Abstract
Informed consent is crucial in research, but potential participants may not all speak the same language, posing questions that have not been examined concerning decisions by institutional review boards (IRBs) and research ethics committees’ (RECs) about the need for researchers to translate consent forms and other study materials. Sixty US IRBs (every fourth one in the list of the top 240 institutions by The National Institutes of Health funding) were contacted, and leaders (eg, chairs) from 34 (response rate=57%) and an additional 12 members and administrators were interviewed. IRBs face a range of problems about translation of informed consent documents, questionnaires and manuals—what, when and how to translate (eg, for how many or what proportion of potential subjects), why to do so and how to decide. Difficulties can arise about translation of specific words and of broader cultural concepts regarding processes of informed consent and research, especially in the developing world. In these decisions, IRBs weigh the need for autonomy (through informed consent) and justice (to ensure fair distribution of benefits and burdens of research) against practical concerns about costs to researchers. At times IRBs may have to compromise between these competing goals. These data, the first to examine when and how IRBs/RECs require researchers to translate materials, thus highlight a range of problems with which these committees struggle, suggesting a need for further normative and empirical investigation of these domains, and consideration of guidelines to help IRBs deal with these tensions.
Institutional review boards (IRBs) and research ethics committees (RECs) need to ensure informed consent, and the fair distribution of the benefits and burdens of research; but potential participants in a study may not all speak the same language, posing questions of when and how IRBs/RECs should require that researchers translate their consent forms and study materials. Such translation can incur challenges and costs to researchers, creating tensions. How these committees deal with these conflicts is unclear.
These issues can arise in both domestic and international research. In the USA, for instance, about 20% of the population speaks a language other than English at home.1 The need for translation of informed consent and other research-related documents for non-English speakers has been well documented.2 Such translation can increase comprehension and uptake of important medical tests and services.3 It has long been recommended that documents be translated and back-translated to verify the accuracy of the translation.4 Nonetheless, the readability of these forms has been questioned,5 and comprehension may remain somewhat limited.6
Anecdotally, IRBs in the USA have often asked researchers to translate all documents when a relatively small number of potential participants may not read and speak English, even though researchers have felt that the likelihood of recruiting such participants was negligible.
But it is not clear how IRBs make decisions about these matters. I have found no studies examining how IRBs or RECs view and make decisions about translation.
Recent research on US IRBs, has explored how these committees make decisions, showing, for instance, that they view research integrity (RI) very broadly.7 Interviewees’ perceptions of RI are related to how they saw and dealt with conflicts of interest,8 relationships with researchers,9 undue influence,10 central IRBs,11 and so-called ‘community’ members,12 variations between IRBs13 and research in the developing world.14 Critical issues also arise concerning how IRBs view many aspects of consent forms, including translation of these and other study documents. This paper examines these topics.
METHODS
In brief, as described elsewhere,7–14 telephone interviews of about 2 h each were conducted with 46 chairs, directors, administrators and members. The leadership of 60 US IRBs (every fourth one in the list of the top 240 institutions by The National Institutes of Health (NIH) funding) were contacted, and IRB leaders from 34 of these institutions (response rate=57%) were included. At times a chair/director as well as an administrator from an institution (eg, if the chair thought that the administrator could better provide particular details) were included. In all, 39 chairs/directors and administrators from these 34 institutions were interviewed. Table 1 summarises the respondents’ sociodemographic information.
Table 1.
Characteristics of the sample (N=46) of IRB chairs, members, and administrators
| Characteristics | Total | % |
|---|---|---|
| Type of IRB staff | ||
| Chairs/co-chairs | 28 | 61 |
| Directors | 1 | 2 |
| Administrators | 10 | 22 |
| Members | 7 | 15 |
| Gender | ||
| Male | 27 | 59 |
| Female | 19 | 41 |
| Institution rank | ||
| 1–50 | 13 | 28 |
| 51–100 | 13 | 28 |
| 101–150 | 7 | 15 |
| 151–200 | 1 | 2 |
| 201–250 | 12 | 26 |
| State vs private | ||
| State | 19 | 41 |
| Private | 27 | 59 |
| Region | ||
| Northeast | 21 | 46 |
| Midwest | 6 | 13 |
| West | 13 | 28 |
| South | 6 | 13 |
| Total number of institutions represented | 34 | |
IRB, institutional review board.
As seen in table 1, the 46 interviewees included 28 chairs/co-chairs; one director; 10 administrators; and 7 members. In all, 59% were male, and 94% were Caucasian. Interviewees were distributed across geographical regions and institutions by ranking in NIH funding. The Columbia University department of psychiatry IRB approved the study, and all participants gave informed consent.
The interviews probed participants’ views of RI, but also shed important light on many other, broader issues that arose concerning IRBs’ decisions, and interactions and relationships with principal investigators (PIs). Online supplementary appendix A presents relevant portions of the semistructured interview guide. Analyses sought to obtain a ‘thick description’—to understand aspects of participants’ decisions, lives and social situations.15 The methodological approach adapted elements from grounded theory,16 using techniques of ‘constant comparison,’ analysing data from different contexts for similarities and differences.
RESULTS
As seen in figure 1 and described more fully below, IRBs faced a series of questions about when, what and how to translate informed consent forms and other study-related documents, why to do so or not and how to decide.
Figure 1.
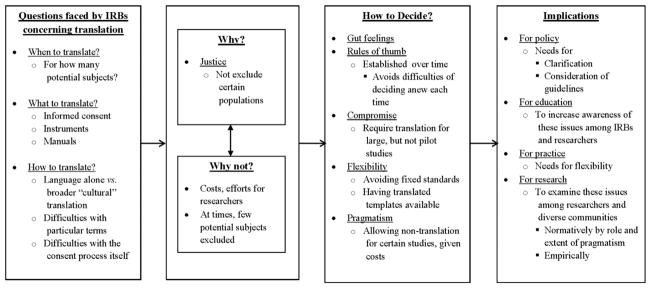
Issues concerning IRBs’ decisions regarding translation of study materials.
Often the need for translation may be clear and straightforward, but challenges and questions can nonetheless arise. Certain studies may be explicitly designed to examine issues among non-English speaking populations, yet IRBs may worry about how well specific terms and broader cultural concepts about informed consent and research are translated. Sites in the developing world may lack an IRB, or have one that is not well functioning, complicating these processes.
For other studies, where non-English speakers constitute only a minority of potential participants, IRBs face other problems of whether and when to require translation, and committees can vary in the thresholds they use in making these determinations. Committees may be uncertain, for instance, if they should require PIs to translate documents even for small numbers of subjects, and if so, how small. Given the relatively high costs to researchers, an IRB may require such effort for larger, but not smaller, pilot studies, yielding to pragmatism rather than pure principalism per se. But some problems remain. As one administrator said,
Some people say that if you have one subject who doesn’t speak English, you need to translate. Our IRB argues about this all the time, because our researchers go into communities that are 11% Hispanic. How many of those people are monolingual in Spanish? If it’s 20%, do we say: “Sorry, you have to translate stuff?” Our scientists are reluctant to start translating everything. But otherwise, you end up excluding people who could benefit from the research, yet don’t speak English. The Feds should come out with a specific formula or cut-off exactly when you’re supposed to translate your documents. IRB26
Problems emerge, too, because not all instruments have been tested and validated in other languages. Hence, IRBs may suggest, but not require, translation. Still, not all IRB members may accept this rationale. As the administrator above added,
We have a really sketchy rule of thumb. We don’t take a heavy stand. We just nudge toward translation. The PIs are real resistant to it. So, we don’t require them to translate, but we’ll discuss it a lot with them, and let them know we’re not happy about it. Translation will be a concern and suggestion, not a requirement. They don’t do it very often. We ask researchers: what will you do if you encounter non-English speaking people? How many do you hope to encounter? IRB26
IRBs may thus have ‘sketchy rules of thumb’ and a wide continuum of degrees and firmness to their positions (eg, from ‘heavy’ to ‘light and flexible’) and requirements for change (eg, from ‘suggestion’ to ‘requirement’).
IRBs may take into account researchers’ budgetary constraints, though at times with ambivalence.
We don’t require pilot and feasibility studies to translate their documents, because the researchers usually have a very small budget—though that’s not a very good reason. A lot of manualized treatments in English just don’t translate well. We also have an Eastern European population, and those treatments haven’t been translated, treated, or validated in those languages. We also look scientifically: how would this affect the cost/benefit ratio? How much effort would it take, and how meaningful would the data be? A lot of IRBs say: “If a person otherwise meets your recruitment criteria, they should be allowed in the study.” IRB26
IRBs may thus recognise that scales require translation and also validation in other languages, complicating these decisions.
Given these vagaries, IRB decisions about whether to require translations may be very subjective, and debated. As one continued,
My gut feeling is that you cannot exclude any subject on the basis of the language they speak. The guidelines say consent forms need to be written in a language that is comprehensible to the person. We haven’t been real rigid with that, but demographics are changing. We’re collaborating a lot more with other geographic areas. So a lot of PIs translate a lot of their documents, or have translators available. But we don’t say you can’t exclude a person anymore because of language. IRB26
IRBs thus wrestle with where to draw these lines, how to decide and how firmly, and are often flexible.
IRBs may seek to help researchers with the need for translation. For instance, an IRB may have standard templates, already translated, that PIs can draw on or use.
We have available a model consent form for behavioral and more than minimal risk research translated into nine different languages, so that researchers don’t have to deny people who really need to be enrolled. IRB16
IRBs may also be flexible in approving studies, even if the researcher has not yet translated all the documents, but will do so. An IRB may decide not to withhold approval, and thereby impede the receipt of research funds, because of the need for translation. IRBs may view the need for translation as less critical than the need to deal with other aspects of a protocol (eg, concerning study design), before being able to approve a study so that the researcher can pursue funding. One IRB for instance approved a protocol for an investigator who still had documents to be translated, but faced a ‘deadline for a small funding institution.’ (IRB8)
Yet other decisions about translation may be among the most difficult that IRBs confront. For instance, institutions with few non-English speakers face particular challenges about whether and when translation is needed, posing abstract notions of justice against added practical and logistical obstacles. IRBs may choose to maintain vague and unspecified cut-off points.
The hardest issue that we have faced is: when is translation necessary. Our minority population is very, very small. So the researchers say, “Of 250 subjects, we expect to have maybe five non-English speakers. It’s too costly.” IRB35
These dilemmas may be hard because they pit justice and participants’ rights against pragmatism and feasibility for researchers. The last time this IRB took a vote was concerning translation.
A scientist studying children in another city’s hospital didn’t want to translate anything into Spanish. We had a feeling that a lot of people there speak Spanish. He just wouldn’t do it, and was pretty upset about it. We voted and barely approved the protocol. The vote was pretty close. We said, “Okay, this is a Phase I feasibility study. We’ll approve it this time in English. But don’t expect to come back here for Phase II without translating.” He was furious. He thought we had a hell of a lot of nerve dictating this. IRB26
IRBs may thus make exceptions for a particular study, but not others. These decisions can also anger PIs, though such frustration may not always be fully justified.
In wrestling with these problems, IRBs may seek compromises. ‘We basically decided not to make it a requirement for small or pilot studies with a small N,’ one interviewee said. ‘We didn’t specify the number. Resistance from researchers made it difficult.’ (IRB35) Here, too, IRBs may acknowledge and incorporate researcher opposition.
Difficulties can emerge since specific words may have different meanings and implications in other cultures. Terms may have different connotations (eg, using slang that may not be appropriate in research), but still get back-translated correctly. Seemingly straightforward medical terms can pose challenges, even in English:
I don’t like the term “asthma” because it will label a child for life. In the US, if you give somebody the label of asthma, they have a pre-condition for the rest of their life. But most asthma goes away! I’d like to find another word to use. But we don’t have another word, so it sort of hangs there as a difficult problem. IRB34
Concerns arise, too, about broader cultural translations—making the informed consent and research practices comprehensible, bridging potentially clashing beliefs. Other societies may view informed consent documents and processes very differently, and investigators may need verbatim translations of words and also understanding and incorporation of these wider meanings. Research in the developing world poses these issues acutely.
A researcher doing ethnographic linguistics research in a Chinese village where people speak a minority language had to translate her consent form and so on. The villagers in China surely thought that this was a bizarre document! But she had to go through the full translation and back translation. IRB7
In some cultures, individuals may be wary of signing documents, which they ordinarily do only when dealing with the police.
IRBs may vary in how much they appreciate and address these difficulties. Committees may seek to be flexible—that is, by allowing oral consent, or seeking additional background about the culture.
A six- or seven-page consent translated into Mayan or Nepali is only part of the problem. Is this going to be meaningful or culturally appropriate to those folks? Otherwise, the translation is a waste of time. So what alternative means might the researchers suggest to show we’re achieving that aim, without us being overly rigid and saying, “Subjects have to sign it. I don’t care”? We try to be flexible, but don’t all have experience in those settings. There’s trust. Maybe key contacts in the community can help us understand what would be appropriate. IRB21
Cultural competence has recently been emphasised as important for physicians—though posing definitional ambiguities—and confronts IRBs as well. IRBs are required to have scientific, but not necessarily cultural, expertise, and must rely on PIs, who may not necessarily have such expertise, either.
Differences in broader cultural views of research endeavours can also pose challenges—that is, about the social and economic contexts and meanings of research itself. One chair fears that individuals in another culture will think about researchers:
“Here comes the White man. You haven’t taken the time to understand us, our needs.” Or, “You’ve collected health data on us for 25 years, and put out peer-reviewed publications. But none of this has helped us with the disease. IRB21
Yet addressing these differences adequately can be difficult.
Consequently, US IRBs may use added caution, seeking assurance that documents have been appropriately translated. Local IRBs in most communities can fulfil certain functions, but may be non-existent or poorly functioning:
We’ll ask, “Is there a local contact here, at least?” We talked about auditing. You hope that we’ll make sure, you know, who did the translation of the materials. We’ll really check. IRB23
CONCLUSIONS
These data highlight how IRBs can face a range of dilemmas about translation of informed consent documents, questionnaires and manuals—what, when and how to translate (eg, for how many or what proportion of potential subjects). In these decisions, IRBs weigh the need for autonomy (through informed consent) and justice (to ensure fair distribution of benefits and burdens of research) against practical challenges and cost. These problems arise in reviewing research conducted both domestically and abroad.
These data suggest, for instance, that IRBs, while not permitting concerns about costs to trump those about autonomy, beneficence or non-maleficence, will nonetheless at times allow these pragmatic obstacles to outweigh concerns about justice (ie, by allowing researchers, when non-English speakers constitute a relatively small minority of potential participants, to conduct studies without translating documents). While the first three of these core principles affect individual participants, justice affects broader groups to which the participant may belong. These data illustrate how, as others have argued, among ethical principles included in the Belmont Report, justice is often approached as secondary to autonomy.17
These data pose larger questions, too, whether, and at what point costs to researchers should outweigh ethical principles. The decision to let costs prevail may reflect pragmatism but raises additional questions of whether, how and when such pragmatism should prevail.
Pragmatism can be important in clinical ethics18 but has received little, if any, attention in research ethics, where the Belmont Report and 45-CFR-4619 mandate principles and do not admit of perceived practical impediments that may legitimately trump these. Arguably, such pragmatism should also be considered in decisions in research ethics.
These data have several implications for future practice, research, policy and education. Standardised informed consent templates could be developed in multiple languages, and be shared among researchers and IRBs. Flexibility may also be helpful, but questions remain of when and how (eg, whether a relatively low percentage of non-English speaking potential participants can justify non-translation, and if so, how low). Further examination and discussion of these questions, as articulated here, is critical, and may assist IRBs in answering them. Further research is needed, for example, to examine among larger samples how often IRBs confront these various dilemmas, what these committees decide, what other factors are involved and how diverse groups of study participants and members of communities being studied also view these tensions and decisions. These interviews raise normative questions as well—whether these decisions to allow costs to outweigh justice, and to adopt various forms of compromise, are ethically justified, and if so, when, to what extent and why. Additional scholarly investigation, both empirical and normative, of when and how oral consent may be permissible for cultural reasons (ie, because signing documents connotes involvement with the police) may also be beneficial.
These data suggest, too, the possible need for clarifications or guidelines from the Office for Human Research Protections, PRIM&R (Public Responsibility in Medicine and Research), or other organisations about these issues, to assist IRBs and researchers deal with them. Educational materials can also be developed to assist IRBs.
This study has several potential limitations. These data are based on in-depth interviews with individual IRB chairs, members and administrators, not direct observation of IRBs meetings, or analysis of IRB records. Future research can also observe IRBs and examine such records. However, such further data may be difficult to collect since, anecdotally, IRBs have at times required that investigators obtain written consent from all IRB members, as well as from the PIs and funders of protocols being discussed at IRB meetings.
In summary, these data, the first to examine when and how IRBs require researchers to translate materials, illustrate how these committees often struggle with a series of problems. These findings highlight the need for enhanced future practice, policy, research and education.
Supplementary Material
Acknowledgments
I thank Patricia Contino for her assistance with this manuscript.
Funding The National Institutes of Health (NIH) (R01-NG04214) and the National Library of Medicine (5-G13-LM009996-02) funded this work.
Footnotes
Competing interests None.
Ethics approval The Columbia University institutional review board.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Readers interested in unpublished data may contact the author.
References
- 1.Linsky A, McIntosh N, Cabral H, et al. Patient-provider language concordance and colorectal cancer screening. J Gen Intern Med. 2010;26(2):142–7. doi: 10.1007/s11606-010-1512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt LM, de Voogd KB. Are good intentions good enough?: informed consent without trained interpreters. J Gen Intern Med. 2007;22:598–605. doi: 10.1007/s11606-007-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavizzo-Mourey R. Improving quality of US health care hinges on improving language services. J Gen Intern Med. 2007;22(Suppl 2):279–80. doi: 10.1007/s11606-007-0382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breslin RW. Back-translation for cross-cultural research. J Cross Cult Psychol. 1970;1:185–216. [Google Scholar]
- 5.McCabe M, Morgan F, Curley H, et al. The informed consent process in a cross-cultural setting: is the process achieving the intended result. Ethn Dis. 2005;15:300–4. [PubMed] [Google Scholar]
- 6.Kithinji C, Kass NE. Assessing the readability of non-English-language consent forms: the case of Kiswahili for research conducted in Kenya. IRB. 2010;32(4):10–15. [PMC free article] [PubMed] [Google Scholar]
- 7.Klitzman R. Views and experiences of IRBs concerning research integrity. J Law Med Ethics. 2011;39(3):513–28. doi: 10.1111/j.1748-720X.2011.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klitzman R. “Members of the same club”: challenges and decisions faced by US IRBs in identifying and managing conflicts of interest. PLoS ONE. 2011;6(7):e22796. doi: 10.1371/journal.pone.0022796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klitzman R. The ethics police? IRBs’ views concerning their power. PLoS One. 2011;6(12):e28773. doi: 10.1371/journal.pone.0028773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klitzman R. How IRBs view and make decisions about coercion and undue influence. J Med Ethics. doi: 10.1136/medethics-2011-100439. Published Online: 14 Sept 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klitzman R. Local IRBs vs. federal agencies: shifting dynamics, systems, and relationships. J Empir Res Hum Res Ethics. 2012;7(3):50–62. doi: 10.1525/jer.2012.7.3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klitzman R. Institutional review board community members: who are they, what do they do, and whom do they represent? Acad Med. 2012;87(7):975–81. doi: 10.1097/ACM.0b013e3182578b54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klitzman R. The myth of community differences as the cause of variations between IRBs. AJOB Prim Res. 2011;2(2):24–33. doi: 10.1080/21507716.2011.601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klitzman R. US IRBs confronting research in the developing world. Dev World Bioeth. 2012;12(2):63–73. doi: 10.1111/j.1471-8847.2012.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geertz C. The interpretation of cultures: selected essays. New York: Basic Books; 1973. [Google Scholar]
- 16.Strauss A, Corbin J. Basics of qualitative research: techniques and procedures for developing grounded theory. Newbury Park, CA: Sage Publications; 1990. [Google Scholar]
- 17.Bayer R, Fairchild AL. The genesis of public health ethics. Bioethics. 2004;18(6):473–92. doi: 10.1111/j.1467-8519.2004.00412.x. [DOI] [PubMed] [Google Scholar]
- 18.Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129–43. doi: 10.1353/ken.1997.0013. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services. Human subjects research protections: enhancing protections for research subjects and reducing burden, delay, and ambiguity for investigators. Fed Regist. 2011;76:44512–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


