Abstract
Morphological alterations of brain structure are generally assumed to be involved in the pathophysiology of obsessive–compulsive disorder (OCD). Yet, little is known about the morphological connectivity properties of structural brain networks in OCD or about the heritability of those morphological connectivity properties. To better understand these properties, we conducted a study that defined three different groups: OCD group with 30 subjects, siblings group with 19 subjects, and matched controls group with 30 subjects. A structural brain network was constructed using 68 cortical regions of each subject within their respective group (i.e., one brain network for each group). Both small-worldness and modularity were measured to reflect the morphological connectivity properties of each constructed structural brain network. When compared to the matched controls, the structural brain networks of patients with OCD indeed exhibited atypical small-worldness and modularity. Specifically, small-worldness showed decreased local efficiency, and modularity showed reduced intra-connectivity in Module III (default mode network) and increased interconnectivity between Module I (executive function) and Module II (cognitive control/spatial). Intriguingly, the structured brain networks of the unaffected siblings showed similar small-worldness and modularity as OCD patients. Based on the atypical structural brain networks observed in OCD patients and their unaffected siblings, abnormal small-worldness and modularity may indicate a candidate endophenotype for OCD.
Keywords: Obsessive–compulsive disorder, Cortical thickness, Brain networks, Modularity, Small-worldness
Introduction
Obsessive–compulsive disorder (OCD) is a highly heritable neuropsychiatric disorder. The first-degree relatives of individuals with OCD have a fivefold increased risk of developing the disorder than the general population (Nestadt et al. 2000; Pauls et al. 1995). There is increasing evidence in recent research that OCD is associated with morphological abnormalities in the cerebral cortex (Fan et al. 2012; Kuhn et al. 2012). Studies of cortical morphology derived from structural magnetic resonance imaging (MRI) have the potential to significantly advance our understanding of the underlying pathophysiology in OCD. Unfortunately, little is currently known about the morphological connectivity properties in OCD patients, and this is a vital step toward a comprehensive understanding of how these brain networks are structurally organized.
Alterations in brain structures and functional activities of canonical fronto-subcortical circuitry have been implicated in the pathophysiological mechanisms of OCD. Structurally, a volume-based meta-analysis on grey matter demonstrated that OCD patients had a reliably smaller volume in both anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC), but an larger thalamic volume than healthy controls, which provides substantial evidence that OCD was related to the structural alteration of the canonical fronto-subcortical circuits (Rotge et al. 2009). These findings were also replicated by a recent voxel-based meta-analysis on grey matter in OCD (Radua and Mataix-Cols 2009). Functionally, a meta-analysis of functional neuroimaging data identified that, compared with healthy controls, OCD patients had significantly different activities in the OFC and caudate nucleus, which are also included in the fronto-subcortical circuits (Whiteside et al. 2004). Based on these documented structural and functional alterations in the fronto-subcortical circuitry, it was proposed that the structural alterations of this circuitry may contribute to the functional disruptions observed in OCD. Recently, several imaging studies have focused on exploring the characteristics of cortical morphology in OCD (Fan et al. 2012; Kuhn et al. 2012). For instance, Kühn et al. found that individuals with OCD exhibited reduced cortical thickness in the ACC and also in several regions within the fronto-parietal network by measuring cortical thickness (Kuhn et al. 2012). But there are still few studies reporting the actual structural connectivity properties within the global cortex in OCD, which may help reveal the characterization of the underlying structural organizations of the brain in OCD patients, and also advance our understanding of how their brains segregate and integrate information.
The structural organization of human cortex network was believed to have features of a complex network, i.e., small-worldness and modularity (Bullmore and Sporns 2009; Chen et al. 2008). In particular, a small-world network is a type of mathematical graph where most nodes can be reached from each other by a small number of steps. This can be characterized by a high degree of local clustering and short-length paths linking individual network nodes (Watts and Strogatz 1998). Small-worldness is a quantitative measure of such small-world network properties. High clustering ensures functionally segregated processing, and short paths provide effective integrity and rapid transfers of information between distant brain regions (Bullmore and Sporns 2009; Sporns et al. 2004). Human brain networks have been consistently demonstrated to have the small-world property, a prominent feature shared by various social, economic, and biological networks (Strogatz 2001). Modularity is the other feature of the human cortical network that is defined as groups of cortical regions that are connected morphologically to achieve the maximum network efficiency. Modularity helps identify relevant substructures that correspond to specific functions, providing a linkage between structure and function in a complex network (Fortunato and Barthelemy 2007). Modularity is a fundamental and ubiquitous feature of complex systems in nature, ranging from social to biological networks (Newman 2006). For example, embryonic tissues are organized with different modules which are considered as repetitive and conserved building blocks that give rise to specific body parts in the adult animal. Within each module, cells often have similar molecular determinants and functional characteristics. Though each module develops and functions relatively independently, there are many interactions between different modules, as the modules undergo temporal and spatial transformation during development. Generally, connectivity is stronger within modules than between modules (Redies and Puelles 2001). Small-worldness and modularity have provided rich quantitative insights into the organization of complex brain networks. For instance, several neuroimaging studies have demonstrated that human brain networks are related to the anatomical modules using diffusion spectrum imaging (Hagmann et al. 2008) and the functional modules using functional MRI (Ferrarini et al. 2009). Moreover, a recent network-based study has detected that neurodegenerative diseases, such as Alzheimer’s disease (AD), also affect the specific large-scale distributed brain systems (Seeley et al. 2009).
Previous studies reported that the structural organizations in the human brain had the properties of modularity (Chen et al. 2008) and small-worldness (He et al. 2007). Atypical morphological features of cortical networks were found in normal aging (Wu et al. 2012) and in some neuropsychiatric diseases, such as AD (He et al. 2008) and schizophrenia (Bassett et al. 2008). Patients with OCD also exhibited abnormal small-world attributions in the functional networks using a resting-state functional MRI (Zhang et al. 2011). However, it is not known for structural networks in OCD patients. Thus, understanding of structural organizational principles in OCD is crucial to the substrate of functional networks.
Due to the common pattern of heritability, both patients with OCD and their unaffected siblings might share a genetic profile associated with vulnerability to OCD. For this reason, siblings might express some traits related to OCD even if they do not fully meet the diagnosis criterion of OCD (Nestadt et al. 2000). Previous studies have identified that unaffected first-degree relatives of patients with OCD have several cognitive and brain structural deficits similar to OCD patients, such as impaired cognitive flexibility and motor inhibition (Chamberlain et al. 2007), delayed response inhibition (Menzies et al. 2007), and abnormal white matter in parietal and frontal regions (Menzies et al. 2008), which can serve as candidate endophenotypes for OCD. Therefore, unaffected siblings can provide rich genetic information for OCD research, i.e., helping to disentangle the state and trait markers of the illness.
The present study aimed to test two hypotheses: (1) whether atypical modularity and small-worldness of structural brain networks exist in patients with OCD and (2) whether similar topological patterns are evident in their unaffected siblings, thus representing a potential biomarker of increased risk for OCD. To test our hypotheses, the entire cerebral cortex of each subject was parcellated into 68 areas. We then calculated the correlation matrix of regional cortical thickness across subjects within each group as a graph that represents the underlying structural cortical network. Finally, the properties of modularity and small-worldness were calculated. We expected that the atypical structural organizations exist in the individuals with OCD and, given the high heritability of OCD, that the similar abnormalities would also be present in their unaffected siblings.
Materials and methods
Subjects
Structural MRI data were acquired for a total of 79 subjects, including 30 OCD patients, 19 siblings, and 30 healthy controls (see Table 1 for demographic information). All subjects were right-handed. Patients with OCD and their unaffected siblings were recruited through Guangzhou Psychiatry Hospital, China, and healthy subjects were recruited by local community and internet advertisements in Guangzhou, China.
Table 1.
Demographic and clinical characteristics for participants
| Characteristics | OCD (N = 30) Mean (SD) |
SIB (N = 19) Mean (SD) |
CON (N = 30) Mean (SD) |
Analysis
|
|
|---|---|---|---|---|---|
| F (df = 2.76) | P value | ||||
| Age (years) | 0.17 | 0.84 | |||
| Male/female | 0.15 | ||||
| Education (years) | 13.7 (3.0) | 14.6 (2.4) | 13.3 (3.7) | 1.10 | 0.34 |
| IQ estimate | 104.2 (16.7) | 104.7 (14.8) | 107.2 (15.7) | 0.30 | 0.74 |
| Age at onset of OCD (years) | 19.5 (5.5) | ||||
| Duration of illness (years) | 8.6 (5.5) | ||||
| Y-BOCS (total) | 27.7 (6.2) | 3.8 (3.7) | 2.0 (2.7) | 279.80 | <0.01 |
| Y-BOCS (obsessions) | 15.7 (3.5) | 1.7 (1.9) | 0.9 (1.2) | 315.46 | <0.01 |
| Y-BOCS (compulsions) | 12.0 (4.9) | 2.2 (2.2) | 1.1 (1.9) | 88.80 | <0.01 |
| OCI-R | 21.9 (12.2) | 9.0 (6.6) | 10.3 (9.3) | 13.73 | <0.01 |
| BDI | 17.0 (13.7) | 3.5 (7.4) | 7.2 (8.0) | 11.54 | <0.01 |
| STAI (state) | 49.9 (17.7) | 29.1 (15.1) | 34.5 (18.1) | 10.08 | <0.01 |
| STAI (trait) | 52.1 (16.8) | 29.4 (17.6) | 34.1 (17.8) | 12.44 | <0.01 |
BDI Beck Depression Inventory, CON healthy control subjects, OCD patients with obsessive–compulsive disorder; OCI-R Obsessive–Compulsive Inventory-Revised, SD standard deviation, SIB siblings of individuals with OCD, STAI state-trait anxiety inventory, Y-BOCS Yale-Brown Obsessive–Compulsive Scale
Patients with OCD were evaluated by a trained clinician (Z.W. P) using the Structured Clinical Interview (SCID) for DSM-IV Axis I Disorders (First et al. 1996). All patients had a primary diagnosis of OCD, excluding hoarding subtypes. Specifically, five of these subjects had comorbid major depressive disorder (three recurrent and two with a single episode, but without psychotic features), and their depressive symptoms were in partial or full remission during clinical evaluation; three had social phobia, one had an eating disorder, and all others had OCD as their sole diagnosis. In addition, patients with comorbid anxiety were included if these symptoms were secondary to their OCD (two had social phobia). According to the OCI-R scores, 16 OCD patients mainly had checking symptoms, 9 for obsessing, 2 for washing, 2 for ordering, and 1 for neutralizing. All patients were received with stable drug treatment, consisting of selective serotonin reuptake inhibitors for the majority of subjects (see Table S1).
The healthy controls and unaffected siblings were interviewed using the SCID for the DSM-IV-TR Axis I disorders, Research Version, Non-Patient edition (SCID-I/NP) (First et al. 2002). Both groups had no history of OCD. Moreover, healthy control subjects reported no history of psychiatric illness within their three degree relatives. Exclusion criteria included psychosis, bipolar disorder, neurological disorder, tic disorder, head injury, serious medical condition, history of drug and/or alcohol addiction, and cardiac pacemakers or other metallic implants or artifacts.
This study was designed in accordance with the Declaration of Helsinki and approved by the ethics committee of Guangzhou Psychiatry Hospital, China. All subjects gave their written informed consent after the procedure had been explained to them.
Clinical assessments
Obsessive–compulsive (OC) symptom severity was evaluated using the Yale-Brown Obsessive–Compulsive Scale (Y-BOCS) (Goodman et al. 1989), and the Obsessive–Compulsive Inventory-Revised (OCI-R) was used to assess OC symptom substyles (Foa et al. 2002; Peng et al. 2011). Symptoms of depression and anxiety were quantified using the Beck Depression Inventory (BDI) (Beck and Steer 1984) and the State-Trait Anxiety Inventory (STAI) (Spielberger 1983), respectively. The Annett Handedness Inventory was used to measure handedness information (Spreen and Strauss 1991). Estimated IQ was assessed with the short form of the Chinese version of the Wechsler Adult Intelligence Scale-Revised (WAIS-R), including four subscales such as information, arithmetic, similarity and digit span (Gong 1992), and subjects with a total score of less than 80 were excluded.
MRI acquisition
MRI scanning was performed on a Signa HDe 1.5-T GE scanner (GE Medical Systems, Milwaukee, WI, USA.) equipped with an 8-channel phased-array head coil at the First Affiliated Hospital of Jinan University, China. Foam pads were used for positioning and immobilization of the subject’s head within the head coil. A high-resolution T1-weighted anatomical image was acquired using a 3-dimensional fast spoiled gradient recalled (FSPGR) sequence with 128 contiguous slices (TR = 8 ms; TE = 1.7 ms; flip angle = 20°; FOV = 240 mm × 240 mm; matrix = 256 × 256; slice thickness = 1.0 mm). Uniform magnetic field was reached before each scanning. No gross abnormalities were observed for any subjects when images were visually inspected by an experienced radiologist (C.Z. S) prior to analysis.
Imaging analysis
Cortical thickness measurement
Freesurfer was used to measure cortical thickness (http://surfer.nmr.mgh.harvard.edu/). In brief, the procedure includes: (1) resampling of all images into isotropic voxel of 1 × 1 × 1 mm3, (2) intensity inhomogeneity correction and skull stripping, (3) segmentation of white matter using a hybrid watershed classifier, (4) searching for the pial surface using a deformable surface algorithm, (5) measuring local cortical thickness as the distance between the inner and the outer cortical surfaces at each vertex (Fischl et al. 1999), and (6) parcellating the brain surface into 68 cortical regions using the Desikan-Killiany cortical atlas (see Tables S2) (Desikan et al. 2006). Cortical thickness measurement results and the brain parcellation map of each subject were used for subsequent structural network construction.
Structural brain network construction
The structural brain network defined in this study was derived from a 68 × 68 correlation matrix computed from 68 regional mean cortical thicknesses for each of the OCD, sibling, and control groups. Structural network construction procedures were thoroughly reported in the previous study (He et al. 2007). In brief, the mean cortical thickness was extracted for each of 68 cortical regions by averaging thickness within the same cortical region. Then, a linear regression was performed at each cortical region to remove the effects of mean cortical thickness, age, gender, and age–gender interaction. The resultant residuals were used to substitute the raw cortical thickness values. Finally, full-weighted structural networks were obtained by calculating Pearson correlation coefficients of the cortical thickness between each pair of regions across all subjects in each group.
Small-world properties
Small-world properties were characterized by the clustering coefficient Cp and the characteristic path length Lp, which measure the extent of interconnectivity of a network at local and global levels, respectively (Watts and Strogatz 1998). Despite the conventional small-world parameters (Cp and Lp), efficiency metrics were also used to provide more biologically sensible properties for brain networks. The global efficiency (Eglob) and local efficiency (Eloc) quantify the extent of the information transmission at the global network and individual node levels, respectively (Latora and Marchiori 2001b). In this study, efficiency metrics were used to measure small-world properties as in (Wu et al. 2012). In brief, a cost threshold range (0.05 ≤ cost ≤ 0.5, step = 0.01) was used to normalize all networks (Achard and Bullmore 2007; He et al. 2009). Then, the small-world regime was defined by: (1) all brain networks were fully connected and (2) resultant brain networks showed sparse and distinguishable properties compared to degree-matched random networks (Bassett et al. 2008). Finally, a range of cost threshold (0.11 ≤ cost ≤ 0.25, step = 0.01) was selected as small-world regime. The analysis procedure is listed in the supplementary material.
Network modularity
A module of a complex network is generally defined as a subset of nodes that are more tightly connected with the other nodes in the same module than with nodes outside the module (Reichardt and Bornholdt 2006). The network modularity of each group was calculated using a modified greedy optimization algorithm (Newman and Girvan 2004) and the detailed procedure was described in the previous study (Chen et al. 2008). Essentially, the correlation map was directly used as a continuously weighted graph G with N (68) nodes and K (2,278, 68 × 67/2) possible weighted edges, where nodes represent cortical regions and edges represent undirected connections between regions. For group comparison, edge weights in the networks of each group were normalized by their total network weight (Chen et al. 2011). In addition, the sum of all connectional weights within one module was defined as an index of intramodule connectivity, and the total connectional weights between two modules as an index of intermodule connectivity. The detailed equations are listed on the supplementary material.
Statistical analysis
To evaluate the significance of the network modularity obtained from the real brain data, we compared it with random networks with the same number of nodes. These random networks were generated from our real network by randomly reassigning the edge weights within the same set of nodes, and repeated 10,000 times. Note that they are still fully connected with switched edge weights. One-sample t test was used to assess whether our network had significantly higher network modularity than random networks.
In order to evaluate the significance of modular connectivity and efficiency metrics among the three groups (OCD patients, siblings, and healthy controls), three between-group comparisons were performed, i.e., OCD vs. Sibling, Sibling vs. Control, and OCD vs. Control. We employed a nonparametric permutation test for the statistical analysis. In brief, for each pair of groups, subjects were randomly reassigned to either group while keeping the number of subjects in each group unchanged. The correlation matrices were recomputed and the network parameters were recalculated. Note that the modular connectivity was directly computed based on the generated weighted networks, while the efficiency metrics were calculated at each threshold level in the small-world regime obtained above (0.11 ≤ cost ≤ 0.25, step = 0.01) (Bullmore et al. 1999). This procedure was repeated 10,000 times. We counted the number of cases where the network parameter difference of groups in permutations was higher than the original difference. This value was considered to be the significance level P, after dividing by the total number of permutations (Wu et al. 2012).
Results
Demographic and clinical characteristics
Table 1 shows the demographic and clinical characteristics of the participants. The three groups were well-matched in age, IQ, gender, years of education, and handedness. There were significant differences in clinical measures (Y-BOCS, OCI-R, BDI, STAI) between the three groups. Post hoc tests found that patients with OCD had higher scores than healthy controls or siblings in total scores for the Y-BOCS, OCI-R, BDI, and STAI. Siblings did not differ from healthy controls in any of these measures.
Small-world property
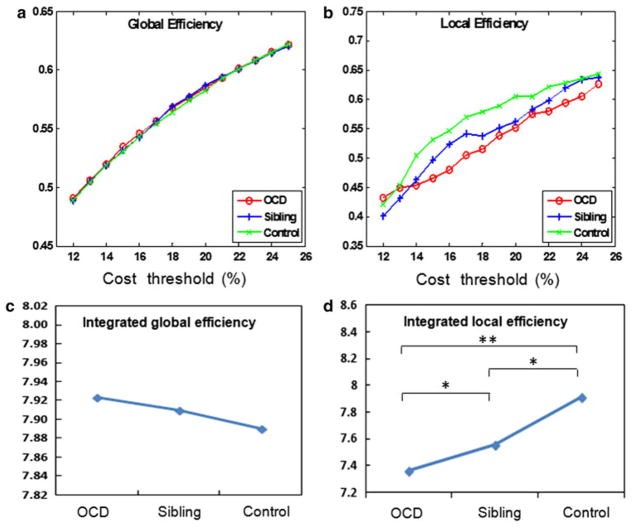
The local and global efficiency curves of structural networks were constructed in three groups using the cost thresholding strategy (0.11 ≤ cost ≤ 0.25, step = 0.01). The efficiency curves were intermediated compared with those of matched random and regular networks. Our results found that the structural networks in three groups showed economical small-world properties. No significant difference was found between the three groups in the threshold levels of global efficiency (Fig. 1a) or in their integrated global efficiency (Fig. 1c). The local efficiency in siblings was significantly lower than that in healthy controls in a majority of costs, but it was significantly higher than that in OCD patients (Fig. 1b). Using the integrated efficiency metrics over the small-world regime, local efficiency properties demonstrated a trendline with positive correlation at OCD patients, siblings, and healthy controls (three between-group P<0.05, Fig. 1d).
Fig. 1.
Economical small-world properties in three groups. a Global efficiency calculated under the cost threshold range of 0.11–0.25; b local efficiency calculated under the cost threshold range of 0.11–0.25; c integrated global efficiency (P>0.05 between groups); and d integrated local efficiency (P<0.05 between groups)
Modularity
Modular organizations
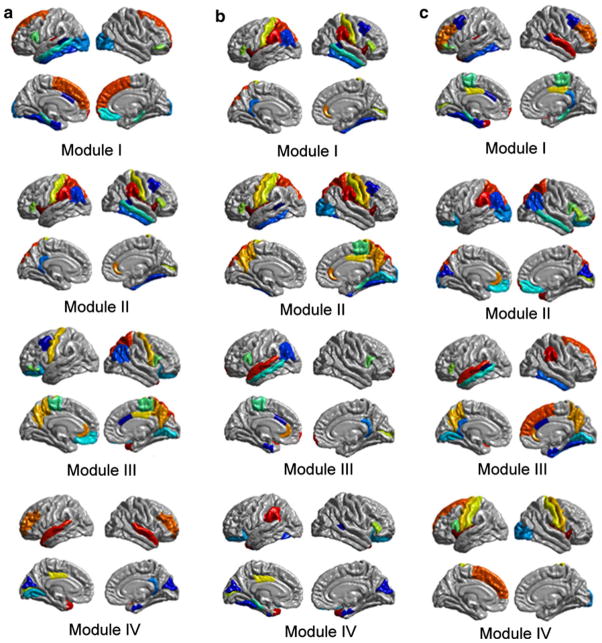
Four functionally oriented modules were detected in each of the three groups (OCD patients, siblings, and healthy controls), and the surface representations for structural modules are shown in Fig. 2. Each group had a different pattern of modular organization. Patients with OCD exhibited four major modules labeled from I to IV, as shown in Fig. 2a. (1) Module I includes 18 regions, such as superior frontal (SF), frontal pole (FP), fusiform gyrus (FUSI), inferior temporal (IT), middle temporal (MT), and lateral occipital (LOCC), which are mainly associated with visual/dorsal attentional function. (2) Module II consists of 18 regions, such as inferior parietal (IP), postcentral gyrus (PSTC), superior parietal (SP), supramarginal (SMAR), pars triangularis (PTRI), and pericalcarine (PERI), which are known to be primarily involved in spatial/language functions. (3) Module III is composed of 19 regions, such as precentral gyrus (PREC) and paracentral lobule (PARC), which are the main components of sensorimotor operations. (4) Module IV includes 13 regions, such as entorhinal, superior temporal (ST), temporal pole (TP), and transverse temporal (TT), which are largely in accordance with auditory functions.
Fig. 2.
Modular organization of the cortical structural network in three groups. a The modular organization of OCD patient’s brain network. Module I: visual/dorsal attentional function. Module II: spatial/language. Module III: sensorimotor. Module IV: auditory. b The modular organization of sibling’s brain network. Module I: cognitive control. Module II: sensorimotor/spatial. Module III: auditory/language. Module IV: visual. c The modular organization of healthy controls brain network. Module I: executive function. Module II: cognitive control/spatial. Module III: default mode network. Module IV: sensorimotor
Unaffected siblings also had four functional modules, as shown in Fig. 2b. (1) Module I includes 19 regions, such as medial orbitofrontal (MOF), pars orbitalis (PORB), and SF, which are mainly associated with cognitive control. (2) Module II consists of 22 regions, such as PREC, PSTC, precuneus, SP, and SMAR, which are known to be primarily involved in sensorimotor/spatial operations. (3) Module III is composed of 13 regions, such as entorhinal, MT, ST, POPE, and PERI, which are the main components of auditory/language functions. (4) Module IV includes 14 regions, such as cuneus, FUSI, and TP, which are largely in accordance with visual functions.
Healthy controls demonstrated four modules in the structural network, as showed in Fig. 2c. (1) Module I includes 23 regions, such as middle frontal (MF), PORB, FP, CAC, isthmus of the cingulate (ISTC), IT, ST, and TP, which are mainly related to executive function. (2) Module II consists of 20 regions, such as lateral orbitofrontal (LOF), IP, SP, and SMAR, which are known to be primarily involved in cognitive control/spatial function. (3) Module III consists of 16 regions, such as SF, IF, precuneus, IT, and MT, which are the main components of the default mode network (DMN). (4) Module IV includes 9 regions, such as PSTC and PREC, which are largely in accordance with the sensorimotor function.
Global brain modularity
The global brain modularity was first calculated in three groups. The cortical networks in the three groups exhibited high modularity when compared with those of the corresponding 10,000 random networks. No significant difference was detected in the global network modularity between the three groups.
Secondly, to compare structural modules among the three groups, the modular organizations in healthy controls were presupposed to represent a more optimized structural organization. Then, structural modules in healthy controls were applied to the OCD patients’ and siblings’ brain network, and the modularity of their networks was recalculated. Compared to healthy controls, patients with OCD and the siblings both exhibited decreased modularity in the global networks (pocd<0.01 and psiblings<0.01), as shown Fig. 3.
Fig. 3.
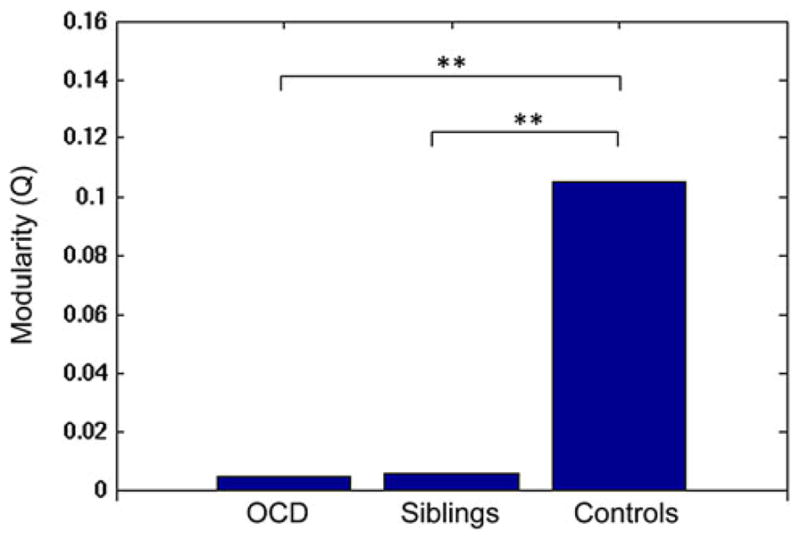
Between-group difference in the network modularity. **P<0.01
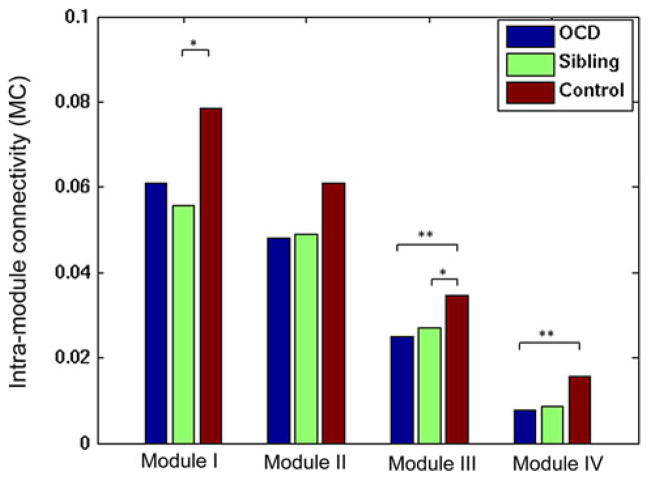
Alterations in the intramodule connectivity
We evaluated the intramodule connectivity in OCD patients and siblings for the four functional modules obtained from healthy controls. Our results found that the patients with OCD showed a significantly reduced modularity in Modules III (pocd = 0.01) and IV (pocd = 0.01) compared to healthy controls, and the siblings showed significantly decreased modularity in Modules I (psiblings = 0.02) and III (psiblings = 0.049). Figure 4 shows these group-level comparisons.
Fig. 4.
Between-group difference in the intramodule connectivity. *P<0.05, **P<0.01
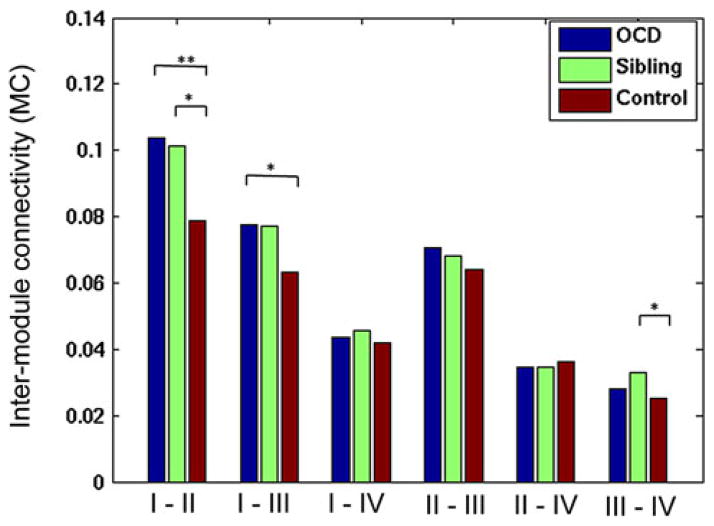
Alterations in the intermodule connectivity
Next, we examined the intermodule connectivity between the three groups using the four functional modules obtained from healthy controls. Compared with the healthy controls, individuals with OCD showed significantly increased intermodule connectivity between Modules I–II (pocd<0.01) and I–III (pocd<0.01), and siblings showed significantly increased modularity between Modules I–II (psiblings = 0.01) and III–IV (psiblings = 0.01). Figure 5 shows the details.
Fig. 5.
Between-group difference in the intermodule connectivity. *P<0.05, **P<0.01
Discussion
Our study is the first to investigate structural brain network modularity and small-world morphological connectivity properties in OCD patients and their unaffected siblings. Our results show that structural brain networks have modularity and small-world properties in all three groups. Of particular interest were the atypical morphological connectivity properties of the structural brain networks found in patients with OCD. Interestingly, unaffected siblings showed similar morphological connectivity patterns as the OCD patients. Our findings provide new insights into the understanding of morphological connectivity properties of structural brain networks in OCD, suggesting that the atypical structural brain networks can serve as a possible hereditary risk indicator for OCD.
Small-world properties of OCD
Our results demonstrated atypical small-world properties when comparing the OCD group to the other two groups. On the one hand, all three groups exhibited economical small-world properties (i.e., high global and local efficiency) in structural brain networks, which is an optimal balance between local specialization and global integration (Strogatz 2001). Our findings also coincided with previous MRI studies about structural cortical networks in humans (Bassett et al. 2008; He et al. 2008; He et al. 2007). The findings provide empirical evidence to support the standpoint that human cortical morphology might be evolved into a complex but efficient network to maximize the power of parallel information processing at a low energy cost. Lower local efficiency in OCD patients may result in insufficient information interactions in the nearby brain regions, which might be responsible for lose of synchronization in the ongoing behaviors. Such a phenomenon may lead to increased desynchronization signals, which will cause more repetitive behaviors to correct these signals aimed to lessen desynchronization. On the other hand, patients with OCD showed decreased local efficiency compared to the healthy controls even though no significant difference was detected in global efficiency. Local efficiency is associated with short-range connections between nearby regions that regulate the modularized information processing or fault-tolerance of a network (Latora and Marchiori 2001a). Given that the small-world model reflects an optimal balance between local specialization and global integration, the reduced local efficiency in OCD group may indicate a disturbance of the normal balance in their structural brain network, which may tend to have a more randomized configuration. Compared to small-world networks, random networks have less modularized information processing or fault-tolerance (Latora and Marchiori 2001b). Similarly, a functional MRI study about small-world architecture also determined that OCD patients exhibited abnormal local functional organization in top-down control networks (Zhang et al. 2011). Therefore, the atypical small-world topological efficiency reported here represents a less optimal network organization in individuals with OCD, providing implications for further understanding the relationship between the network topology and the pathology of this disorder.
Modular organization of OCD
Our findings indicate that patients with OCD demonstrate atypical modularity in structural cortical networks. Modular organization in the structural brain networks exists in all three groups. Most importantly, the modular organization in cortical networks was consistent with the pre-stated small-world properties, reflecting higher local and global efficiency than the comparable random and regular networks. Moreover, the modules in structural brain networks were overlapping on some functional domains. For example, executive function, DMN, cognitive control, and sensorimotor function were detected in healthy controls. Our findings were consistent with several recent studies on the modular organizations in structural cortical networks (Wu et al. 2012; Zhang et al. 2011), which reported that human structural brain networks maintained an optimal balance between local specialization and global integration of the information process.
Nonetheless, patients with OCD exhibited atypical modular features relative to the same modular structures of healthy controls. First, OCD patients showed significantly decreased modularity compared to healthy controls. Previous studies found that patients with OCD had various morphological alterations such as decreased grey matter volume (Pujol et al. 2004) and reduced cortical thickness (Shin et al. 2007). Due to the structural abnormalities, it is supposed that the reduced modularity in OCD patients may arise from the reorganization of the brain network to compensate for the OCD-related focal alterations, such as widespread cortical thinning, and then maintain stable cognitive functions. Second, the composition of modular organizations in OCD patients was quite different from that of healthy controls. For instance, a cognitive control network was detected in healthy controls as seen in Fig. 2c, but failed to be detected in OCD patients. This implies that a patient with OCD may have a dysfunctional cognitive control network, where cognitive control is an ability that modulates our thoughts and actions to achieve internal goals while still allowing the flexibility to adjust these goals with changing task demands. The deficits of cognitive control have often been reported in OCD patients and suggested to be involved in intrusiveness of obsessive thoughts (Cocchi et al. 2012; Kocak et al. 2011; Viard et al. 2005). Third, we observed notably decreased connectivity within Module III (DMN) and Module IV (sensorimotor network) in the individuals with OCD (see Fig. 4). The DMN is thought to be the backbone of the intrinsic functional architecture (Biswal et al. 2010) and is mainly associated with self-referential mental activity (Gusnard et al. 2001). There is an increasing evidence demonstrating that OCD is associated with disrupted DMN connectivity using a resting-state functional MRI (Fitzgerald et al. 2010; Harrison et al. 2009), suggesting that OCD patients have dysfunctions of self-referential cognitive processes. Fitzgerald et al. detected that a lack of self-relevance contributed to many symptoms of OCD (Fitzgerald et al. 2010). Thus, disrupted connectivity of the DMN may be involved in the psychopathological symptoms of OCD. Our findings advanced the understanding of abnormal DMN in OCD from a novel view of structural organization.
Similarly, patients with OCD exhibited notably reduced modularity in the sensorimotor network. A recent functional MRI study found that OCD patients exhibited increased functional connectivity in the sensorimotor cortex during a cognitive control task, reflecting compensatory processes enacted to cope with task demands (Cocchi et al. 2012). Ahmari et al. found that OCD patients have deficits in pre-pulse inhibition, which is thought to reflect abnormalities in the processing and integration of sensory and motor information (Ahmari et al. 2012). Therefore, our results confirmed their findings and also extended their works to the level of large-scale structural brain networks. Fourth, as seen in Fig. 5, OCD patients exhibited significantly increased connectivity between Modules I (executive function)–II (cognitive control/spatial) and Modules II (cognitive control/spatial)–III (DMN). Cumulative studies have demonstrated that OCD patients are related to executive function and cognitive control deficits for their difficulties in changing strategies when demands or rules of a task change or in set-shifting between task (Cavedini et al. 2010; Viard et al. 2005). Recently, an fMRI meta-analysis found that the domains in executive function were indeed associated with increased activity in the superordinate cognitive control network (Niendam et al. 2012). Therefore, the increased connectivity between executive module and cognitive control module reported here may result from inappropriate signals that are integrated in the choice and execution of behaviors aimed to control the uncontrolled thoughts and behaviors.
The DMN is closely related to the cognitive control networks. The DMN is deactivated during cognitive task performance, which is considered to accompany the shift in one’s focus of attention between the states in self-directed mental activity at rest to an external focus of attention when performing tasks (Harrison et al. 2008). In addition, the less deactivation magnitude of DMN is related with more task performance errors (Christoff et al. 2009). Recently, fMRI study reported that OCD patients showed abnormal functional connectivity patterns in the DMN during a cognitive control task. This finding suggested that OCD patients’ initial engagement in the task was accompanied by more persistent task-unrelated mental activity (Cocchi et al. 2012). Taken together, the increased connectivity between cognitive control module and DMN module may reflect that OCD patients have difficulty in inhibiting the external stimuli (uncontrolled and recurrent thoughts, impulses or images) because they pay more attention to the distressing stimuli and attend less on internal thoughts. In addition, a recent functional MRI study identified that cognitive control, sensorimotor, and default mode networks interacted abnormally in OCD patients during the transition from rest to task (Cocchi et al. 2012). Previous studies have also demonstrated that the intermodule connections contributed to informational communication between different modules and facilitated the cognitive integrity in the whole brain network (Chen et al. 2008; He et al. 2009). Though the mechanism of increased intermodule connection is still unclear, one interpretation of our findings is that the increased intermodule connections in OCD patients compensate for the decreased intramodule modularity. An assessment of functional interconnectivity of those modules would conceivably help clarify the nature of the alterations reported here.
Morphological vulnerability in structural cortical networks in unaffected siblings
Intriguingly, we observed that unaffected siblings exhibited similar morphological patterns to OCD patients, though they did not show OCD symptoms and were free of psychotropic medication. The economical small-world properties were detected in the structural brain networks of siblings, and they also showed decreased local efficiency, but not global efficiency, when compared to healthy controls (see Fig. 1). On the other hand, siblings demonstrated reduced whole brain modularity, intra-connectivity within the DMN and increased interconnectivity between Modules I–II, which were similar to the patterns in OCD patients. Previous studies have identified that the first-degree relatives of OCD patients showed some similar morphological alterations with OCD patients. Abnormal white matter (Menzies et al. 2008) and grey matter (Menzies et al. 2007) were detected in the first-degree relatives of OCD patients. Due to the morphological alterations, relatives also exhibited some similar neurocognitive dysfunctions to OCD patients, such as executive function (Cavedini et al. 2010), cognitive flexibility, and motor inhibition (Chamberlain et al. 2007), which are mainly related to the abovementioned modules that had significant discrepancy between siblings and healthy controls. Based on the nonoptimal balance between local specialization and global integration in siblings, our results provide novel structural evidence to understand how the morphological alterations in brain networks underlie the specific cognitive deficits serving as endophenotypes for OCD. Taking all these findings together, atypical small-world properties and modularity in both OCD patients and their unaffected siblings support the hypothesis that OCD qualities are heritable and state-independent features, reflecting a possible trait marker for OCD.
Our findings should be considered in light of three limitations. First, the present study had a relatively small sample size. Previous studies have found that different OCD symptom dimensions may be supported by different neural mechanisms (Mataix-Cols et al. 2004). Therefore, findings in the current study need to be verified across different OCD subgroups in a larger sample. Second, all patients with OCD were being treated with psychotropic medications at the time of MRI examination, and the potential effect of these medications on cortical thickness cannot yet be determined. Future longitudinal research should directly investigate whether medication alters cortical thickness in drug-naïve patients with OCD. Third, several methodological issues should be considered, such as the biological nature of the morphological correlations and the relationship between structural disruptions in the brain networks and functional deficits. Future studies focusing on distinct functions within each structural module would be more appreciated.
Conclusion
In conclusion, we performed a comprehensive evaluation of brain network properties on OCD patients, siblings, and control subjects. Regional cortical thickness measurements were used to construct structural brain networks, and atypical small-world properties and modularity were identified in OCD patients, implying the loss of optimal balance between local specialization and global integration. Most interestingly, unaffected siblings shared similar characteristics of structural brain networks as OCD patients. The obtained results supported the idea that atypical small-worldness and modular organization of structural brain networks represented a candidate endophenotype of OCD that may indicate a vulnerability to the disorder.
Supplementary Material
Acknowledgments
This study was partly supported by National Institutes of Health (EB006733, EB008374, EB009634, AG041721), the National Science Fund China Young Investigator Award (81088001), the National Key Technologies R&D Program (2012BAI36B01), National Natural Science Foundation of China (81201049), The Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX2-EW-J-8), and also by a grant from the initiation fund of the CAS/SAFEA International Partnership Programme for Creative Research Teams to Raymond Chan.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-013-0602-y) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare no conflict of interest.
Contributor Information
Ziwen Peng, Email: pengziwen8@gmail.com, Department of Radiology and BRIC, University of North Carolina, 130 Mason Farm Road, Chapel Hill, NC 27599-7513, USA. Department of Psychology, South China Normal University, Guangzhou, China.
Feng Shi, Department of Radiology and BRIC, University of North Carolina, 130 Mason Farm Road, Chapel Hill, NC 27599-7513, USA.
Changzheng Shi, Medical Imaging Center, The First Affiliated Hospital of Jinan University, Guangzhou, China.
Qiong Yang, Guangzhou Psychiatry Hospital, Guangzhou, China.
Raymond C. K. Chan, Neuropsychology and Applied Cognitive Neuroscience Laboratory, Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China
Dinggang Shen, Email: dgshen@med.unc.edu, Department of Radiology and BRIC, University of North Carolina, 130 Mason Farm Road, Chapel Hill, NC 27599-7513, USA. Department of Brain and Cognitive Engineering, Korea University, Seoul, Korea.
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:174–183. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:1216–1223. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Zorzi C, Piccinni M, Cavallini MC, Bellodi L. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biol Psychiatry. 2010;67:1178–1184. doi: 10.1016/j.biopsych.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, Sahakian BJ. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb Cortex. 2008;18:2374–2381. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Gong G, Evans AC. Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. NeuroImage. 2011;56:235–245. doi: 10.1016/j.neuroimage.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L, Harrison BJ, Pujol J, Harding IH, Fornito A, Pantelis C, Yucel M. Functional alterations of large-scale brain networks related to cognitive control in obsessive-compulsive disorder. Hum Brain Mapp. 2012;33:1089–1106. doi: 10.1002/hbm.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fan Q, Palaniyappan L, Tan L, Wang J, Wang X, Li C, Zhang T, Jiang K, Xiao Z, Liddle PF. Surface anatomical profile of the cerebral cortex in obsessive-compulsive disorder: a study of cortical thickness, folding and surface area. Psychol Med. 2012:1–11. doi: 10.1017/S0033291712001845. [DOI] [PubMed] [Google Scholar]
- Ferrarini L, Veer IM, Baerends E, van Tol MJ, Renken RJ, van der Wee NJ, Veltman DJ, Aleman A, Zitman FG, Penninx BW, van Buchem MA, Reiber JH, Rombouts SA, Milles J. Hierarchical functional modularity in the resting-state human brain. Hum Brain Mapp. 2009;30:2220–2231. doi: 10.1002/hbm.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MBSR, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) APA Press; 1996. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Biometrics Research Department. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Stern ER, Angstadt M, Nicholson-Muth KC, Maynor MR, Welsh RC, Hanna GL, Taylor SF. Altered function and connectivity of the medial frontal cortex in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68:1039–1047. doi: 10.1016/j.biopsych.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess. 2002;14:485–496. [PubMed] [Google Scholar]
- Fortunato S, Barthelemy M. Resolution limit in community detection. Proc Natl Acad Sci USA. 2007;104:36–41. doi: 10.1073/pnas.0605965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YX. Manual of Wechsler Adult Intelligence Scale: Chinese version. Chinese Map Press; Changsha: 1992. [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, Soriano-Mas C, Yucel M, Pantelis C, Cardoner N. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA. 2008;105:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, Deus J, Alonso P, Yucel M, Pantelis C, Menchon JM, Cardoner N. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s Disease. J Neurosci. 2008;28:4756–4766. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, Evans A. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132:3366–3379. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocak OM, Ozpolat AY, Atbasoglu C, Cicek M. Cognitive control of a simple mental image in patients with obsessive–compulsive disorder. Brain Cogn. 2011;76:390–399. doi: 10.1016/j.bandc.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Kaufmann C, Simon D, Endrass T, Gallinat J, Kathmann N. Reduced thickness of anterior cingulate cortex in obsessive- compulsive disorder. Cortex. 2012 doi: 10.1016/j.cortex.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001a;87(19):198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001b;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, del Campo N, Sahakian BJ, Robbins TW, Bullmore E. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- Menzies L, Williams GB, Chamberlain SR, Ooi C, Fineberg N, Suckling J, Sahakian BJ, Robbins TW, Bullmore ET. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am J Psychiatry. 2008;165:1308–1315. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle M, Bienvenu OJ, 3rd, Liang KY, LaBuda M, Walkup J, Grados M, Hoehn-Saric R. A family study of obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57:358–363. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci USA. 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MEJ, Girvan M. Finding and evaluating community structure in networks. Phys Rev E. 2004;69:026113. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP, 2nd, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152:76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- Peng ZW, Yang WH, Miao GD, Jing J, Chan RC. The Chinese version of the Obsessive-Compulsive Inventory-Revised Scale: replication and extension to non-clinical and clinical individuals with OCD symptoms. BMC Psychiatry. 2011;11:129. doi: 10.1186/1471-244X-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive- compulsive disorder. Arch Gen Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Redies C, Puelles L. Modularity in vertebrate brain development and evolution. BioEssays. 2001;23:1100–1111. doi: 10.1002/bies.10014. [DOI] [PubMed] [Google Scholar]
- Reichardt J, Bornholdt S. When are networks truly modular? Physica D. 2006;224:20–26. [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YW, Yoo SY, Lee JK, Ha TH, Lee KJ, Lee JM, Kim IY, Kim SI, Kwon JS. Cortical thinning in obsessive compulsive disorder. Hum Brain Mapp. 2007;28:1128–1135. doi: 10.1002/hbm.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger DC. Manual for the State-Trait Anxiety Inventory (STAI) Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford University Press; New York: 1991. [Google Scholar]
- Strogatz SH. Exploring complex networks. Nature. 2001;410:268–276. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- Viard A, Flament MF, Artiges E, Dehaene S, Naccache L, Cohen D, Mazet P, Mouren MC, Martinot JL. Cognitive control in childhood-onset obsessive-compulsive disorder: a functional MRI study. Psychol Med. 2005;35:1007–1017. doi: 10.1017/s0033291704004295. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Kinomura S, Goto R, Okada K, Kawashima R, He Y, Evans AC, Fukuda H. Age-related changes in topological organization of structural brain networks in healthy individuals. Hum Brain Mapp. 2012;33:552–568. doi: 10.1002/hbm.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wang J, Yang Y, Wu Q, Li B, Chen L, Yue Q, Tang H, Yan C, Lui S, Huang X, Chan RC, Zang Y, He Y, Gong Q. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci. 2011;36:23–31. doi: 10.1503/jpn.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.