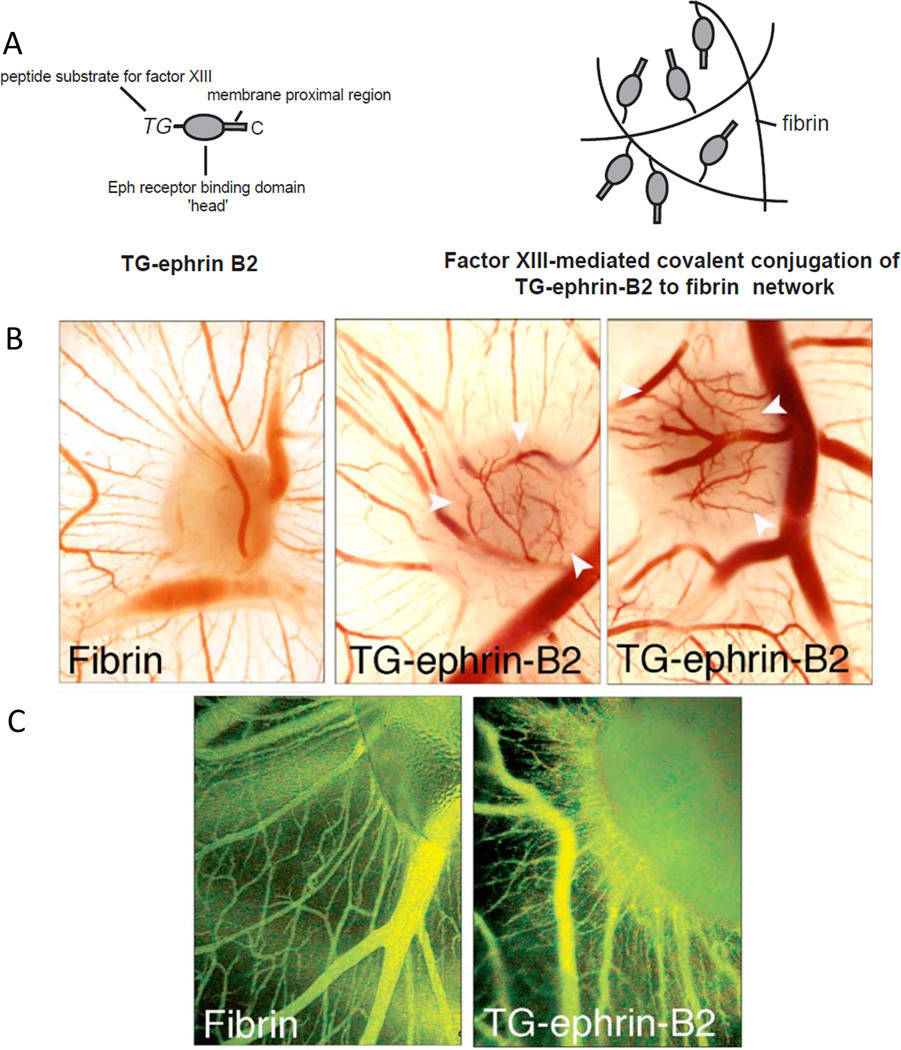
Figure 3. Engineering fibrin for controlled growth factor presentation.
(A) The recombinant protein TG-ephrin B2, the full length ectodomain of ephrin B2 fused to a transglutaminase-sensitive (TG) motif, was conjugated to fibrin via factor XIIIa through the TG region of the protein. (B) Modifying fibrin in this way improved the angiogenic response of acellular fibrin implants in a chick chorioallantoic membrane (CAM) model, producing localized angiogenesis at the implant site. Pure fibrin implants showed little vessel ingrowth. (B) In vivo examination of microvascular growth on the growing CAM revealed strong and specific induction of new vessels by ephrin-B2 from fibrin grafts. Blood vessels were monitored by in vivo fluorescence microscopy performed after Intravenous injections of FITC-dextran, and showed larger diameters of vessels induced by ephrin-B2 indicative of arterioles or venules; by contrast, no changes of the regular vessel pattern of the CAM were observed in response to plain fibrin. Figure adapted from [88] with permission from Elsevier.
Additional methods of engineering fibrin for controlled release and protein sequestration have emerged as well. In one example, heparin was directly attached to fibrin matrices via carbodiimide chemistry at a molar ratio of approximately 1:1, and then used to control the release of bone morphogenetic protein-2 for bone regenerative therapy [94]. In another example, fibrin knob:pocket interactions were engaged by using custom peptides, which were also conjugated to a fibronectin protein fragment. Characterization of the fibrin gels generated using this method showed improved mechanical properties when the “b” pocket was engaged by its conjugate peptide with no decrease in the angiogenic potential of the gels [95]. Despite the fact that fibrin itself has been studied for over a century, these and other examples collectively illustrate the ability to customize it in innovative new ways for specific purposes.
7.2 Chemical Modification of Fibrin(ogen)
Despite fibrin’s versatility in a wide range of regenerative medicine applications, its relatively weak mechanical properties may limit its utility for some applications. One potential means to improve mechanical properties is to conjugate fibrinogen with polyethylene glycol (PEG), either with [96] or without [97, 98] retaining the protein’s thrombin-mediated clottability. PEGylation provides an approach to better control the scaffold material properties, maintain the biological activity of the native protein, and provide a mechanism for sequestering growth factors within the gel for drug delivery. We have shown that PEG-fibrinogen supports cell adhesion, spreading, proliferation, and differentiation of some cell types in vitro [98, 99]; however, its potential to support complex morphogenetic processes, such as angiogenesis, remains unrealized in our hands. A similar PEGylated fibrin material has been used to deliver mesenchymal stem cells into the heart, validating the use of these types of gels for cell therapies in vivo [100]. Similarly, PEG-peptide conjugates which mimic the knob:hole interactions of fibrin have also been used to modify fibrin matrix properties non-covalently by disrupting native knob:hole interactions and decreasing the gel modulus [101]. In this strategy, the fibrin(ogen) molecule was unmodified, leaving its biological functionality intact. It has further been shown that these types of interactions do not disrupt angiogenesis, and thus may lead to improved control of gel mechanics in variety of fibrin-based biomedical applications [102–104].
8. Conclusion
Fibrin is an elegant and prototypical material characterized by a long list of features: fibrillar architecture, considerable extensibility, strain stiffening, multiple adhesive domains, susceptible to multiple proteases, and abilities to sequester and release growth factors. These characteristics enable fibrin to outperform all synthetic material platforms engineered to date, particularly in terms of its ability to support angiogenesis. However, these impressive features also complicate the use of fibrin for fundamental studies as it is typically impossible to decouple fibrin’s multiple instructive cues in an attempt to ascertain how cells respond to any one particular input; thus, the use of fibrin (or any natural material for that matter) for fundamental works requires caution with respect to interpretation. On one hand, efforts to modify fibrin using natural and synthetic means to enhance its function from the “top-down” continue to be well-served by an improved understanding of its structure-property relationships, yielding potential new applications without the need to engineer a new material from scratch. On the other hand, an improved understanding of fibrin’s intricacies and complexities continue to inspire “bottom-up” designs of new material platforms. Together, these two paradigms ensure that fibrin, a classic material with an already long history of utility, will continue to be a staple in the biomaterials community.
Acknowledgments
We gratefully acknowledge financial support from the National Institutes of Health (R01-HL085339 and R21-DE021537). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 2.Clark RA. The molecular and cellular biology of wound repair. Springer; 1996. [Google Scholar]
- 3.Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11–30. doi: 10.1111/j.1749-6632.2001.tb03491.x. [DOI] [PubMed] [Google Scholar]
- 4.Pechik I, Yakovlev S, Mosesson MW, Gilliland GL, Medved L. Structural basis for sequential cleavage of fibrinopeptides upon fibrin assembly. Biochemistry. 2006;45:3588–3597. doi: 10.1021/bi0525369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laudano AP, Doolittle RF. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci U S A. 1978;75:3085–3089. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 7.Lord ST. Fibrinogen and fibrin: scaffold proteins in hemostasis. Current opinion in hematology. 2007;14:236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- 8.Ferry JD. The Mechanism of Polymerization of Fibrinogen. Proc Natl Acad Sci U S A. 1952;38:566–569. doi: 10.1073/pnas.38.7.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorkun OV, Veklich YI, Medved LV, Henschen AH, Weisel JW. Role of the alpha C domains of fibrin in clot formation. Biochemistry. 1994;33:6986–6997. doi: 10.1021/bi00188a031. [DOI] [PubMed] [Google Scholar]
- 10.Weisel JW, Nagaswami C, Makowski L. Twisting of fibrin fibers limits their radial growth. Proc Natl Acad Sci U S A. 1987;84:8991–8995. doi: 10.1073/pnas.84.24.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siebenlist KR, Meh DA, Mosesson MW. Plasma factor XIII binds specifically to fibrinogen molecules containing gamma chains. Biochemistry. 1996;35:10448–10453. doi: 10.1021/bi9606206. [DOI] [PubMed] [Google Scholar]
- 12.Mosesson MW, Siebenlist KR, Hainfeld JF, Wall JS. The covalent structure of factor XIIIa crosslinked fibrinogen fibrils. J Struct Biol. 1995;115:88–101. doi: 10.1006/jsbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- 13.Standeven KF, Ariens RA, Grant PJ. The molecular physiology and pathology of fibrin structure/function. Blood reviews. 2005;19:275–288. doi: 10.1016/j.blre.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Shen LL, Hermans J, McDonagh J, McDonagh RP, Carr M. Effects of calciumion and covalent crosslinking on formation and elasticity of fibrin cells. Thromb Res. 1975;6:255–265. doi: 10.1016/0049-3848(75)90073-0. [DOI] [PubMed] [Google Scholar]
- 15.Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647–654. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- 16.Medved L, Nieuwenhuizen W. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb Haemost. 2003;89:409–419. [PubMed] [Google Scholar]
- 17.Marder VJ, Francis CW. Plasmin degradation of cross-linked fibrin. Ann N Y Acad Sci. 1983;408:397–406. doi: 10.1111/j.1749-6632.1983.tb23260.x. [DOI] [PubMed] [Google Scholar]
- 18.Hajjar KA, Harpel PC, Jaffe EA, Nachman RL. Binding of plasminogen to cultured human endothelial cells. J Biol Chem. 1986;261:11656–11662. [PubMed] [Google Scholar]
- 19.Kroon ME, Koolwijk P, van Goor H, Weidle UH, Collen A, van der Pluijm G, et al. Role and localization of urokinase receptor in the formation of new microvascular structures in fibrin matrices. Am J Pathol. 1999;154:1731–1742. doi: 10.1016/S0002-9440(10)65429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheresh DA, Berliner SA, Vicente V, Ruggeri ZM. Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell. 1989;58:945–953. doi: 10.1016/0092-8674(89)90946-x. [DOI] [PubMed] [Google Scholar]
- 22.Gailit J, Clarke C, Newman D, Tonnesen MG, Mosesson MW, Clark RA. Human fibroblasts bind directly to fibrinogen at RGD sites through integrin alpha(v)beta3. Exp Cell Res. 1997;232:118–126. doi: 10.1006/excr.1997.3512. [DOI] [PubMed] [Google Scholar]
- 23.Chalupowicz DG, Chowdhury ZA, Bach TL, Barsigian C, Martinez J. Fibrin II induces endothelial cell capillary tube formation. J Cell Biol. 1995;130:207–215. doi: 10.1083/jcb.130.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odrljin TM, Francis CW, Sporn LA, Bunce LA, Marder VJ, Simpson-Haidaris PJ. Heparin-binding domain of fibrin mediates its binding to endothelial cells. Arterioscler Thromb Vasc Biol. 1996;16:1544–1551. doi: 10.1161/01.atv.16.12.1544. [DOI] [PubMed] [Google Scholar]
- 25.Bunce LA, Sporn LA, Francis CW. Endothelial cell spreading on fibrin requires fibrinopeptide B cleavage and amino acid residues 15–42 of the beta chain. J Clin Invest. 1992;89:842–850. doi: 10.1172/JCI115663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bach TL, Barsigian C, Yaen CH, Martinez J. Endothelial cell VE-cadherin functions as a receptor for the beta15-42 sequence of fibrin. J Biol Chem. 1998;273:30719–30728. doi: 10.1074/jbc.273.46.30719. [DOI] [PubMed] [Google Scholar]
- 27.Martinez J, Ferber A, Bach TL, Yaen CH. Interaction of fibrin with VE-cadherin. Ann N Y Acad Sci. 2001;936:386–405. doi: 10.1111/j.1749-6632.2001.tb03524.x. [DOI] [PubMed] [Google Scholar]
- 28.Yakovlev S, Medved L. Interaction of fibrin(ogen) with the endothelial cell receptor VE-cadherin: localization of the fibrin-binding site within the third extracellular VE-cadherin domain. Biochemistry. 2009;48:5171–5179. doi: 10.1021/bi900487d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vernon RB, Angello JC, Iruela-Arispe ML, Lane TF, Sage EH. Reorganization of basement membrane matrices by cellular traction promotes the formation of cellular networks in vitro. Lab Invest. 1992;66:536–547. [PubMed] [Google Scholar]
- 31.Nehls V, Drenckhahn D. A novel, microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvasc Res. 1995;50:311–322. doi: 10.1006/mvre.1995.1061. [DOI] [PubMed] [Google Scholar]
- 32.Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875–2888. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 33.Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 34.Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC, et al. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11:257–266. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 35.Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CC, Jeon NL, et al. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J. 2008;94:1930–1941. doi: 10.1529/biophysj.107.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112(Pt 19):3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 37.Bayless KJ, Salazar R, Davis GE. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am J Pathol. 2000;156:1673–1683. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15:253–264. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrion B, Huang CP, Ghajar CM, Kachgal S, Kniazeva E, Jeon NL, et al. Recreating the perivascular niche ex vivo using a microfluidic approach. Biotechnol Bioeng. 2010;107:1020–1028. doi: 10.1002/bit.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trkov S, Eng G, Di Liddo R, Parnigotto PP, Vunjak-Novakovic G. Micropatterned three-dimensional hydrogel system to study human endothelial-mesenchymal stem cell interactions. J Tissue Eng Regen Med. 2010;4:205–215. doi: 10.1002/term.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moya ML, Hsu YH, Lee AP, Hughes CC, George SCIn. Vitro Perfused Human Capillary Networks. Tissue Eng Part C Methods. 2013 doi: 10.1089/ten.tec.2012.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeon JH, Ryu HR, Chung M, Hu QP, Jeon NL. In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip. 2012;12:2815–2822. doi: 10.1039/c2lc40131b. [DOI] [PubMed] [Google Scholar]
- 43.Allen P, Melero-Martin J, Bischoff J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J Tissue Eng Regen Med. 2011;5:e74–e86. doi: 10.1002/term.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicosia RF, Ottinetti A. Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol. 1990;26:119–128. doi: 10.1007/BF02624102. [DOI] [PubMed] [Google Scholar]
- 45.Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 46.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 47.Ghajar CM, Kachgal S, Kniazeva E, Mori H, Costes SV, George SC, et al. Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res. 2010;316:813–825. doi: 10.1016/j.yexcr.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 2011;14:47–59. doi: 10.1007/s10456-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kachgal S, Carrion B, Janson IA, Putnam AJ. Bone marrow stromal cells stimulate an angiogenic program that requires endothelial MT1-MMP. J Cell Physiol. 2012;227:3546–3555. doi: 10.1002/jcp.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 51.Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bott K, Upton Z, Schrobback K, Ehrbar M, Hubbell JA, Lutolf MP, et al. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials. 2010;31:8454–8464. doi: 10.1016/j.biomaterials.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 53.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 54.Hanjaya-Putra D, Bose V, Shen YI, Yee J, Khetan S, Fox-Talbot K, et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118:804–815. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller JS, Shen CJ, Legant WR, Baranski JD, Blakely BL, Chen CS. Bioactive hydrogels made from step-growth derived PEG-peptide macromers. Biomaterials. 2010;31:3736–3743. doi: 10.1016/j.biomaterials.2010.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon JJ, Saik JE, Poche RA, Leslie-Barbick JE, Lee SH, Smith AA, et al. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010;31:3840–3847. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sokic S, Papavasiliou G. Controlled proteolytic cleavage site presentation in biomimetic PEGDA hydrogels enhances neovascularization in vitro. Tissue engineering Part A. 2012;18:2477–2486. doi: 10.1089/ten.tea.2012.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 60.Ehrbar M, Rizzi SC, Schoenmakers RG, Miguel BS, Hubbell JA, Weber FE, et al. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules. 2007;8:3000–3007. doi: 10.1021/bm070228f. [DOI] [PubMed] [Google Scholar]
- 61.Guthold M, Liu W, Sparks EA, Jawerth LM, Peng L, Falvo M, et al. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem Biophys. 2007;49:165–181. doi: 10.1007/s12013-007-9001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W, Jawerth LM, Sparks EA, Falvo MR, Hantgan RR, Superfine R, et al. Fibrin fibers have extraordinary extensibility and elasticity. Science. 2006;313:634. doi: 10.1126/science.1127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferry JD, Morrison PR. Preparation and properties of serum and plasma proteins; the conversion of human fibrinogen to fibrin under various conditions. Journal of the American Chemical Society. 1947;69:388–400. doi: 10.1021/ja01194a066. [DOI] [PubMed] [Google Scholar]
- 64.Brown AE, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science. 2009;325:741–744. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winer JP, Oake S, Janmey PA. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS One. 2009;4:e6382. doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumoto T, Sasaki J, Alsberg E, Egusa H, Yatani H, Sohmura T. Three-dimensional cell and tissue patterning in a strained fibrin gel system. PLoS One. 2007;2:e1211. doi: 10.1371/journal.pone.0001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morin KT, Tranquillo RT. Guided sprouting from endothelial spheroids in fibrin gels aligned by magnetic fields and cell-induced gel compaction. Biomaterials. 2011;32:6111–6118. doi: 10.1016/j.biomaterials.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 68.Sander EA, Barocas VH, Tranquillo RT. Initial fiber alignment pattern alters extracellular matrix synthesis in fibroblast-populated fibrin gel cruciforms and correlates with predicted tension. Ann Biomed Eng. 2011;39:714–729. doi: 10.1007/s10439-010-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kotlarchyk MA, Shreim SG, Alvarez-Elizondo MB, Estrada LC, Singh R, Valdevit L, et al. Concentration independent modulation of local micromechanics in a fibrin gel. PLoS One. 2011;6:e20201. doi: 10.1371/journal.pone.0020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kniazeva E, Weidling JW, Singh R, Botvinick EL, Digman MA, Gratton E, et al. Quantification of local matrix deformations and mechanical properties during capillary morphogenesis in 3D. Integr Biol (Camb) 2012;4:431–439. doi: 10.1039/c2ib00120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gjorevski N, Nelson CM. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr Biol (Camb) 2010;2:424–434. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sumpio BE, Chang R, Xu WJ, Wang XJ, Du W. Regulation of tPA in endothelial cells exposed to cyclic strain: role of CRE, AP-2, and SSRE binding sites. The American journal of physiology. 1997;273:C1441–C1448. doi: 10.1152/ajpcell.1997.273.5.C1441. [DOI] [PubMed] [Google Scholar]
- 74.Diamond SL, Eskin SG, McIntire LV. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science. 1989;243:1483–1485. doi: 10.1126/science.2467379. [DOI] [PubMed] [Google Scholar]
- 75.Cummins PM, von Offenberg Sweeney N, Killeen MT, Birney YA, Redmond EM, Cahill PA. Cyclic strain-mediated matrix metalloproteinase regulation within the vascular endothelium: a force to be reckoned with. Am J Physiol Heart Circ Physiol. 2007;292:H28–H42. doi: 10.1152/ajpheart.00304.2006. [DOI] [PubMed] [Google Scholar]
- 76.Ceccarelli J, Cheng A, Putnam AJ. Mechanical Strain Controls Endothelial Patterning During Angiogenic Sprouting. Cellular and Molecular Bioengineering. 2012;5:463–473. doi: 10.1007/s12195-012-0242-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nat Med. 1996;2:824–826. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- 78.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 79.Spotnitz WD. Fibrin sealant: past, present, and future: a brief review. World journal of surgery. 2010;34:632–634. doi: 10.1007/s00268-009-0252-7. [DOI] [PubMed] [Google Scholar]
- 80.Dunn CJ, Goa KL. Fibrin sealant: a review of its use in surgery and endoscopy. Drugs. 1999;58:863–886. doi: 10.2165/00003495-199958050-00010. [DOI] [PubMed] [Google Scholar]
- 81.Radosevich M, Goubran HI, Burnouf T. Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox sanguinis. 1997;72:133–143. doi: 10.1046/j.1423-0410.1997.7230133.x. [DOI] [PubMed] [Google Scholar]
- 82.Jackson MR. Fibrin sealants in surgical practice: An overview. American journal of surgery. 2001;182:1S–7S. doi: 10.1016/s0002-9610(01)00770-x. [DOI] [PubMed] [Google Scholar]
- 83.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 84.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 85.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CC, et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15:1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grainger SJ, Carrion B, Ceccarelli J, Putnam AJ. Stromal Cell Identity Influences the In Vivo Functionality of Engineered Capillary Networks Formed by Co-delivery of Endothelial Cells and Stromal Cells. Tissue Eng Part A. 2013;19:1209–1222. doi: 10.1089/ten.tea.2012.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjugate chemistry. 1999;10:75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 88.Zisch AH, Zeisberger SM, Ehrbar M, Djonov V, Weber CC, Ziemiecki A, et al. Engineered fibrin matrices for functional display of cell membrane-bound growth factorlike activities: study of angiogenic signaling by ephrin-B2. Biomaterials. 2004;25:3245–3257. doi: 10.1016/j.biomaterials.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 89.Sakiyama-Elbert SE, Panitch A, Hubbell JA. Development of growth factor fusion proteins for cell-triggered drug delivery. Faseb J. 2001;15:1300–1302. doi: 10.1096/fj.00-0564fje. [DOI] [PubMed] [Google Scholar]
- 90.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF--fibrin matrices for endothelialization. Journal of controlled release : official journal of the Controlled Release Society. 2001;72:101–113. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 91.Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. Faseb J. 1999;13:2214–2224. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- 92.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. Journal of controlled release : official journal of the Controlled Release Society. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 93.Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama-Elbert SE. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft matter. 2010;6:5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang HS, La WG, Bhang SH, Jeon JY, Lee JH, Kim BS. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng Part A. 2010;16:1225–1233. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- 95.Soon AS, Stabenfeldt SE, Brown WE, Barker TH. Engineering fibrin matrices: the engagement of polymerization pockets through fibrin knob technology for the delivery and retention of therapeutic proteins. Biomaterials. 2010;31:1944–1954. doi: 10.1016/j.biomaterials.2009.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barker TH, Fuller GM, Klinger MM, Feldman DS, Hagood JS. Modification of fibrinogen with poly(ethylene glycol) and its effects on fibrin clot characteristics. Journal of biomedical materials research. 2001;56:529–535. doi: 10.1002/1097-4636(20010915)56:4<529::aid-jbm1124>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 97.Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 98.Peyton SR, Kim PD, Ghajar CM, Seliktar D, Putnam AJ. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials. 2008;29:2597–2607. doi: 10.1016/j.biomaterials.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim PD, Peyton SR, VanStrien AJ, Putnam AJ. The influence of ascorbic acid, TGF-beta1, and cell-mediated remodeling on the bulk mechanical properties of 3-D PEG-fibrinogen constructs. Biomaterials. 2009;30:3854–3864. doi: 10.1016/j.biomaterials.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A. 2008;14:1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 101.Soon AS, Lee CS, Barker TH. Modulation of fibrin matrix properties via knob:hole affinity interactions using peptide-PEG conjugates. Biomaterials. 2011;32:4406–4414. doi: 10.1016/j.biomaterials.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stabenfeldt SE, Gourley M, Krishnan L, Hoying JB, Barker TH. Engineering fibrin polymers through engagement of alternative polymerization mechanisms. Biomaterials. 2012;33:535–544. doi: 10.1016/j.biomaterials.2011.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang G, Wang X, Wang Z, Zhang J, Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12:9–19. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 104.Zhang G, Drinnan CT, Geuss LR, Suggs LJ. Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta biomaterialia. 2010;6:3395–3403. doi: 10.1016/j.actbio.2010.03.019. [DOI] [PubMed] [Google Scholar]