Abstract
Background
The once-daily (QD), prolonged-release formulation of tacrolimus has been shown to improve adherence versus twice-daily (BD) tacrolimus. Treatment nonadherence in transplant recipients has been associated with poor graft outcomes.
Methods
This open-label, parallel-group study randomized adults with end-stage renal disease undergoing primary kidney transplantation or retransplantation to an initial dose of tacrolimus BD 0.2 mg/kg per day (Arm 1; n=309), QD 0.2 mg/kg per day (Arm 2; n=302), QD 0.3 mg/kg per day (Arm 3; n=304) all with mycophenolate mofetil and corticosteroids (tapered) over 24 weeks, or tacrolimus QD 0.2 mg/kg per day with mycophenolate mofetil, basiliximab, and corticosteroids given only perioperatively (Arm 4; n=283). The primary composite endpoint (efficacy failure; per protocol set) was defined as graft loss, biopsy-confirmed acute rejection, or graft dysfunction at week 24. Graft dysfunction was defined as estimated glomerular filtration rate Modification of Diet in Renal Disease-4 formula of less than 40 mL/min/1.73 m2. The prespecified noninferiority margin was 12.5%.
Results
The per protocol set included 976 patients: 237, 263, 246, and 230 patients in Arms 1 to 4, respectively. Noninferiority of the composite endpoint was demonstrated for Arm 2 versus Arm 1; Kaplan–Meier estimates of efficacy failure were 42.2% and 40.6%, respectively (difference, −1.6%; 95% confidence interval [CI], −12.2% to 9.0%). Noninferiority to Arm 1 was not confirmed for Arm 3 (difference, −3.5%; 95% CI, −13.6% to 6.6%) or Arm 4 (difference, −7.1%; 95% CI, −16.1% to 1.9%). Graft dysfunction (estimated glomerular filtration rate <40 mL/min/1.73 m2) was the main determinant of composite-endpoint efficacy failure across all arms.
Conclusions
In patients representative of the European kidney transplant population, tacrolimus QD-based immunosuppression (0.2 mg/kg/day), without induction, showed similar efficacy to 0.2 mg/kg per day tacrolimus BD.
Keywords: Tacrolimus, Kidney transplantation, Extended-criteria donor, Composite endpoint for graft failure
Supplemental digital content is available in the article.
Tacrolimus has a narrow therapeutic index resulting in a tightly defined range of optimal drug exposure (1, 2). A prolonged-release formulation, providing once-daily (QD) dosing, allows tacrolimus to be absorbed over a greater proportion of the gastrointestinal tract. Tacrolimus QD reduces variability in bioavailability and delivers more consistent blood concentration, which may improve long-term patient outcomes (3–5). QD morning dosing is convenient for patients and improves treatment adherence (6–9). Pharmacokinetic data demonstrated that mean tacrolimus exposure (AUC0–24) on day 1 was approximately 30% lower with tacrolimus QD versus twice-daily dosing (BD) at equivalent starting doses. Mean exposure on day 4 was comparable between regimens (10).
Optimising immunoSuppression After Kidney transplantation with ADVAGRAF (OSAKA) assessed the noninferiority of immunosuppressive protocols with tacrolimus QD versus tacrolimus BD in kidney transplantation. Various starting doses of tacrolimus QD were employed in consideration of differences in mean exposure early after transplantation. A tacrolimus QD steroid-avoidance regimen was included.
This was one of the largest randomized clinical studies ever conducted in kidney transplantation, and the first European large-scale, open-label study to use the novel composite primary endpoint of efficacy failure (graft loss, biopsy-confirmed acute rejection [BCAR], and graft dysfunction), as recommended by the European Medicines Agency (11).
RESULTS
Patient and Donor Demographics
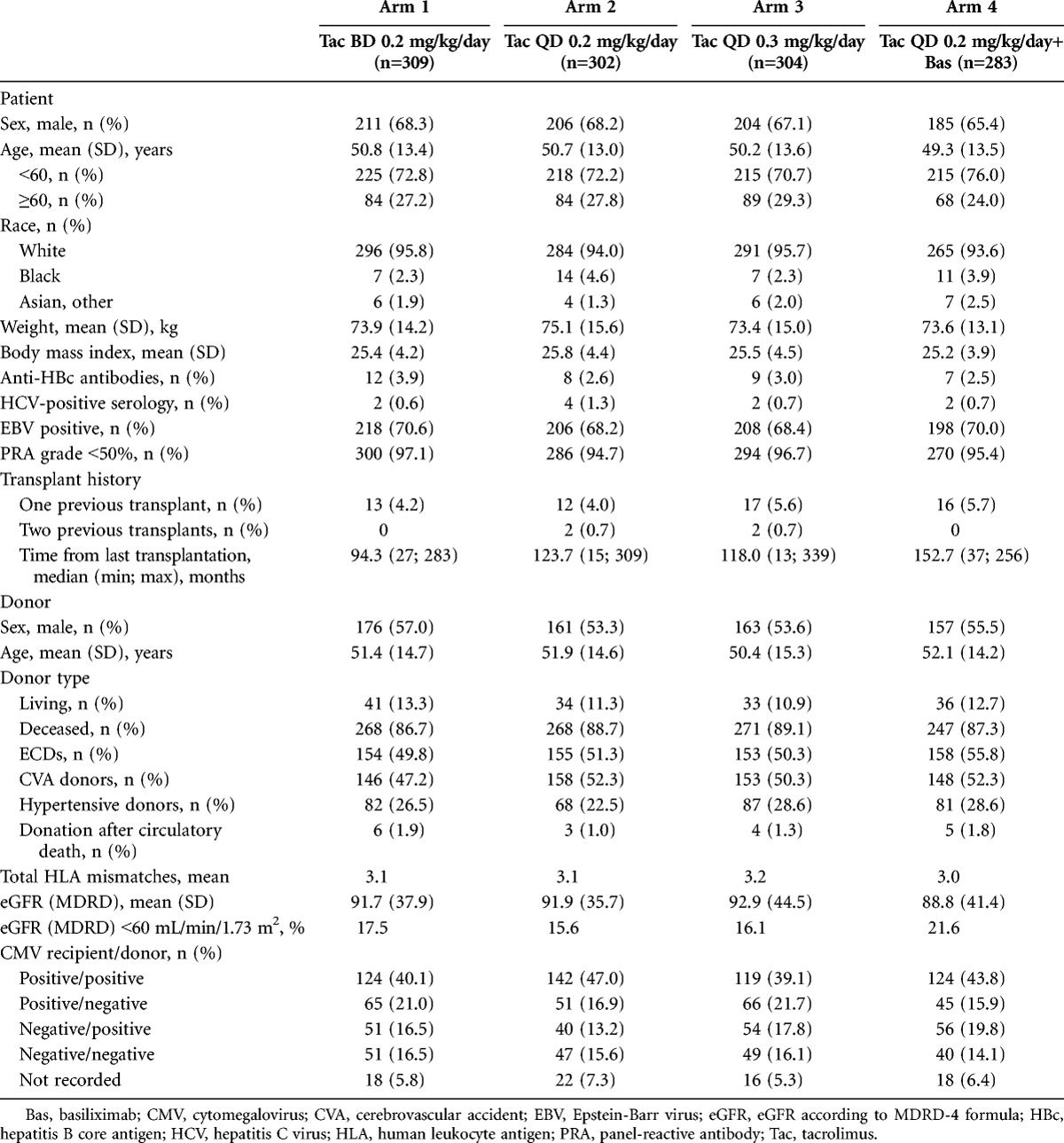
A total of 1214 patients received one or more dose of study medication and were included in the safety analysis set (SAF), 1198 patients were included in the full analysis set (FAS), and 976 in the per protocol set (PPS) (Fig. 1). A total of 222 patients were excluded from the PPS due to major protocol violations; the most commonly reported protocol violation was a deviation of the initial dose of study drug that was either greater than or less than 10% of the recommended dose (62, 30, 49, and 43 patients in Arms 1–4, respectively). A total of 959 (79.0%) patients completed the study; the main reason for discontinuation was adverse events (Fig. 1). Baseline characteristics were comparable between groups (Table 1).
FIGURE 1.
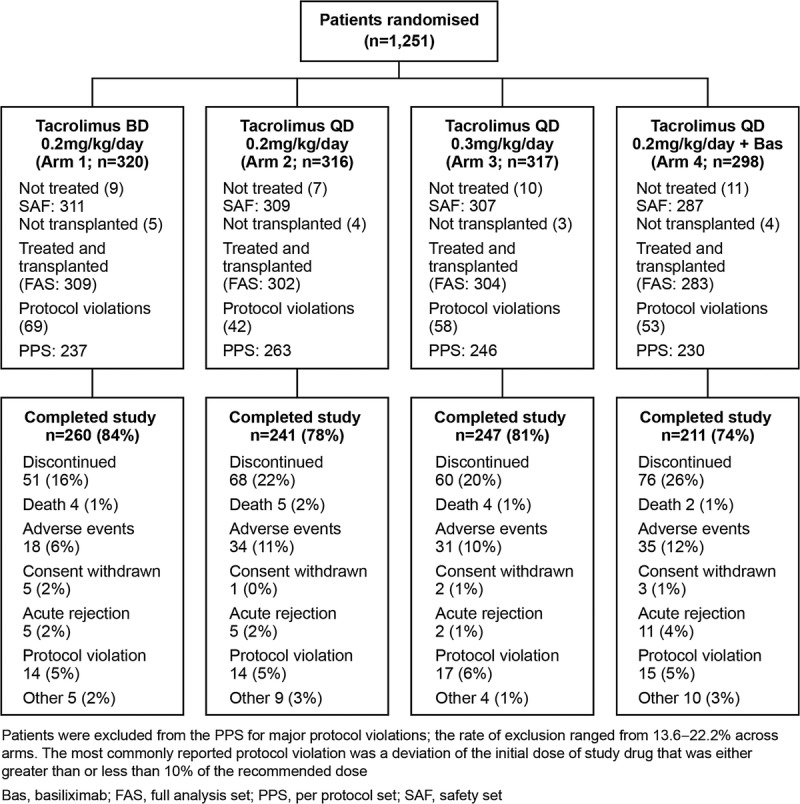
Patient populations and reasons for discontinuation. Patients were excluded from the PPS for major protocol violations; the rate of exclusion ranged from 13.6% to 22.2% across arms. The most commonly reported protocol violation was a deviation of the initial dose of study drug that was either greater than or less than 10% of the recommended dose. Bas, basiliximab; FAS, full anaysis set; PPS, per protocol set; SAF, safety set.
TABLE 1.
Patient and donor demographics and transplantation information (FAS)
Dosing and Exposure
Median tacrolimus trough concentrations were higher with tacrolimus QD 0.3 mg/kg versus the other regimens on days 1 and 7, consistent with higher initial dosing in the protocol; trough concentrations were similar from day 14 onwards. At week 24, median tacrolimus trough concentrations were 7.7 to 8.3 ng/mL across arms. Mean total tacrolimus dose on day 1 (FAS) was 0.158, 0.164, 0.218, and 0.170 mg/kg per day in Arms 1 to 4, respectively. Tacrolimus QD doses were generally higher than BD at each time point, and doses decreased throughout the study in all arms (see Table S1, SDC, http://links.lww.com/TP/A858). Mycophenolate mofetil (MMF) doses were similar across groups throughout the study, and corticosteroid dosage decreased over time in Arms 1 to 3 (see Table S1, SDC, http://links.lww.com/TP/A858) in line with the tapering schedule (see Figure S1, SDC, http://links.lww.com/TP/A858). Contrary to the study protocol, in Arm 4, a total of 44 patients received “maintenance” corticosteroids to prevent further rejection and/or stabilize graft function.
Primary Endpoint (PPS)
Noninferiority was established for efficacy failure rates between Arms 2 and 1; Kaplan–Meier estimates of efficacy failure rates were 42.2% (111 of 263) versus 40.6% (96 of 237), respectively (difference, −1.6%; 95% confidence interval [CI], −12.2% to −9.0%). Noninferiority of efficacy failure between tacrolimus 0.3 mg/kg per day QD (Arm 3: 44.2% [108 of 246]) and Arm 1 was not achieved (difference, −3.5%; 95% CI, −13.6% to −6.6%). Noninferiority for efficacy failure was also not established for Arm 4 (48.2% [110 of 230]) versus Arm 1 (difference, −7.1%; 95% CI, −16.1% to −1.9%). The dominant reason for efficacy failure in all arms was graft dysfunction at week 24; 56 of 237 (23.6%), 72 of 263 (27.4%), 60 of 246 (24.4%), and 72 of 230 (31.3%) patients in Arms 1 to 4, respectively (Fig. 2A).
FIGURE 2.
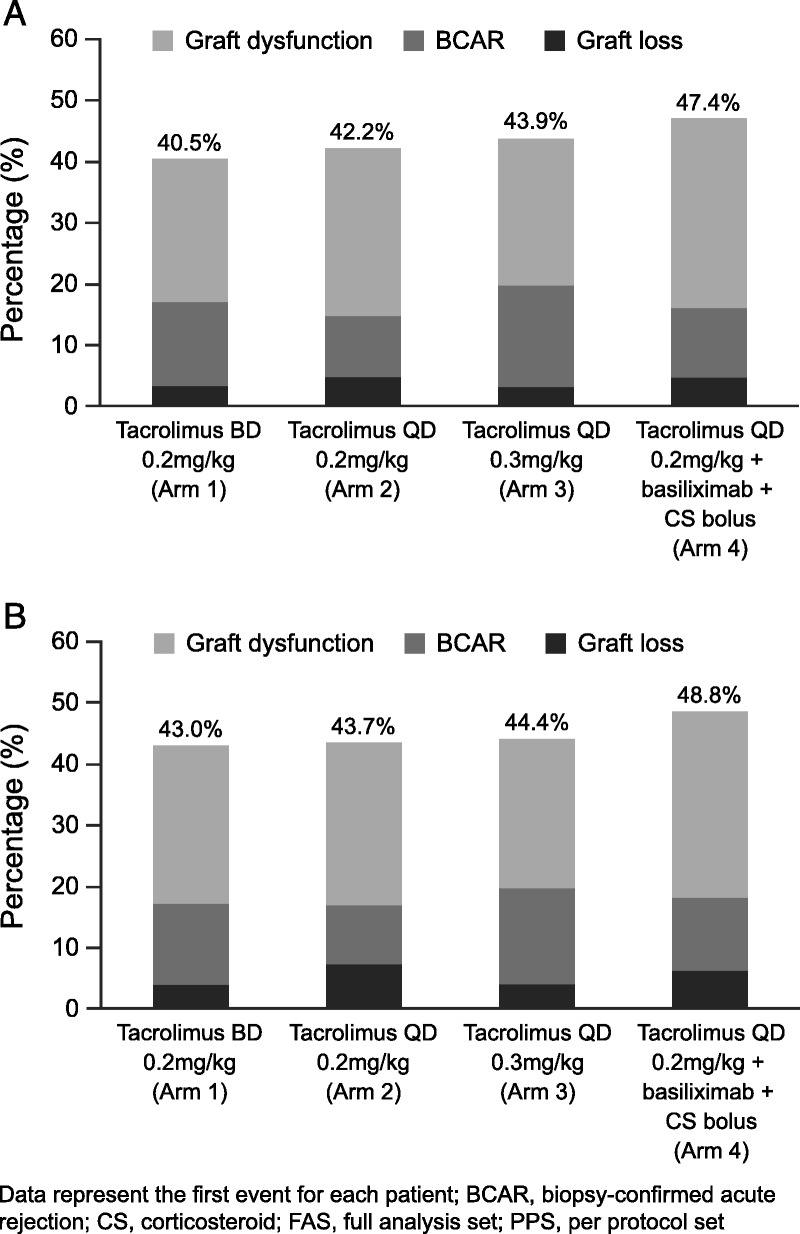
Primary endpoint: (A) efficacy failure rates (PPS) and (B) efficacy failure rates (FAS). Data represent the first event for each patient. BCAR, biopsy-confirmed acute rejection; CS, corticosteroid; FAS, full analysis set; PPS, per protocol set.
Secondary Endpoints (FAS)
Kaplan–Meier estimates of efficacy failure rates for the FAS demonstrated the noninferiority of Arm 2 versus Arm 1 (43.7% [132 of 302] vs. 43.3% [133 of 309]; difference, −0.4%; 95% CI, −10.0% to −9.3%) and did not show noninferiority for Arm 4 (49.4% [138 of 283]) versus Arm 1 (95% CI, −13.7% to −2.4%) (Fig. 2B). However, Arm 3 (44.6% [135 of 304]) did show noninferiority versus Arm 1 for efficacy failure (difference, −1.3%; 95% CI, −10.3% to −7.7%). In total, 7.4% of patients experienced graft loss; 18, 29, 20, and 23 patients in Arms 1–4, respectively. The main reasons were technical complications (n=31), nonfunctioning graft (n=19), death with functioning graft (n=17), and infection (n=8).
The number of patients who experienced BCAR was low with tacrolimus initiated at 0.2 mg/kg per day (Arm 1: 13.6% [42 of 309], Arm 2: 10.3% [31 of 302], and Arm 4: 12.7% [36 of 283]). A comparable incidence of BCAR (16.1% [49 of 304]) was observed with tacrolimus initiated at 0.3 mg/kg per day. The majority of BCARs were corticosteroid-sensitive and mild to moderate in severity (see Table S2, SDC, http://links.lww.com/TP/A858). The incidence of Banff grade 3 (severe) BCAR was low and did not differ significantly between groups (P=0.479, Fisher’s exact test). In total, 20.1%, 18.5%, 25.0%, and 24.0% of patients received antirejection treatment in Arms 1 to 4, respectively.
At week 24, kidney function (estimated glomerular filtration rate [eGFR]) was similar in Arms 1 to 3 but lower in Arm 4 (Fig. 3A). The least-squares mean values for eGFR were 48.3, 45.7, and 45.9 mL/min/1.73 m2 in Arms 1 to 3, respectively, versus 41.7 mL/min/1.73 m2 in Arm 4 (P<0.001). A similar pattern was observed with creatinine clearance (see Figure S2, SDC, http://links.lww.com/TP/A858). Exploratory analyses on completers showed that mean (SD) eGFR was 50.5 (18.5), 49.8 (14.7), 49.6 (16.9), and 46.3 (14.7) mL/min/1.73 m2 in Arms 1 to 4, respectively (P=0.035, analysis of variance).
FIGURE 3.
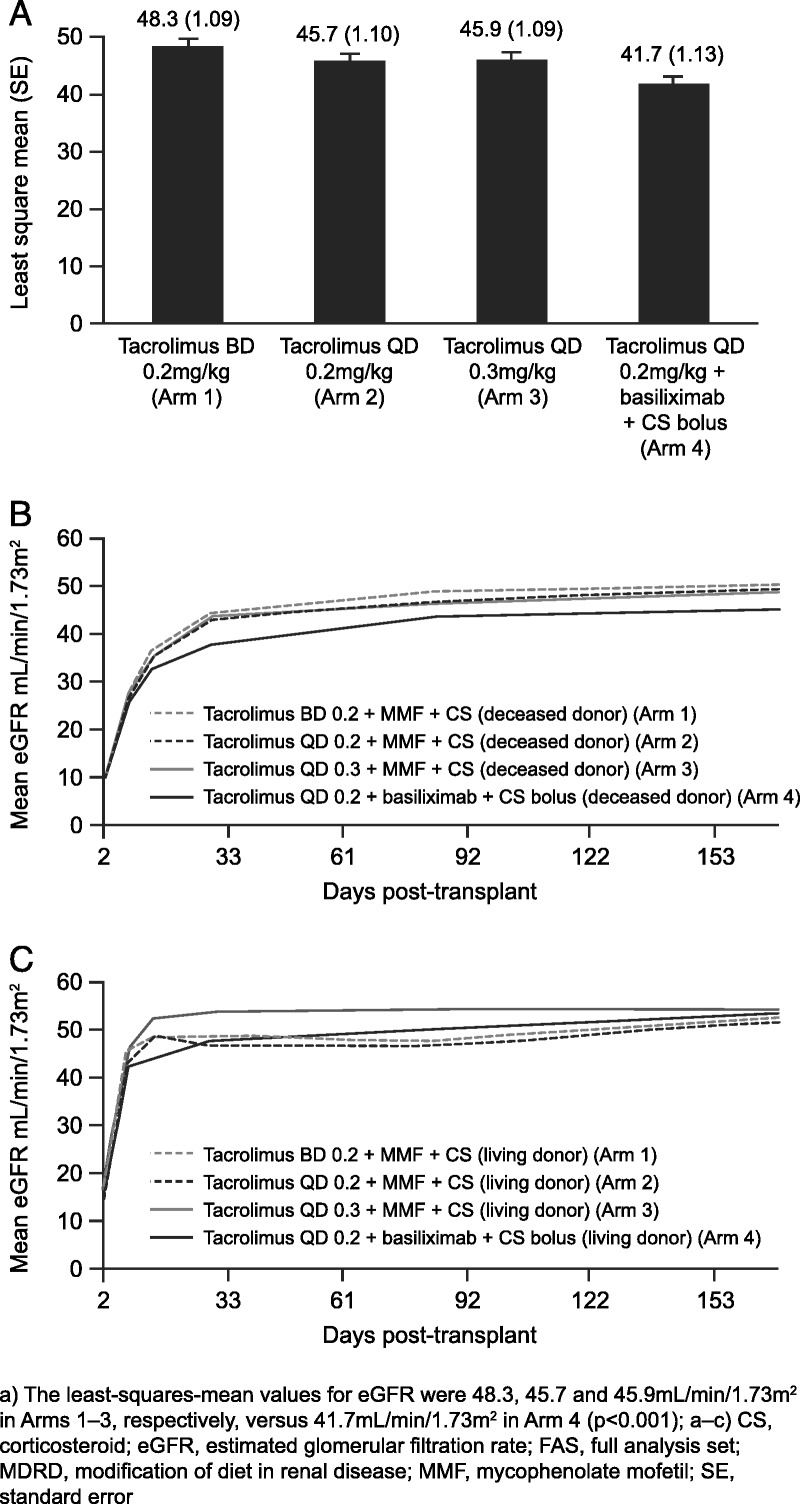
Measurements of (A) eGFR at 24 weeks (FAS), (B) time course of eGFR in patients who received a kidney from a deceased donor (FAS), and (C) time course of eGFR in patients who received a kidney from a living donor (FAS). The least-squares mean values for eGFR were 48.3, 45.7, and 45.9 mL/min/1.73 m2 in Arms 1 to 3, respectively, versus 41.7 mL/min/1.73 m2 in Arm 4 (P<0.001). Bas, basiliximab; CS, corticosteroid; MMF, mycophenolate mofetil; Tac, tacrolimus.
Recipients of kidneys from living donors tended to have better kidney function early after transplantation versus patients who received deceased-donor organs, because there was no delayed graft function (one or more dialysis during week 1) with living donation. This difference diminished over time (Fig. 3B,C). In total, 51.8% of donors met the definition for “extended criteria” (Table 1); differences between the groups were not statistically significant. Kidney dysfunction was significantly higher among recipients of organs from extended-criteria donors (ECDs) (315 of 620 [50.8%]), compared with recipients of organs from standard-criteria donors (SCDs) (140 of 578 [24.2%]; P=0.0001).
Kaplan–Meier estimates of graft survival at week 24 were 94.1% (291 of 309) in Arm 1 versus 90.4% (273 of 302), 93.4% (281 of 304), and 91.8% (260 of 283) in Arms 2 to 4, respectively. The incidence of delayed graft function in deceased-donor transplant recipients was similar in all arms (Arm 1: 14.2% [38 of 268], Arm 2: 13.8% [37 of 268], Arm 3: 14.4% [39 of 271], and Arm 4: 15.4% [37 of 247]). Twenty-four deaths were reported during the study or after premature study withdrawal. The most common causes were cardiovascular events or infection. Two cases were considered to be possibly related to treatment (pneumonia, Arm 1; respiratory distress and multiple organ failure, Arm 2). Kaplan–Meier estimates of patient survival (week 24) were 98.0% (303 of 309), 97.3% (294 of 302), 97.7% (297 of 304), and 98.9% (280 of 283) for Arms 1 to 4, respectively.
Safety
Rates of treatment-emergent adverse events ranged from 94% to 96% across arms (see Table S3, SDC, http://links.lww.com/TP/A858). Most were of mild/moderate severity (~70% [830 of 1214]). Incidence of malignancy was low (1, 2, 3, and 3 patients in Arms 1–4, respectively). There was one incidence of Pneumocystis jiroveci infection (Arm 1) and no cases of progressive multifocal leukoencephalopathyor posttransplantation lymphoproliferative disorder. The occurrence of new-onset diabetes mellitus (NODM) was comparable between groups: 16.1%, 13.2%, 18.3%, and 12.6% in Arms 1 to 4, respectively (see Table S3, SDC, http://links.lww.com/TP/A858). Ongoing diabetes and antidiabetic therapy were significantly lower in Arm 4 versus Arm 1 (P=0.042 and 0.024, chi-square test).
DISCUSSION
Data from OSAKA showed that, in adult de novo kidney transplantation, the efficacy of QD morning administration of prolonged-release tacrolimus 0.2 mg/kg was noninferior to a BD immunosuppressive regimen based on the same tacrolimus starting dose, in regimens without induction therapy. Increasing the starting dose of tacrolimus QD (0.3 mg/kg/day, Arm 3) did not increase efficacy. In particular, the incidence of BCAR was not improved. Noninferiority of Arm 2 versus Arm 1 was supported by Kaplan–Meier analyses and measurements of kidney function.
Previous randomized controlled studies have shown similar efficacy with tacrolimus QD and BD in kidney transplantation. A large, open-label study in 638 de novo kidney transplant recipients showed that tacrolimus QD and BD were noninferior to cyclosporine (plus MMF, corticosteroids, and basiliximab induction) for the primary endpoint of efficacy failure (death, graft loss, BCAR, or lost to follow-up) (12). A more recent study compared tacrolimus QD and BD (0.2 mg/kg/day) with low-dose MMF and steroids (without antibody induction) in 667 de novo kidney transplant recipients (13). Although BCAR event rates at week 24 (the primary efficacy endpoint) with tacrolimus QD did not differ significantly from BD therapy (20.4% vs. 15.8%), the criteria for treatment noninferiority were not met. Tacrolimus QD and BD showed similar patient and graft survival at 1 year after transplantation, efficacy failure rates did not differ between treatments, and safety profiles were similar.
Acute rejection rates in OSAKA were low and comparable with 0.2 mg/kg per day tacrolimus QD versus BD. These data indicate that a higher starting dose of tacrolimus QD is unnecessary to achieve comparable efficacy to tacrolimus BD. The acute rejection rates observed with Arm 2 further support 0.2 mg/kg per day tacrolimus QD plus MMF and corticosteroids as the optimal QD treatment regimen in this study. Interestingly, the acute rejection rates with tacrolimus 0.2 mg/kg per day without induction therapy were similar to those reported in the ELITE-Symphony Study after initiation with 0.1 mg/kg per day tacrolimus BD plus daclizumab (Symphony: 12.3%) (14).
The scale of OSAKA allows meaningful analyses of the effect of organ quality on the primary composite outcome. In this study, graft dysfunction (eGFR <40 mL/min/1.73 m2) was the main driver for the primary composite outcome, which was strongly influenced by donor-related factors. Approximately 50% (620 of 1198) of patients received kidneys from ECDs, reflecting the worsening quality of donor organs available in Europe. Subsequently, there was an unexpectedly higher proportion of patients than anticipated who met the <40 mL/min/1.73 m2 threshold, and this contributed to the seemingly high efficacy failure rate. However, the difference in renal function observed between the patients who received organs from ECDs and SCDs early after transplantation diminished over time, indicating that the immunosuppressive regimens used (including the initial dosing before transplantation) were appropriate for this patient population. In a previous study comparing tacrolimus QD and BD, efficacy failure (BCAR, graft loss, or death but excluding graft dysfunction) was 18.9% (55 of 291) in patients receiving tacrolimus BD and 22.5% (63 of 280) for QD (PPS) (13). In hind sight, using the chronic kidney disease predefined eGFR categories may have resulted in a more balanced primary composite endpoint.
As donor demographics have such a strong influence on outcomes, it is essential that donor information is readily available for consideration in any indirect comparisons between studies. This study was designed to reflect real-world practice in Europe where there is a substantial proportion of deceased-donor and ECD kidney transplants performed (in OSAKA: 88% deceased donors, mean donor age, 51.5 years). In comparison, in BENEFIT, 42% of kidneys were from deceased donors, with mean ages of 42 years for living donors and 38 years for deceased donors (15). In ELITE-Symphony, 64% of kidneys were from deceased donors and mean donor age was approximately 45 years (14).
In OSAKA, kidney function was lowest in the steroid-avoidance arm (Arm 4). Interestingly, in a subanalysis of patients who received organs from SCDs, the incidence of renal dysfunction was comparable between arms (24.5%, 22.4%, 21.9%, and 28.8% in Arms 1–4, respectively; P=0.5011). This implies that the higher incidence of renal dysfunction in Arm 4 was predominantly associated with organs from ECDs. Steroid use has been shown to confer immediate posttransplantation kidney protection (16); consequently, steroid avoidance may have a greater impact on kidneys from ECDs compared with SCDs. A steroid-avoidance regimen with tacrolimus QD at the lower starting dose of 0.2 mg/kg may therefore be feasible for the SCD subpopulation (with low immunologic risk) with the potential for a reduced incidence of ongoing diabetes and requirement for antidiabetic treatment.
OSAKA was conducted in a high number of kidney recipients who can be considered to be representative of the European transplant population. Graft dysfunction was the main driver for the primary efficacy endpoint, most likely as a result of there being approximately 50% of patients receiving kidneys from ECDs, thus making the true interpretation of treatment effect on recipients who received kidneys from SCDs more challenging. A marginally higher dropout rate and a higher number of protocol violations than originally assumed may also have influenced some of the study outcomes in this trial. To overcome this, and to confirm the robustness of the data, the endpoints were analyzed for both the PPS and FAS. Other limitations of this study include a low immunologic risk population, not assessing treatment adherence and short study duration (24 weeks). It would also be interesting to assess the calcineurin inhibitor-related long-term toxicities between regimens (such as renal impairment or the development of NODM after transplantation) over a longer treatment period.
In conclusion, our results show that a modern tacrolimus QD-based immunosuppressive regimen using a starting dose of 0.2 mg/kg per day is of similar efficacy to the current standard tacrolimus 0.2 mg/kg per day BD-based therapy in de novo kidney transplantation. Increasing the starting dose of tacrolimus QD to 0.3 mg/kg per day offered no efficacy or other clinical advantage.
MATERIALS AND METHODS
Study Design (NCT00717470; ClinicalTrials.gov)
This randomized, open-label, parallel-group study was conducted at 110 centers in 22 countries (May 2008–March 2010), in accordance with the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonisation guidelines, and the applicable laws and regulations. An independent ethics committee from each study center granted approval before initiation. Written informed consent was obtained from all participants.
Eligible patients were 18 years or older with end-stage renal disease who received a primary kidney/retransplantation (unless the graft was lost due to rejection within 12 months after first transplantation). Kidneys were transplanted from deceased/living donors with compatible ABO blood types; there was no restriction on donor age or in cold ischemia time.
Randomization and Masking
The randomization sequence was prepared by Astellas Pharma Europe Ltd. (Surrey, UK). Randomization was coordinated centrally by Cenduit Interactive Response Technologies using an interactive voice response system to randomize eligible patients to one of four treatment arms (1:1:1:1) and allocate patient numbers. Treatment allocation was stratified according to study center and age group.
Procedure
A preoperative dose of tacrolimus BD (0.1 mg/kg, Arm 1) or QD (0.1 mg/kg, Arms 2 and 4; 0.15 mg/kg, Arm 3) was given within 12 hr before reperfusion and within 3 hr of anesthesia when possible. The second dose of tacrolimus BD or QD was administered postoperatively but not within 4 hr of the first dose or more than 12 hr after reperfusion. Patients who received a living-donor organ were allowed predosing with tacrolimus BD or QD within 72 hr of perfusion (Arms 1, 2, and 4: maximum 0.2 mg/kg and Arm 3: maximum 0.3 mg/kg). Subsequent oral doses of tacrolimus BD and QD were allowed after day 1 and adjusted based on clinical efficacy and safety, taking account of recommended whole-blood trough concentrations (see Figure S1, SDC, http://links.lww.com/TP/A858). Tacrolimus trough concentrations were determined by the local laboratories.
Treatment continued for 24 weeks. In Arm 1, tacrolimus BD commenced at a daily dose of 0.2 mg/kg; QD was initiated at a daily dose of 0.2 mg/kg in Arms 2 and 4 and 0.3 mg/kg in Arm 3. Corticosteroids were administered in Arms 1 to 3 in the following doses: day 0, ≤500 mg (bolus); day 1, 125 mg (bolus); days 2 to 14, 20 mg per day; days 15 to 28, 15 mg per day; days 29 to 42, 10 mg per day; days 43 to 84, 5 mg per day; and days 85 to 168, ≤5 mg per day. All patients received MMF (1 g preoperatively then 1 g BD for 14 days and 0.5 g BD thereafter; see Figure S1, SDC, http://links.lww.com/TP/A858). Basiliximab was administered to patients in Arm 4 only; these patients also received a steroid bolus (<500 mg) on day 0.
A kidney biopsy was performed before initiation of antirejection therapy if clinical and/or laboratory signs indicated rejection and was evaluated by a local histopathologist following Banff ‘97 criteria (17).
Primary Efficacy Variable
Efficacy failure rate was defined as incidence and time to first occurrence of one of the following: (a) graft loss (retransplantation, nephrectomy, death, or dialysis ongoing at study end or at time of discontinuation, unless superseded by follow-up information that indicated graft survival); (b) BCAR, diagnosed locally; and (c) graft dysfunction (eGFR Modification of Diet in Renal Disease [MDRD]-4, <40 mL/min/1.73 m2, capped at 60 mL/min/1.73 m2) at week 24. Analysis was undertaken on the PPS as per the protocol. The threshold for graft dysfunction was based on estimates from previously reported studies (18–20): it was expected that mean eGFR would be approximately 50 to 55 mL/min/1.73 m2. On this basis, it was anticipated that BCAR and graft dysfunction would have a similar impact on the composite endpoint.
Secondary Endpoints
Secondary endpoints included eGFR and creatinine clearance (Cockcroft–Gault) at week 24, assessment of BCAR, and graft and patient survival. Adverse events were monitored throughout. NODM was defined as fasting blood glucose of 7 mmol/L or more from day 2 onwards.
Statistical Analysis (SAS Version 9.1.3)
The planned sample size of 1200 patients (300 per group) provided 80% power to detect a difference of 12.5% or more between groups, assuming a failure rate of 30% and a 6% dropout rate for the composite primary efficacy endpoint. Failure rate was based on an expected early death/graft loss rate in de novo renal transplantation of 5% to 7.5%, an expected BCAR rate using a tacrolimus-based regimen of 10% to 17.5%, and an expected rate of renal dysfunction at week 24 in the range of 10% to 15% based on an eGFR threshold of less than 40 mL/min/1.73 m2. Adjusting for overlap resulting from patients who experience more than one of the events (BCAR and graft loss ~2.5% or BCAR and renal dysfunction ~5%), the estimated frequency of the composite endpoint was approximately 30% (range, 25%–35%).
Primary analyses of efficacy data were undertaken on the PPS: randomized patients who received one or more dose of study medication were transplanted, and did not have a major protocol violation. To determine the robustness of the data, these analyses were also assessed on the FAS (secondary endpoint): all randomized patients who had a transplant and received one or more dose of tacrolimus. All other efficacy data were assessed on the FAS. Safety analyses were based on all randomized patients who received one or more dose of tacrolimus. Analyses were stratified by treatment group.
To prove noninferiority, a 12.5% difference in the composite endpoint was considered to be within the range in which a meaningful clinical difference in efficacy between the treatment regimens becomes apparent (based on the 1200 patient sample size; 80% power to detect a difference). Noninferiority was demonstrated if the lower limit of the 95% CI for the difference in efficacy failure rate was above -12.5% for each tacrolimus QD arm versus the BD arm (PPS and FAS). The Bonferroni–Holm method was applied for multiple comparisons. Kaplan–Meier analyses were used to determine efficacy failure rates; the reported percentages represent Kaplan–Meier estimates, and the plain incidences are given in brackets. Kidney function and blood lipid parameters were evaluated using analysis of covariance. P<0.05 was considered statistically significant.
Ancillary Analysis
Although the study protocol did not define ECDs or SCDs, a post hoc analysis was performed. ECDs (modified Crystal City criteria) (21) included living or deceased donors aged 60 years or older or who were 50 to 60 years old with one or more other risk factor (cerebrovascular accident as reason for death, hypertension, or serum creatinine >1.5 mg/dL) and donation after circulatory death. All other donors were, by default, SCDs. Where values for any of these variables were missing, donors were assumed not to have met that criterion. For all comparisons, FAS data were analyzed using Pearson’s chi-square test.
ACKNOWLEDGMENTS
The authors thank Stefan Schleibner and Roland Wolf (AMS-Advanced Medical Services, Munich, Germany) for their statistical support during the study and in the preparation of the manuscript, and Nina Heath and Anisha Mehra (iS Health) for their editorial support in the preparation of the article. Editorial support was funded by Astellas Pharma Europe Ltd.
APPENDIX
This study was performed in 110 centers in 22 countries: Argentina, Austria, Belgium, Czech Republic, France, Germany, Greece, Hungary, Ireland, Italy, The Netherlands, Norway, Poland, Portugal, Romania, Russian Federation, Slovakia, South Africa, Spain, Sweden, Switzerland, and United Kingdom.
Footnotes
This study was funded by Astellas Pharma Europe Ltd.
B.B. has served on advisory boards for Astellas, BMS, and Novartis and has participated in clinical trials sponsored by Astellas, BMS, Genzyme, Novartis, Roche, and Wyeth. J.L.K. has received funds and consulting fees from Astellas, Roche, Novartis, and BMS. O.V. has received lecture and consultancies fees from Astellas. N.K. has received honoraria and travel grants from Astellas, Novartis, Pfizer, Roche, Genzyme, and Amgen.
Trial identification number and registry: NCT00717470; www.clinicaltrials.gov
L.A. has served on advisory boards fro BMS, Novartis and received travel grants from BMS, Astellas, Roche and Novartis.
E-mail: kamar.n@chu-toulouse.fr
L.A. and B.B. contributed equally to this work. All authors were involved in drafting of the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors were likewise involved in the study conception and design, acquisition of data, or analysis and interpretation of data.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
Received 3 January 2013. Revision requested 25 January 2013.
Accepted 24 June 2013.
REFERENCES
- 1.European Medicines Agency. Committee for Medicinal Products for Human Use (2012). CHMP Pharmacokinetics Working Party (PKWP). Questions and answers: positions on specific questions addressed to the Pharmacokinetics Working Party. London. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002963.pdf. Accessed May 2012.
- 2. Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol 2007; 2: 374. [DOI] [PubMed] [Google Scholar]
- 3. Wu MJ, Cheng CY, Chen CH, et al. Lower variability of tacrolimus trough concentration after conversion from Prograf to Advagraf in stable kidney transplant recipients. Transplantation 2011; 92: 648. [DOI] [PubMed] [Google Scholar]
- 4. Borra LC, Roodnat JI, Kal JA, et al. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 2010; 25: 2757. [DOI] [PubMed] [Google Scholar]
- 5. Stevenson KS, Glen J, Stevens KK, et al. High tacrolimus intrapatient variability is associated with acute rejection and graft loss (abstract MO-021). Transpl Int 2011. (Suppl 2); 24: 111. [Google Scholar]
- 6. Guirado L, Cantarell C, Franco A, et al. Efficacy and safety of conversion from twice-daily to once-daily tacrolimus in a large cohort of stable kidney transplant recipients. Am J Transplant 2011; 11: 1965. [DOI] [PubMed] [Google Scholar]
- 7. Morales JM, Varo E, Lázaro P. Immunosuppressant treatment adherence, barriers to adherence and quality of life in renal and liver transplant recipients in Spain. Clin Transplant 2012; 26: 369. [DOI] [PubMed] [Google Scholar]
- 8. Kuypers DR, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation 2013; 95: 333. [DOI] [PubMed] [Google Scholar]
- 9. Beckebaum S, Jacob S, Sweid D, et al. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int 2011; 24: 666. [DOI] [PubMed] [Google Scholar]
- 10. Wlodarczyk Z, Squifflet JP, Ostrowski M, et al. Pharmacokinetics for once-versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant 2009; 9: 2505. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency. Committee for Medicinal Products for Human Use (2008). Guideline on Clinical Investigation of Immunosuppressants for Solid Organ Transplantation. London. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003593.pdf. Accessed May 2012.
- 12. Silva HT, Jr, Yang HC, Abouljoud M, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 2007; 7: 595. [DOI] [PubMed] [Google Scholar]
- 13. Krämer BK, Charpentier B, Bäckman L, et al. for the Tacrolimus Prolonged Release Renal Study Group. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant 2010; 10: 2632. [DOI] [PubMed] [Google Scholar]
- 14. Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357: 2562. [DOI] [PubMed] [Google Scholar]
- 15. Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010; 10: 535. [DOI] [PubMed] [Google Scholar]
- 16. De Greef KE, Ysebaert DK, Vercauteren SR, et al. Effect of immunosuppression on damage, leukocyte infiltration, and regeneration after severe warm ischemia/reperfusion renal injury. Transplant Proc 2002; 34: 791. [DOI] [PubMed] [Google Scholar]
- 17. Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713. [DOI] [PubMed] [Google Scholar]
- 18. Rostaing L, Cantarovich D, Mourad G, et al. Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation 2005; 79: 807. [DOI] [PubMed] [Google Scholar]
- 19. Vítko S, Klinger M, Salmela K, et al. Two corticosteroid-free regimens—tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil—in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation 2005; 80: 1734. [DOI] [PubMed] [Google Scholar]
- 20. Vítko S, Wlodarczyk Z, Kyllönen L, et al. Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. Am J Transplant 2006; 6: 531. [DOI] [PubMed] [Google Scholar]
- 21. Rosengard BR, Feng S, Alfrey EJ, et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant 2002; 2: 701. [DOI] [PubMed] [Google Scholar]


