Abstract
Spleen cells treated with mitogens produce a potent bone-resorbing factor called osteoclast-activating factor (OAF). To examine the relationship between the bone-resorbing factor and other protein factors produced by spleen cells, the colony-stimulating factor (CSF), the differentiation-inducing factor (DIF), the macrophage fusion factor (MFF), and the macrophage growth factor (MGF) were purified from 2.68 liters of conditioned medium of mouse spleen cell cultures treated with concanavalin A. Purification was performed successively by DEAE-cellulose, Blue Sepharose, and Sephadex G-150 column chromatography and high-pressure liquid chromatography (HPLC). The DIF was successfully separated from CSF and MGF on HPLC. CSF coincided with MGF on HPLC, but MFF disappeared before application to HPLC. Only the DIF exhibited bone-resorbing activity, whereas CSF and MGF did not. The DIFs purified from L929 cells and Ehrlich ascites tumors similarly exhibited bone-resorbing activity. The DIFs purified from spleen cells and Ehrlich ascites tumor cells exhibited neither interleukin 1 (IL-1) activity nor tumor necrosis factor (TNF) activity, though the unfractionated conditioned medium from spleen cells did exhibit them. In the light of recent reports that IL-1 beta and TNF also stimulate bone resorption, the term OAF should refer to a generic activity rather than a single factor.
Full text
PDF


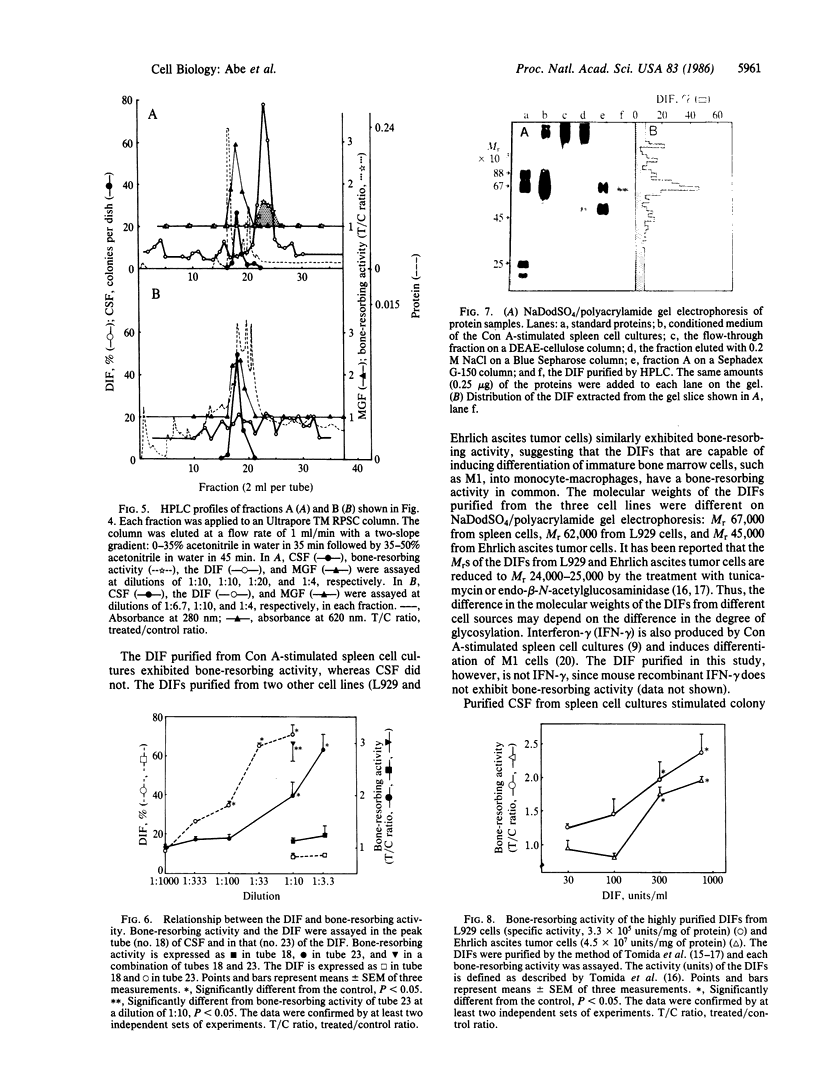

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe E., Miyaura C., Tanaka H., Shiina Y., Kuribayashi T., Suda S., Nishii Y., DeLuca H. F., Suda T. 1 alpha,25-dihydroxyvitamin D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5583–5587. doi: 10.1073/pnas.80.18.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- David J. R. Mediators produced by sensitized lymphocytes. Fed Proc. 1971 Nov-Dec;30(6):1730–1735. [PubMed] [Google Scholar]
- Dewhirst F. E., Stashenko P. P., Mole J. E., Tsurumachi T. Purification and partial sequence of human osteoclast-activating factor: identity with interleukin 1 beta. J Immunol. 1985 Oct;135(4):2562–2568. [PubMed] [Google Scholar]
- FISCHMAN D. A., HAY E. D. Origin of osteoclasts from mononuclear leucocytes in regenerating newt limbs. Anat Rec. 1962 Aug;143:329–337. doi: 10.1002/ar.1091430402. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Vignery A., Gershon R. K., Baron R. Thymus-derived lymphocytes and their interactions with macrophages are required for the production of osteoclast-activating factor in the mouse. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2181–2185. doi: 10.1073/pnas.81.7.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. E., Raisz L. G., Simmons H. A., Oppenheim J. J., Mergenhagen S. E. Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes. Science. 1972 Sep 1;177(4051):793–795. doi: 10.1126/science.177.4051.793. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Pringle J. A., Chambers T. J. Identification of human osteoclasts with monoclonal antibodies. N Engl J Med. 1985 Apr 4;312(14):923–924. doi: 10.1056/NEJM198504043121418. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., Twardzik D. R., D'Souza S. M., Hargreaves W. R., Todaro G. J., Mundy G. R. Stimulation of bone resorption in vitro by synthetic transforming growth factor-alpha. Science. 1985 May 24;228(4702):1007–1009. doi: 10.1126/science.3859011. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luben R. A., Mundy G. R., Trummel C. L., Raisz L. G. Partial purification of osteoclast-activating factor from phytohemagglutinin-stimulated human leukocytes. J Clin Invest. 1974 May;53(5):1473–1480. doi: 10.1172/JCI107696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura C., Abe E., Nomura H., Nishii Y., Suda T. 1 alpha,25-Dihydroxyvitamin D3 suppresses proliferation of murine granulocyte-macrophage progenitor cells (CFU-C). Biochem Biophys Res Commun. 1982 Oct 29;108(4):1728–1733. doi: 10.1016/s0006-291x(82)80111-3. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Raisz L. G., Cooper R. A., Schechter G. P., Salmon S. E. Evidence for the secretion of an osteoclast stimulating factor in myeloma. N Engl J Med. 1974 Nov 14;291(20):1041–1046. doi: 10.1056/NEJM197411142912001. [DOI] [PubMed] [Google Scholar]
- Parker J. W., Metcalf D. Production of colony-stimulating factor in mitogen-stimulated lymphocyte cultures. J Immunol. 1974 Feb;112(2):502–510. [PubMed] [Google Scholar]
- Postlethwaite A. E., Jackson B. K., Beachey E. H., Kang A. H. Formation of multinucleated giant cells from human monocyte precursors. Mediation by a soluble protein from antigen-and mitogen-stimulated lymphocytes. J Exp Med. 1982 Jan 1;155(1):168–178. doi: 10.1084/jem.155.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Insogna K. L., Vignery A. M., Stewart A. F., Broadus A. E., D'Souza S. M., Bertolini D. R., Mundy G. R., Rodan G. A. Factors associated with humoral hypercalcemia of malignancy stimulate adenylate cyclase in osteoblastic cells. J Clin Invest. 1983 Oct;72(4):1511–1515. doi: 10.1172/JCI111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina Y., Miyaura C., Tanaka H., Abe E., Yamada S., Yamamoto K., Ino E., Takayama H., Matsunaga I., Nishii Y. Mechanism of the differentiating action of 25-hydroxyvitamin D3 endoperoxides in human myeloid leukemia cells (HL-60). J Med Chem. 1985 Sep;28(9):1153–1158. doi: 10.1021/jm00147a006. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Cifone M., Heard P. M., Defendi V. Factors regulating macrophage production and growth: identity of colony-stimulating factor and macrophage growth factor. J Exp Med. 1976 Mar 1;143(3):631–647. doi: 10.1084/jem.143.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lazzaro M., Singer F. R., Roberts A. B., Derynck R., Winkler M. E., Levine L. Alpha and beta human transforming growth factors stimulate prostaglandin production and bone resorption in cultured mouse calvaria. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4535–4538. doi: 10.1073/pnas.82.13.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida M., Yamamoto-Yamaguchi Y., Hozumi M. Characterization of a factor inducing differentiation of mouse myeloid leukemic cells purified from conditioned medium of mouse Ehrlich ascites tumor cells. FEBS Lett. 1984 Dec 10;178(2):291–296. doi: 10.1016/0014-5793(84)80619-5. [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto-Yamaguchi Y., Hozumi M. Preparation and neutralization characteristics of an antibody to the factor inducing differentiation of mouse myeloid leukemic cells. FEBS Lett. 1983 Jan 24;151(2):281–285. doi: 10.1016/0014-5793(83)80087-8. [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto-Yamaguchi Y., Hozumi M. Purification of a factor inducing differentiation of mouse myeloid leukemic M1 cells from conditioned medium of mouse fibroblast L929 cells. J Biol Chem. 1984 Sep 10;259(17):10978–10982. [PubMed] [Google Scholar]
- Tomida M., Yamamoto Y., Hozumi M. Stimulation by interferon of induction of differentiation of mouse myeloid leukemic cells. Cancer Res. 1980 Aug;40(8 Pt 1):2919–2924. [PubMed] [Google Scholar]
- Yamamoto Y., Tomida M., Hozumi M. Production by mouse spleen cells of factors stimulating differentiation of mouse myeloid leukemic cells that differ from the colony-stimulating factor. Cancer Res. 1980 Dec;40(12):4804–4809. [PubMed] [Google Scholar]



