Abstract
Purpose of review
To describe the current status of testing Schwann cell transplantation as a therapy for human spinal cord injury (SCI).
Recent findings
Transplanted Schwann cells have reparative effects in the damaged spinal cord. A few clinical studies have reported that Schwann cell transplantation appears safe. Compared with allogeneic cell transplants, autologous cells do not require immune suppression, but the workload of cell manufacturing is greater. Preclinical Schwann cell transplant studies conducted at the University of Miami in 2009–2012 supported an investigational new drug approved by the Food and Drug Administration. A Phase 1 safety study has been initiated.
Summary
Spinal cord repair after severe SCI requires that axonal regeneration and myelination occur in a context of reduced inhibition, enhanced plasticity, and new circuit formation. Evolving clinical experience with Schwann cell transplantation may provide a basis upon which additionally combined therapeutics can be tested to increase the extent of repair after SCI. Safety is the primary consideration when ex-vivo manipulated cells are introduced into the damaged nervous system. Preclinical studies across several species have not indicated safety concerns regarding Schwann cells. Initial clinical reports from studies in Iran and China are suggestive of clinical safety, although more rigorous characterization of the implanted cells is needed.
Keywords: autologous, cell culture, Schwann cell, spinal cord injury, transplant
INTRODUCTION
Schwann cell transplantation for spinal cord injury (SCI) is at an early stage of clinical testing following preclinical development. Food and Drug Administration (FDA) approval of an investigational new drug application (IND) to undertake a Phase 1 safety and feasibility study in patients after subacute SCI was based on the following milestones: detailed characterization of manufactured human Schwann cell batches, pivotal preclinical safety studies, development of clinical cell injection methodology, and adequate outcome assessment methods to make a valid appraisal of feasibility and safety. Schwann cells are being tested for repair in the central nervous system (CNS) because they support axonal regeneration in the peripheral nervous system (PNS). Following nerve injury, Schwann cells dedifferentiate [1▪,2], secrete growth-promoting trophic molecules and axon growth-promoting extracellular matrix such as laminin, which support axonal growth cone elongation. Regenerated axons are then myelinated, restoring rapid action potential conduction and function. The biology of peripheral nerve regeneration across many species, including man, is similar and has been studied for more than a century [3]. Experimental studies have tested whether Schwann cells would allow normally nonregenerative damaged CNS axons to regenerate within the traumatically injured spinal cord and myelinate damaged axons [4]. Autologous transplantation of Schwann cells should not require immune suppression and eliminates the risk of transmission of undetected allograft donor abnormalities.
Box 1.
no caption available
Few clinical trials have yet reported on the effects of transplanted Schwann cells in SCI or other potential applications such as nerve repair (Table 1[5–7]). Investigators in Iran reported two studies: the first with four patients with chronic thoracic SCI transplanted with autologous Schwann cells [5] followed for 1 year and a second report of 33 patients with 2-year follow-up [6]. They described a method to cultivate human Schwann cells using initial serum starvation followed by exposure to autologous serum without growth factors [8], arguing that this technique may be safer than the use of artificial mitogens. A Chinese study enrolled six patients with chronic SCI and reported their results after following the patients for 5 years. In that study, the sural nerve was predegenerated by cutting it within the body a week before removing it for cell culture [7]. This step may accelerate the rate of cell division in culture. These clinical studies found a low incidence of adverse events that could be linked to the transplants. Although the studies were not conducted under rigorous FDA oversight, the lack of reported serious adverse events in the patients is reassuring. Clinicaltrials.gov lists only one current study testing Schwann cell transplantation, NCT01739023, a Phase 1 clinical study of the ‘Safety and Tolerability of Autologous Human Schwann Cells (ahSCs) in Subjects With Subacute SCI’ with which the authors are associated.
Table 1.
Reported clinical trials of Schwann cell transplantation for SCI
| Characteristics | Saberi et al. [5,6] | Zhou et al. [7] |
| Age, number of patients | 23–50, n = 33 | 7–44, n = 6 |
| Injury level | T6–T9 | C5–T12 |
| Injury severity | ASIA A–C | ASIA A–C |
| Time after injury | Average 4.1 years | 1–20 Months |
| Surgical decompression at the time of transplantation | No | Yes |
| Cell purification | ‘Starvation’ method | Differential adhesion |
| Dose | 300 μl (3–4.5 million cells) | 200 μl (5 million cells) |
| Cell delivery | 5–6 injections per side, within, rostral and caudal to the injury site | 6–7 injections per side adjacent to injury site |
| Posttransplant rehabilitation | Not stated | Yes, duration not specified |
| Adverse effects | One transient neurological worsening, one wound breakdown and one infected cell culture | None |
| Follow-up period | 2 years | 5–7 years |
| Neurological change | Improved light touch sensory scores, minimal improvement in pin-prick sensation and motor scores. Improved bladder sensation and control of urination in some patients. | Recovery in all patients in motor, sensory and autonomic measures. |
| Functional change | Nonsignificant increase in FIM scores | Improvement in FIM scores |
| MRI | No concerning changes from preop to follow-up were detected | No concerning changes from preop to follow-up were detected |
FIM, functional independence measure; SCI, spinal cord injury.
SCHWANN CELLS AND PERIPHERAL NERVE REPAIR
Spontaneous recovery after nerve injuries, although imperfect, provides the core rationale to test Schwann cells in the CNS. Even when nerves are completely transected, the careful interposition of nerve graft segments derived from noncritical nerves can support axonal regeneration, leading to the recovery of muscle function and sensation[9–12]. In these clinical grafting procedures, the donor nerve grafts often do not match the size of the injured nerve stumps and there has been interest in using fabricated tubes with diameters similar to the injured nerve stumps, filled with cultured Schwann cells, to span the injured nerve gap. Animal experiments have demonstrated the feasibility of this approach [13,14] using human Schwann cells. The success and reproducibility of these experiments in nerves led to successful testing of tubing biomaterials and Schwann cells within the spinal cord [15,16] in complete transection models.
PERIPHERAL NERVE GRAFTS VERSUS SCHWANN CELL TRANSPLANTATION FOR CENTRAL NERVOUS SYSTEM REPAIR
Several investigators studied whether peripheral nerve grafts (PNGs) transplanted into continuity with spinal cord or brain tissues [17] could support CNS axonal growth. Regeneration into nerve grafts [18] confirmed the ability of some classes of damaged CNS axons to regenerate if the tissue environment is permissive. Grafts depleted of Schwann cells by freezing did not support CNS axonal growth, establishing that viable Schwann cells were essential [19]. The suitability of PNGs for transplantation into damaged regions of nontransected spinal cord is limited by their structure and the need to manipulate injured spinal cord tissue to create a suitable interface with the nerve grafts. Suspensions of Schwann cells cultured from peripheral nerve biopsies can be delivered with less surgical spinal tissue manipulation and have several other advantages over the use of PNGs: Schwann cells can be highly characterized for phenotypic markers, purified to remove fibroblasts, and expanded exponentially to provide the large cell numbers that are necessary for adequate engraftment in spinal cord injuries. Transplanted Schwann cells can fill the injury region, migrate and insinuate to the unique dimensions of each injury, and form bridging tissue. Advances in tissue culture were necessary to permit the reliable derivation of human Schwann cell cultures from donor nerves [20–22]. The sural nerve is the most commonly harvested nerve from which human Schwann cells are derived because of its superficial location, adequate length, and the modest consequences of removing it [23].
HOW SCHWANN CELL TRANSPLANTATION DIFFERS FROM THE ENDOGENOUS SCHWANN CELL RESPONSE AFTER INJURY?
Normally, the spinal cord is segregated from the associated peripheral tissues such as the nerve roots and pia mater by the glial limiting membrane (GLM), formed by astrocyte foot-processes and extracellular matrix at the brain and spinal cord surface. Schwann cells are present at the dorsal root entry and ventral root exit spinal cord interfaces where nerve roots join the spinal cord, but they are not found within the parenchyma because of the specialized GLM in these areas [24,25]. Following various injuries including SCI, the GLM is transiently disrupted and Schwann cells spontaneously enter the spinal cord [26]. In people, this leads to the formation of neuromatous structures within the injury site called ‘Schwannosis’ [27,28]. On the basis of the current information, it does not appear that Schwannosis has a significant role in the repair of damaged central axons. Schwannosis differs from Schwann cell neoplasms such as Schwannoma because of the presence of normally formed myelin and axons [29] in the former and their absence in the latter. Transplanted Schwann cells may be placed into the spinal cord at a specific location and time point after injury distinct from the endogenous Schwann cell response. The fact that no adverse effect has been attributed to naturally occurring Schwannosis is an important argument for the inherent safety of Schwann cell transplantation.
PRECLINICAL DATA SUPPORTING SCHWANN CELL TRANSPLANTATION IN HUMANS WITH SPINAL CORD INJURY
A PubMed search of animal experimental studies was performed using Endnote X5 with the search terms, ‘Schwann’, and ‘spinal cord injury’ in the abstract fields. The references positive for these two search terms (413) were screened for those in which Schwann cells were directly implanted into the spinal cord regardless of the injury model. Review articles were excluded. This reduced the total number of citations to 72. Of these studies, 16 tested transplantation of genetically unmodified Schwann cells in the most relevant injury model (contusion) as one arm in the study. To summarize the observations from these 16 studies, it was consistently found that some proportion of Schwann cells engrafted and supported axonal sprouting and myelination. None of the prior studies was performed in compliance with Good Laboratory Practice (GLP), the standard expected by the United States. FDA [30▪▪,31] and none used Schwann cells that were prepared to current Good Manufacturing Process (cGMP) standards. Therefore, the prior published studies were not suitable as pivotal studies to support an IND application.
The authors and their colleagues at the Miami Project to Cure Paralysis conducted detailed toxicity studies that were designed to support an IND application to conduct a safety study of autologous Schwann cell transplantation in subacute SCI. The IND was submitted to the FDA in September 2011 and approved in July 2012. Three animal models were used: rodents, minipigs, and primates. The rodent studies were designed for robust statistical analysis of cell survival and engraftment, whereas those in the larger animals addressed the issues related to the transplant methodology and used autologous cell preparations. Together, these studies demonstrated long-term cell survival and the absence of abnormal cellular formations throughout the brain and spinal cord.
RESEARCH DESIGNS IN CELL THERAPY CLINICAL TRIALS
Despite extensive preclinical research, the effects of a therapeutic in humans with the target disease cannot be fully predicted until rigorous clinical testing occurs with adequate long-term follow-up. Control groups are problematic in cell therapy trials because it is unrealistic to place research individuals at the risk of surgical exposure, anesthesia, and postsurgical recovery in order to perform a noncellular control injection. Another approach could be to randomize individuals to two or more treatment groups, such as two different cell types, or the combination of cell therapy plus another biological therapy versus cell therapy alone. In these situations, similar risks and the existence of equipoise could justify such research designs. However, most cell therapies for SCI are at the Phase 1 safety study stage of development, and it would be complex from a regulatory and informed consent point-of-view to perform these comparisons until clinical data regarding the individual therapies are available. Thus, if control groups in early studies are used, they are generally prospectively matched individuals assigned to the best standard care [32▪▪]. Relevant data registries can provide important comparator information for the incidence of adverse events [33▪▪] and anticipated neurological outcomes [34].
The first cell therapy for SCI conducted under an FDA approved IND that has been reported is the Proneuron study [32▪▪]. This Phase 2 study of autologous activated macrophage transplantation was terminated prior to full enrollment for financial reasons, as was the subsequent FDA approved Phase 1 Geron study of embryonic stem cell transplantation [35–38], indicating the difficulty of maintaining financial support of cell therapy trials in neurological diseases. Another FDA approved cell therapy study that has published safety data is the Neuralstem amyotrophic lateral sclerosis (ALS) study, in which neural stem cells are implanted [39▪▪,40] into the spinal cord of patients with advanced ALS to slow the disease progression. Cell therapies for SCI are particularly expensive because of the use of surgery and anesthesia, the need for in-hospital acute care and rehabilitation, advanced imaging, cell manufacturing costs, and extensive follow-up.
CLINICAL ISSUES
In the next section, we address the issues that have been most important to the initiation of our clinical study.
Summary of enrollment criteria for our Phase 1 safety study
The Miami Project study selects for those patients with the least risk to be harmed neurologically by the cell transplantation. Thus, patients with thoracic SCI with neurologically complete injuries are enrolled because their prospect for natural recovery is minimal [34]. Injuries at the thoracic spinal cord level were selected because loss of function in nearby spinal segments as a complication would be less harmful than in the cervical spinal cord. Important exclusion criteria include the inability to adequately image the SCI and implantation site using MRI. The configuration of spinal fixation instrumentation [41] may generate MRI artifacts obscuring the injury and transplant site precluding critical safety evaluations to assess for the formation of an intraspinal mass. Another important consideration in this study is to determine that transplanted Schwann cells do not exacerbate harmful neuroplasticity. After SCI, neuropathic pain is common [42,43], and cell transplantation could theoretically exacerbate this problem [44]. Thus, patients who develop severe neuropathic pain are excluded from transplantation.
Cell manufacturing
Each autologous cell culture is unique. This poses a challenge to generate cell products that are sufficiently similar, so that their effects after transplantation may be compared. It is expected that individual variations in genotype, anatomy, and life history mean that each donor nerve is different (Table 2[5–7]). Even when carefully replicated procedures such as nerve dissection, seeding onto laminin-coated surfaces, and exposure to media components and growth factors are identical, the growth kinetics of the culture and its cellular composition may vary to some extent. In some patients, it may not be possible to obtain adequate cultures for transplantation and this must be explained during informed consent. Furthermore, assessment of the number of failed cultures compared with successful cultures is an important aspect of the determination of the feasibility of the autologous transplant program. The Schwann cell manufacturing capacity is exponential, such that millions of cells can be generated from a modest segment of donor nerve. Although Schwann cells undergo dedifferentiation in cell culture and may exhibit considerable plasticity [45], there is no current evidence that cell culture leads to cellular changes that impair their ability to function as myelinating and regeneration-promoting cells after implantation into the injured spinal cord (unpublished data, IND 14856; Fig. 1).
Table 2.
Advantages and disadvantages of autologous and allogeneic cell transplants for transplantation in spinal cord injury
| Issue | Autologous | Allogeneic |
| Cost | Per batch costs are high as single preparation for one patient | Development and batch validation costs are high, but per vial costs are relatively low |
| Availability | Limited by the success of autologous cell culture | Cryopreserved stocks |
| Risk of host immune rejection | Considered to be minimal | Substantial, immune suppression required |
| Biomarkers of survival and function | None | Evidence of host cellular or antibody immune response to allograft |
| Expansion of cell culture | Limited by senescence at >passage 5–6 | Allogenic 'stem’ cells can be expanded for a greater number of passages |
FIGURE 1.
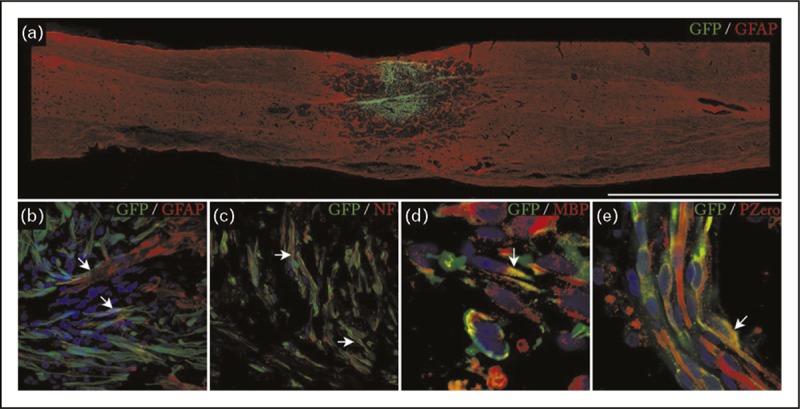
Survival, integration, and formation of myelin by autologous SCs transplanted into the site of thoracic contusive SCI in minipigs. This figure is from the dataset submitted to the FDA in support of the clinical trial NCT01739023 (IND 14856). The transplanted cells have been transduced with a lentivirus to express green fluorescent protein (GFP), allowing their detection within the tissue. (a) Overall appearance of a transplant occupying the injury site, bar = 5000 μm. (b) Integration of transplanted SCs and astrocytes labeled with antiglial fibrillary acidic protein (GFAP) at 7 days after transplantation. (c) Ensheathment of host axons labeled with neurofilament (NF) by aSCs. (d and e) Identification of the characteristic myelin proteins MBP and P0 within 30 days of transplantation. SCs, Schwann cells; SCI, spinal cord injury.
Injection methodology
Cells are implanted into the damaged spinal cord by direct injection. There are several variables to consider in the development of a safe injection method. Transplant injections have the potential to create damage to preserved spinal tissue in several ways and associated injury must be minimized. The most damaging injections are those of large volumes, delivered rapidly, with poor control over motion of the needle and tissue interface [46]. In the clinical environment, it is important to consider all contingencies that might add risk during the cellular transplantation because unpredictable events such as, for example, electrical power failure or anesthetic emergencies, including cardiopulmonary instability, although uncommon, do occur. Therefore, the ability to terminate the injection and exit the spinal cord rapidly is necessary. A rigid needle within spinal cord tissue can cause serious injury if there is loss of control of the position of the needle because of operator error, injection device dysfunction, or inadvertent patient motion. Currently, there are three main approaches to make spinal cord injections: free hand needle injections, fixed platform injections, and floating cannula injections [47,48]. Each method has specific merits and limitations. We currently use a fixed platform injection apparatus.
Dose of cells
Selecting the optimal clinical cell dose is a challenging task because of the complex effects of cells compared to more conventional drugs. For example, after most cell injections, some cells will die and engender some degree of inflammation. Thus, both beneficial and harmful events may occur simultaneously after transplantation. The best cell dose is a function of the final result in the tissue and may not be based solely on a single tissue effect. There is a limited ability to monitor toxicological endpoints in SCI patients receiving cell transplants other than worsening of the neurological injury density or level. Complications of SCI such as neuropathic pain and spasticity occur to some extent in most patients and linking these endpoints to the cell dose may be difficult. The formation of abnormal tissue or tumors may occur independent of the cell dose. In our IND development, we have focused on learning the maximum tolerated dose that can be delivered to the spinal cord in animals and not cause additional injury that is evident by clinical examination, neurophysiology, postinjection MRI, or histology. We found that the minipig SCI model was very useful for dose tolerance studies because of its human-like neural axis dimensions. On the basis of large animal testing, we determined a well tolerated dose at which to initiate the study and successive larger doses that may exert a superior therapeutic effect.
Outcome measures
In our current study, the most important outcomes are the feasibility of the autologous transplant strategy and the safety of the procedures and cellular implant. Impairment of residual neurological function could occur as a result of the surgical implantation procedure or because of the biological effect of the cell transplant. In patients with complete thoracic SCI, such changes are measured using sensory testing, with the neurological level as the endpoint. This level is defined as the last at which sensory perception is normal on both sides. The outcome measures we are utilizing are listed in Table 3[5–7].
Table 3.
Clinical outcome measures in NCT01739023, a Phase 1 clinical study of the safety and tolerability of autologous human Schwann cells (ahSCs) in patients with subacute SCI
| Measures of neurological function | INSCSCI assessment of neurological level and severity |
| Autonomic testing | |
| Bowel and bladder datasets | |
| Evoked potential testing | |
| Measures of disability | SCIM III |
| FIM | |
| SF-12 | |
| Patient global impression of change | |
| Pain assessments | NPSI, pain drawing, LANSS pain scale, ISCI basic pain dataset |
| Spasticity | Modified Ashworth |
| Neuroimaging | Contrast-enhanced MRI |
| Intraoperative ultrasound |
The need for surrogate markers of cell survival, engraftment, and effect
It is desirable to have clinical tests that allow the effect of cell implantation to be followed longitudinally, especially to determine cell survival and biological activity. This is important because a clear impact on neurological recovery may not occur with cell grafts alone and will likely require future combination therapies. The paucity of surrogate markers is not unique to Schwann cell transplantation, but is a general problem facing the CNS cell therapy field. The doses of transplanted cells are relatively small compared with the overall cell death that occurs after SCI, potentially masking the ability to detect Schwann-cell-specific markers of cell death and survival. For Schwann cells, the issue is further complicated because of the fact that endogenous Schwann cells enter the regions of SCI and may have similar biological activity. Because allografts require immune suppressive drugs to avoid cellular rejection, formation of antiallograft antibodies is a useful biomarker that is not available for autografts to determine a definite host response. It is likely that progress in this area will require the development of well tolerated molecular markers that the transplanted cells can uniquely express and which do not impair their biological activity in the long term.
CONCLUSION
Autologous Schwann cell transplantation is a reasonable treatment approach to the repair of spinal cord injuries based on the role of Schwann cells in peripheral nerve repair, the endogenous Schwann cell's response to spinal cord injury, and the feasibility of preparing and delivering the cells. More clinical experience is required to determine the safety and efficacy. It is probable that future studies will combine Schwann cell transplantation with additional therapies to amplify the reparative effects.
Acknowledgements
The Phase 1 Safety trial of Schwann cell transplantation for subacute spinal cord injury is supported by the Miami Project to Cure Paralysis and the Bounniconti Fund. The stereotaxic injector is supplied by Geron corporation. Dr. Ed Wirth provided valuable input regarding the use of the stereotaxic injector. Key study personnel include: Dr Allan Levi MD, PhD, Co-PI; Dr Dalton Dietrich PhD, Sponsor; Dr Kim Anderson PhD; Dr Diana Cardenas MD; Dr Mary Bunge PhD; Dr Damien Pearse PhD; Dr Pat Wood PhD; Ms Aisha Khan MBA PhD; Dr Marina Dididze MD PhD; Dr Paula Monje, PhD; and Mr Anil Lalwani, Project Manager.
Conflicts of interest
J.G. is co-PI of the study SC NCT01739023, a Phase 1 clinical study of the ‘Safety and Tolerability of Autologous Human Schwann Cells (ahSCs) in Subjects With Subacute SCI’.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
Correspondence to James Guest, MD, PhD, Department of Neurological Surgery, The Miami Project to Cure Paralysis, Lois Pope LIFE Centre, Miller School of Medicine, 1095 NW 14th Terrace, Miami, FL 33136, USA. Tel: +1 305 243 8185; e-mail: jguest@med.miami.edu
REFERENCES
- 1▪.Arthur-Farraj PJ, Latouche M, Wilton DK, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012; 75:633–647 [DOI] [PMC free article] [PubMed] [Google Scholar]; An important molecular study that shows that c-Jun expression is the key event that causes Schwann cells to dedifferentiate into a repair phenotype.
- 2.Mirsky R, Jessen KR. The neurobiology of Schwann cells. Brain Pathol 1999; 9:293–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell WH, Huber GC. A physiological, histological and clinical study of the degeneration and regeneration in peripheral nerve fibres after severance of their connections with the nerve centres. J Physiol 1892; 13:335–406311 [PMC free article] [PubMed] [Google Scholar]
- 4.Bunge MB, Wood PM. Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury. Handb Clin Neurol 2012; 109:523–540 [DOI] [PubMed] [Google Scholar]
- 5.Saberi H, Moshayedi P, Aghayan HR, et al. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes. Neurosci Lett 2008; 443:46–50 [DOI] [PubMed] [Google Scholar]
- 6.Saberi H, Firouzi M, Habibi Z, et al. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J Neurosurg Spine 2011; 15:515–525 [DOI] [PubMed] [Google Scholar]
- 7.Zhou XH, Ning GZ, Feng SQ, et al. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow-up. Cell Transplant 2012; 21 (Suppl. 1):S39–S47 [DOI] [PubMed] [Google Scholar]
- 8.Aghayan HR, Arjmand B, Norouzi-Javidan A, et al. Clinical grade cultivation of human Schwann cell, by the using of human autologous serum instead of fetal bovine serum and without growth factors. Cell Tissue Bank 2012; 13:281–285 [DOI] [PubMed] [Google Scholar]
- 9.Eaton DA, Hirsch BE, Mansour OI. Recovery of facial nerve function after repair or grafting: our experience with 24 patients. Am J Otolaryngol 2007; 28:37–41 [DOI] [PubMed] [Google Scholar]
- 10.Garozzo D, Ferraresi S, Buffatti P. Surgical treatment of common peroneal nerve injuries: indications and results. A series of 62 cases. J Neurosurg Sci 2004; 48:105–112discussion 112 [PubMed] [Google Scholar]
- 11.Trumble TE, Vanderhooft E, Khan U. Sural nerve grafting for lower extremity nerve injuries. J Orthop Trauma 1995; 9:158–163 [DOI] [PubMed] [Google Scholar]
- 12.Wood MB. Peroneal nerve repair. Surgical results. Clin Orthop Relat Res 1991; 267:206–210 [PubMed] [Google Scholar]
- 13.Levi AD, Guenard V, Aebischer P, Bunge RP. The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J Neurosci 1994; 14:1309–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi AD, Sonntag VK, Dickman C, et al. The role of cultured Schwann cell grafts in the repair of gaps within the peripheral nervous system of primates. Exp Neurol 1997; 143:25–36 [DOI] [PubMed] [Google Scholar]
- 15.Guest JD, Rao A, Olson L, et al. The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp Neurol 1997; 148:502–522 [DOI] [PubMed] [Google Scholar]
- 16.Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol 1995; 351:145–160 [DOI] [PubMed] [Google Scholar]
- 17.Fishman PS, Nilaver G, Kelly JP. Astrogliosis limits the integration of peripheral nerve grafts into the spinal cord. Brain Res 1983; 277:175–180 [DOI] [PubMed] [Google Scholar]
- 18.Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature 1980; 284:264–265 [DOI] [PubMed] [Google Scholar]
- 19.Smith GV, Stevenson JA. Peripheral nerve grafts lacking viable Schwann cells fail to support central nervous system axonal regeneration. Exp Brain Res 1988; 69:299–306 [DOI] [PubMed] [Google Scholar]
- 20.Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res 1979; 165:105–118 [DOI] [PubMed] [Google Scholar]
- 21.Casella GT, Bunge RP, Wood PM. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia 1996; 17:327–338 [DOI] [PubMed] [Google Scholar]
- 22.Levi AD, Bunge RP, Lofgren JA, et al. The influence of heregulins on human Schwann cell proliferation. J Neurosci 1995; 15:1329–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilton DA, Jacob J, Househam L, Tengah C. Complications following sural and peroneal nerve biopsies. J Neurol Neurosurg Psychiatry 2007; 78:1271–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraher JP. The transitional zone and CNS regeneration. J Anat 2000; 196 (Pt 1):137–158 [PubMed] [Google Scholar]
- 25.Fraher JP. The transitional zone and CNS regeneration. J Anat 1999; 194 (Pt 2):161–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duncan ID, Hammang JP, Gilmore SA. Schwann cell myelination of the myelin deficient rat spinal cord following X-irradiation. Glia 1988; 1:233–239 [DOI] [PubMed] [Google Scholar]
- 27.Bruce JH, Norenberg MD, Kraydieh S, et al. Schwannosis: role of gliosis and proteoglycan in human spinal cord injury. J Neurotrauma 2000; 17:781–788 [DOI] [PubMed] [Google Scholar]
- 28.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol 2005; 192:384–393 [DOI] [PubMed] [Google Scholar]
- 29.Kanakis DN, Kamphausen T, Van de Nes J. A 32-year-old male with brainstem lesions. Brain Pathol 2013; 23:101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪▪.FDA Guidance for industry: preclinical assessment of investigational cellular and gene therapy products, DRAFT. 2012; http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/default.htm [Google Scholar]; An important update on FDA's expectations for preclinical data to support cellular therapy clinical trials.
- 31.Bosse R, Kulmburg P, von Kalle C, et al. Production of stem-cell transplants according to good manufacturing practice. Ann Hematol 2000; 79:469–476 [DOI] [PubMed] [Google Scholar]
- 32▪▪.Lammertse DP, Jones LA, Charlifue SB, et al. Autologous incubated macrophage therapy in acute, complete spinal cord injury: results of the phase 2 randomized controlled multicenter trial. Spinal Cord 2012; 50:661–671 [DOI] [PubMed] [Google Scholar]; The first reported study of cell therapy in acute spinal cord injury.
- 33▪▪.Grossman RG, Frankowski RF, Burau KD, et al. Incidence and severity of acute complications after spinal cord injury. J Neurosurg Spine 2012; 17:119–128 [DOI] [PubMed] [Google Scholar]; An important multicentre prospective study that provides baseline data to anticipate the incidence and type of complications following acute spinal cord injury.
- 34.Zariffa J, Kramer JL, Fawcett JW, et al. Characterization of neurological recovery following traumatic sensorimotor complete thoracic spinal cord injury. Spinal Cord 2011; 49:463–471 [DOI] [PubMed] [Google Scholar]
- 35.Alper J. Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol 2009; 27:213–214 [DOI] [PubMed] [Google Scholar]
- 36.Frantz S. Embryonic stem cell pioneer Geron exits field, cuts losses. Nat Biotechnol 2012; 30:12–13 [DOI] [PubMed] [Google Scholar]
- 37.Lebkowski J. GRNOPC1: the world's first embryonic stem cell-derived therapy Interview with Jane Lebkowski. Regen Med 2011; 6:11–13 [DOI] [PubMed] [Google Scholar]
- 38.Wirth E3rd, Lebkowski JS, Lebacqz K. Response to Frederic Bretzner et al. Target populations for first-in-human embryonic stem cell research in spinal cord injury. Cell Stem Cell 2011; 8:476–478 [DOI] [PubMed] [Google Scholar]
- 39▪▪.Glass JD, Boulis NM, Johe K, et al. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells 2012; 30:1144–1151 [DOI] [PubMed] [Google Scholar]; The first completed study of neural stem cells transplanted into the human spinal cord.
- 40.Riley J, Federici T, Polak M, et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery 2012; 71:405–416discussion 416 [DOI] [PubMed] [Google Scholar]
- 41.Stradiotti P, Curti A, Castellazzi G, Zerbi A. Metal-related artifacts in instrumented spine. Techniques for reducing artifacts in CT and MRI: state of the art. Eur Spine J 2009; 18 (Suppl. 1):102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann R, Schaefer C, Sadosky A, et al. Burden of spinal cord injury-related neuropathic pain in the United States: retrospective chart review and cross-sectional survey. Spinal Cord 2013; 51:564–570 [DOI] [PubMed] [Google Scholar]
- 43.Margot-Duclot A, Tournebise H, Ventura M, Fattal C. What are the risk factors of occurrence and chronicity of neuropathic pain in spinal cord injury patients? Ann Phys Rehabil Med 2009; 52:111–123 [DOI] [PubMed] [Google Scholar]
- 44.Macias MY, Syring MB, Pizzi MA, et al. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp Neurol 2006; 201:335–348 [DOI] [PubMed] [Google Scholar]
- 45.Monje PV, Soto J, Bacallao K, Wood PM. Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state. J Biol Chem 2010; 285:31024–31036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guest J, Benavides F, Padgett K, et al. Technical aspects of spinal cord injections for cell transplantation. Clinical and translational considerations. Brain Res Bull 2011; 84:267–279 [DOI] [PubMed] [Google Scholar]
- 47.Federici T, Hurtig CV, Burks KL, et al. Surgical technique for spinal cord delivery of therapies: demonstration of procedure in Gottingen minipigs. J Vis Exp 2012; 70:e4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riley JP, Raore B, Taub JS, et al. Platform and cannula design improvements for spinal cord therapeutics delivery. Neurosurgery 2011; 69:ons147–ons154discussion ons155 [DOI] [PubMed] [Google Scholar]


