Abstract
Prader-Willi syndrome (PWS) is one of the most commonly recognized causes of early-onset childhood obesity. Individuals with PWS have significant hyperphagia and decreased recognition of satiety. The exact etiology of the hyperphagia remains unknown and, therefore, untreatable. We conducted a pilot, open-label study of response to metformin in 21 children with PWS and six with early morbid obesity (EMO). Participants had significant insulin resistance and glucose intolerance on oral glucose tolerance testing (OGTT) and were started on metformin for these biochemical findings. We administered the Hyperphagia Questionnaire to parents of patients before and after starting metformin treatment. Both the PWS and EMO groups showed significant improvements in food-related distress, anxiety, and ability to be redirected away from food on the Hyperphagia Questionnaire. In the PWS group, improvements were predominantly seen in females. Within the PWS group, responders to metformin had higher 2-h glucose levels on OGTT (7.48 mmol/L vs. 4.235 mmol/L; p=0.003) and higher fasting insulin levels (116 pmol/L vs. 53.5 pmol/L; p=0.04). Additionally, parents of 5/13 individuals with PWS and 5/6 with EMO reported that their child was able to feel full while on metformin (for many this was the first time they had ever described a feeling of fullness). Metformin may improve sense of satiety and decrease anxiety about food in some individuals with PWS and EMO. Positive response to metformin may depend on the degree of hyperinsulinism and glucose intolerance. Nonetheless, the results of this pilot study bear further investigation.
Keywords: metformin, obesity, Prader-Willi syndrome
Introduction
Prader-Willi syndrome (PWS), a complex genetic disorder, is caused by the absence of normally active paternally expressed genes from the chromosome 15q11-q13 region. PWS is an imprinted condition with approximately 70% of the cases due to a de novo deletion in the paternally inherited chromosome 15 q11-q13 region, 25% from a maternal uniparental disomy of chromosome 15 (UPD), and the remaining 5% from either microdeletions or epimutations of the imprinting center in the 15q11-q13 region (i.e., imprinting defects) (1, 2). Features of PWS include poor feeding in infancy often associated with failure to thrive, with obesity beginning around age 2, hyperphagia, hypotonia, developmental and cognitive delay, behavioral problems, sleep abnormalities, and neuroendocrine abnormalities (1, 2). Hyperphagia in PWS is highly stressful for both children and their parents, and, together with the obesity, is a life-threatening element of PWS. Indeed, complications of obesity remain the leading cause of death in adults with this syndrome (3).
The specific etiology of the hyperphagia in PWS is unknown, but abnormalities in appetite and satiety are typically ascribed to hypothalamic dysfunction (4, 5). Individuals with PWS also show increased neuronal reward circuitry activation in response to food, especially high-calorie foods, both pre- and post-meal (6-9). They also have high levels of ghrelin (an orexogenic hormone) and lower levels of insulin and PYY (anorexogenic hormone), which are thought to contribute to their appetite abnormalities (1, 10, 11). Adipokines produced by the excess adipose tissue in individuals with PWS may also play a role in their regulating food intake (12, 13).
Individuals with childhood-onset obesity and increased visceral fat also have adipokine abnormalities that may worsen their obesity. Insulin and leptin resistance are common in long-standing childhood obesity, which result in impaired perception of satiety (14, 15). Some studies have found that insulin resistance can precede the development of obesity (16, 17). Both insulin resistance and childhood obesity have been associated with structural brain abnormalities as well as cognitive abnormalities (18, 19) underscoring the critical needs for treatments of insulin resistance and obesity in these children. Studies in mice indicate that insulin resistance and cognitive decline can occur simultaneously (20), while studies in humans indicate a correlation between insulin resistance and decreased brain volume, increased fatty acid uptake in the brain, and decreased cognitive performance and executive function (21-24). Studies are ongoing to investigate whether metformin treatment for individuals with type 2 diabetes will decrease the incidence of dementia or other cognitive impairments in this population (25).
Many children who develop diabetes require treatment with insulin, which can increase weight gain. Although metformin has been used for treatment of type 2 diabetes in PWS, thus far only one case report has shown that metformin treatment resulted in weight loss in this population (26-28). Among children with obesity and insulin resistance, metformin treatment has been shown to increase insulin sensitivity and decrease body mass index (BMI), blood pressure, and lipid levels (29, 30). This present, open-label pilot study assessed whether similar benefits of metformin therapy are seen in children with PWS or early-onset obesity who are at risk for type 2 diabetes based on glucose intolerance and insulin resistance on oral glucose tolerance testing (OGTT).
Methods
Participants
The study included 21 individuals with genetically confirmed PWS (10 males, 11 females; 16 paternal deletion, five maternal UPD) and 10 with early-onset obesity of unknown etiology [early morbid obesity (EMO); five males, five females]. Participants ranged between 7 and 17 years of age, with a mean of 11.18 years (Table 1). These patients were recruited from a multi-site longitudinal study of PWS and early morbid obesity, and were seen clinically at the University of Florida. All patients underwent an OGTT and were clinically started on metformin therapy for treatment of glucose intolerance and insulin resistance. Patients were provided with routine clinical care and follow-up, and were thus on metformin for variable lengths of time (range, 6 month to 3 years). Follow-up questionnaires were completed after the participants had been on metformin for at least 3 months or longer. This study was approved by the Institutional Review Board at the University of Florida and all parents signed informed consent and children provided assent if they were able.
Table 1.
Characteristics of participants with PWS or EMO who stayed on metformin therapy.
| PWS (n=14) | EMO (n=6) | |
|---|---|---|
| Age | 11.21 (3.81) | 11.16 (4.35) |
| IQ | 84.00 (15.60) | 90.25 (23.76) |
| BMI Z-score | 1.70 (1.2) | 2.59 (0.63) |
| Glucose fasting, mmol/L | 4.87 (0.47) | 4.70 (0.07) |
| Glucose 2 h, mmol/L | 6.63 (2.10) | 6.1 (1.71) |
| Insulin fasting | 98.13 (51.7) | 70.6 (16.3) |
| Insulin maximum | 830.2 (753.7) | 603.2 (281.3) |
Values are shown as mean (SD).
Prior to beginning metformin, all children had a standardized OGTT with glucose and insulin levels drawn at fasting and at 1 and 2 h after glucola ingestion. Body fat was measured using a dual-energy X-ray absorptiometry (General Electric, Fairfield, CT) scanner at the University of Florida, and BMI standard deviation (BMI SDS) was calculated using growth charts from the Centers for Disease Control and Prevention.
Parents completed the Hyperphagia Questionnaire (31) before and after their child’s treatment with metformin, providing an index of the effect of metformin on appetite and satiety. This 11-item questionnaire uses a five-point Likert scale to assess preoccupation with food, as well as hyperphagic drive, behavior, and severity. The questionnaire has good psychometric properties. Based on clinical observations, we included three additional items on the questionnaire that assessed children’s anxiety related to food and whether they left food on their plate or reported feeling full.
Results
Treatment drop outs
After baseline testing and beginning metformin, seven of the ten males with PWS developed a marked worsening of behavioral problems within 1-2 days of taking metformin. Anecdotally, these behavior problems included severe emotional lability, possible seizure activity, and worsening of food seeking. All seven parents of these males immediately stopped the medication and reported that behavioral symptoms improved once off the medication. Understandably, none of these parents returned the follow-up Hyperphagia Questionnaire. None of the females with PWS experienced such negative reactions. The three PWS males remaining on metformin did not experience any marked improvement or worsening in hyperphagic behaviors. In contrast to the PWS male dropouts, all children with EMO stayed on metformin, yet four parents of these children (two males, two females) did not complete the follow-up survey. These parents reported that a lack of any noticeable change in food-related behaviors led them to not return the survey.
Hyperphagia Questionnaire
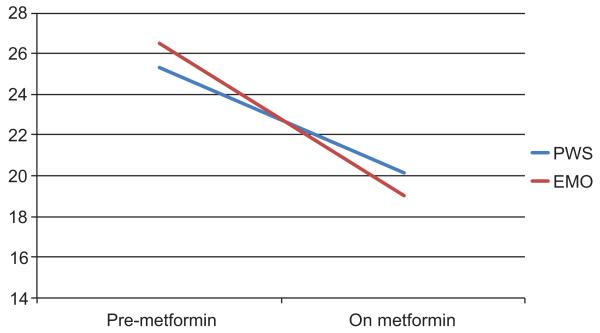
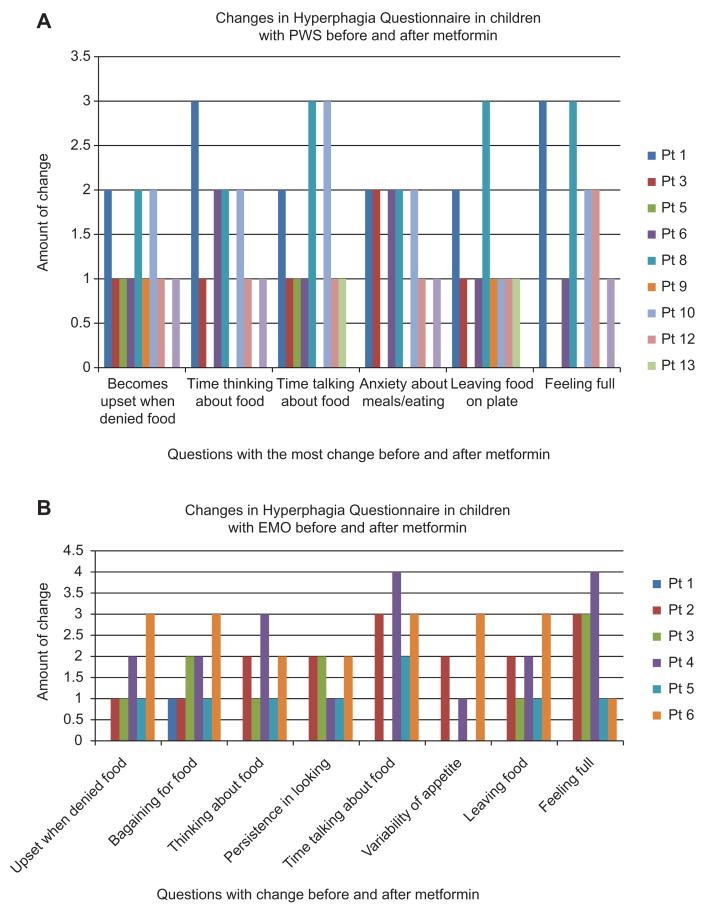
Based on matched t-tests, significant reductions were found in the total hyperphagia scores in both the PWS and EMO groups [PWS: t (13)=3.08, p<0.009; EMO: t (5)=3.76, p<0.01]. Figure 1 depicts the mean hyperphagia scores before and after metformin treatment for each group. As differences were significant in total Hyperphagia Questionnaire scores, we followed up with analyses of specific items on this measure, using a more conservative p<0.01 as summarized in Table 2. Examining specific items on the Hyperphagia Questionnaire, Table 2 shows that significant improvements were noted in several behaviors, including food-related anxiety and preoccupations, being upset when denied food, sense of satiety, and leaving food on their plates (Figure 2).
Figure 1.
Mean Hyperphagia Questionnaire total scores.
Table 2.
Comparison of mean scores and SDs on the Hyperphagia Questionnaire before and after metformin therapy.
| PWS |
EMO |
|||||
|---|---|---|---|---|---|---|
| Pre Tx | On Tx | p (t-test) | Pre Tx | On Tx | p (t-test) | |
| Upset when denied desired food | 3.14 (1.29) | 2.21 (0.97) | 4.76 | 2.83 (1.47) | 1.50 (0.54) | 3.16a |
| Bargains to get more food | 3.43 (1.55) | 2.64 (1.27) | 2.80b | 4.33 (0.81) | 2.67 (0.83) | 5.00b |
| Preoccupied with food | 3.07 (1.21) | 2.21 (0.70) | 3.12b | 4.00 (0.89) | 2.50 (0.55) | 3.40b |
| Persistence in asking for food | 3.36 (1.44) | 2.35 (0.93) | 3.61b | 3.83 (0.75) | 2.50 (0.54) | 4.00b |
| Feels full after eating | 2.21 (1.25) | 2.50 (1.22) | −0.81 | 1.67 (0.82) | 3.67 (0.52) | −3.87b |
| Leaves food on plate | 1.92 (0.92) | 2.43 (1.01) | −2.46a | 1.33 (0.52) | 3.50 (1.37) | −2.90a |
| Distress if stopped from food talk, behaviors | 2.71 (1.38) | 1.86 (0.86) | 3.38b | 2.67 (1.63) | 1.66 (0.52) | 1.94 |
| Time spent in food talk, behaviors | 2.50 (1.22) | 1.50 (0.52) | 3.73b | 3.33 (1.97) | 1.33 (0.52) | 2.98a |
| Degree of food interferes with daily routines | 2.50 (1.01) | 1.71 (0.61) | 3.29b | 3.00 (1.41) | 1.50 (0.84) | 3.50a |
| Total hyperphagia | 20.71 (6.09) | 14.59 (4.57) | 3.18b | 24.00 (7.72) | 13.66 (1.75) | 3.87b |
p<0.05,
p<0.01.
Figure 2.
Mean changes in Hyperphagia Questionnaire total scores.
Correlates of treatment response
Within the PWS group, no differences in treatment responses were found between those with maternal UPD vs. paternal deletions. As well, age at the time of metformin therapy did not have any significant effects on treatment response. However, all of those in the PWS group who responded positively to metformin were females. In contrast, among EMO families who returned the survey, the one treatment non-responder was a female. Despite positive effects on the drive for food, metformin resulted in only a minimal weight loss effect in those with EMO (mean 1.2 kg) and in no weight loss in those with PWS. However, all of those who stopped the metformin treatment experienced variable degrees of weight gain in the study period.
OGTT and metformin response
Differences in the total Hyperphagia Questionnaire scores, indexing the magnitude of treatment response, were correlated with the four glucose or insulin levels. In the PWS group, fasting glucose levels were positively correlated with greater responses to metformin [r (13)=0.54, p<0.05]. Examined another way, pre-treatment 2-h glucose levels on OGTT were significantly higher in participants with PWS who experienced improvements vs. nominal changes in food-related symptoms (7.48 mmol/L vs. 4.24 mmol/L; p=0.003). No other significant relationships were found between treatment response and glucose or insulin levels.
Because impaired satiety is a putative mechanism associated with hyperphagia in PWS, we conducted follow-up exploratory analyses with two items on the Hyperphagia Questionnaire that tap satiety, specifically if the child reports feeling full, or leaves food uneaten on his/her plate., PWS participants with improvements in satiety had higher 2-h glucose (8.1 mmol/L vs. 5.17 mmol/L; p=0.002) and higher 2-h insulin levels (986.2 pmol/L vs. 373.6 pmol/L; p=0.01) of OGTT than those who did not note any differences in satiety. On metformin, five children with PWS and two with EMO began leaving food on their plate; previously, they never left any uneaten food. However, there were no significant differences in fasting or 2-h glucose and insulin levels between those who did vs. those who did not leave food on their plates.
Discussion
Metformin therapy may improve hyperphagia, food-related anxiety, and sense of satiety in some individuals with PWS and EMO. Unexpectedly, only females with PWS showed positive responses to metformin treatment. Seven of ten males experienced sharp increases in problem behaviors within 1–2 days of starting metformin, and three males had neither a positive nor a negative response to treatment. The differential response to metformin therapy between boys and girls may simply be coincidental or related to sampling, or it may represent a true difference. Gender effects in the PWS phenotype are either rarely reported or quite subtle, which contrasts with the striking gender differences we observed in response to metformin. In the general population, it is known that females tend to have more insulin resistance than males, beginning as young as age 5, so perhaps the adverse response to metformin in some males with PWS indicates that they developed hypoglycemia or more blood glucose lability in response to metformin than females (32). However, gender differences have not been reported in child or adult psychiatric patients who are increasingly placed on metformin to curb weight gain associated with atypical antipsychotic or other psychotropic medications [e.g., Ref. (33)]. Further studies are needed to explain the gender differences in response to metformin that we observed in PWS.
It is unclear what mechanisms are associated with metformin-related improvements in food-related anxiety, hyperphagia, and satiety in females with PWS and in children with EMO. However, it is known that obesity results in both leptin and insulin resistance. Resistance to both leptin and insulin at the blood-brain barrier decreases the levels of these satiety hormones that reach the hypothalamus and thus allows for unregulated accrual of adipose tissue (34, 35). Leptin or insulin sensitizers therefore should improve satiety by allowing increased levels of these satiety hormones to reach their receptors in the hypothalamus. Metformin is an insulin sensitizer, and improving the sensitivity to insulin at the blood-brain barrier may allow enough insulin to reach its receptors in the hypothalamus to stimulate satiety.
Metformin stimulates AMP-activated protein kinase (AMPK) in the liver and the muscle (36, 37). AMPK is a regulator of cellular and whole-body homeostasis and acts as a sensor of energy status. Once AMPK is activated a switch from anabolic to catabolic pathways occurs. The increased AMPK stimulates glucose uptake in the muscle, induces hepatic fatty acid oxidation, and inhibits hepatic glucose production. However, metformin inhibits AMPK activity in the hypothalamus, which results in decreased neuropeptide Y expression (38). Neuropeptide Y is a potent orexigen, and the inhibiting function of metformin on AMPK activity may lead to decreased food-related anxiety and increased ability to feel satiated. Metformin also increases hypothalamic leptin receptor expression, thus increasing the central sensitivity to leptin which may also mediate decreased food intake in treated individuals (39).
Many gut hormones influence AMPK activity, including glucagon-like peptide 1, leptin, and insulin (40, 41). Insulin and leptin receptors are present in both α-melanocyte-stimulating hormone neurons and neuropeptide Y neurons in animals (42). In non-obese individuals, the circulating leptin and insulin levels cross the blood-brain barrier, bind to their receptors, and, consequently, determine the long-term energy and adiposity profile (42). However, many individuals with obesity, including the obesity caused by PWS, have leptin resistance at the level of the blood-brain barrier, resulting in low leptin levels in the central nervous system (43). Many individuals with obesity also have insulin resistance at the blood-brain barrier, but individuals with PWS have low circulating insulin levels (44). Thus, the low leptin and insulin levels available to enter the central nervous system in individuals with PWS or EMO may result in constitutively activated AMPK, which results in increased food intake.
This study had several limitations. First, participants were treated clinically in an open-label trial, so they were thus aware that they were receiving a specific medication. Second, the sample size was relatively small. Even so, the positive responses to metformin treatment identified in this open-label trial justify the need for future studies with larger numbers of participants who are randomly assigned to treatment or placebo groups. Third, we were only able to measure appetite and satiety-regulating hormones prior to treatment, and future studies need to examine hormonal profiles both before and after metformin treatment. Finally, we were not able to formally follow participants over an extended period of time, which may be related to the lack of significant weight changes in either the PWS or the EMO group. Children on metformin may show declines in BMI in the long-term, yet given their hyperphagia, even stability in weight in children with PWS or EMO can be construed as a clinically significant outcome.
Even with these limitations, the results of this open-label trial suggest that further long-term, placebo-controlled trials should be performed to determine whether metformin treatment can result in sustained improvements in hyperphagia and food-related anxiety in PWS. Spontaneous comments from families of children with PWS who responded well to metformin underscore the positive impact of these improvements.
-
–
“She seems to be not so focused on food as she was” (13-year-old, UPD);
-
–
“She has less appetite as she is not finishing her lunch at school and that has NEVER happened before” (9-year-old, deletion);
-
–
“ Her obsession with food has significantly decreased. Nothing else has changed in our routine. – She hasn’t been asking for food between our scheduled meals/snacks. She doesn’t obsess about food when she sees or hears me preparing a meal” (7-year-old, deletion); and
-
–
“ She is still acting like an improved and different person in many ways: she has been asking for her meal or snack at appropriate times. She continues to ask only once and not obsess over it ” (8-year-old, deletion).
Food-seeking behaviors remain a life-threatening feature of PWS, and controlling food-related behaviors, anxiety, and preoccupations represents a significant management challenge for families. Metformin has the potential to be an important part of treatment for those with PWS or EMO who have associated glucose intolerance and insulin resistance, while also providing families some help in managing life-threatening hyperphagic behaviors. Nonetheless, currently, the only successful method for controlling weight in individuals with PWS is constant supervision and strict environmental controls to limit access to food, so metformin treatment should not be viewed as an alternative to this effective treatment, but rather, perhaps, an adjunctive therapy for some individuals with PWS.
Contributor Information
Jennifer L. Miller, Division of Endocrinology, Department of Pediatrics, University of Florida, Gainesville, FL, USA.
Tiffany D. Linville, Department of Pediatrics, University of Florida, Gainesville, FL, USA
Elisabeth M. Dykens, Department of Psychology, Vanderbilt University, Nashville, TN, USA
References
- 1.Cassidy SB, Driscoll DJ. Prader-Willi syndrome. Eur J Hum Genet. 2009;17:3–13. doi: 10.1038/ejhg.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler MG, Fischer W, Kibiryeva N, Bittel DC. Array comparative genomic hybridization (aCGH) analysis in Prader-Willi syndrome. Am J Med Genet A. 2008;146:854–60. doi: 10.1002/ajmg.a.32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, Cassidy SB, Schrander JJ, et al. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A. 2004;124A:333–8. doi: 10.1002/ajmg.a.20371. [DOI] [PubMed] [Google Scholar]
- 4.Davies W, Lynn PM, Relkovic D, Wilkinson LS. Imprinted genes and neuroendocrine function. Front Neuroendocrinol. 2008;29:413–27. doi: 10.1016/j.yfrne.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Goldstone AP. The hypothalamus, hormones, and hunger: alterations in human obesity and illness. Prog Brain Res. 2006;153:57–73. doi: 10.1016/S0079-6123(06)53003-1. [DOI] [PubMed] [Google Scholar]
- 6.Holsen LM, Zarcone JR, Brooks WM, Butler MG, Thompson TI, et al. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring) 2006;14:1028–37. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller JL, James GA, Goldstone AP, Couch JA, He G, et al. Enhanced activation of reward mediating prefrontal regions in response to food stimuli in Prader-Willi syndrome. J Neurol Neurosurg Psychiatry. 2007;78:615–9. doi: 10.1136/jnnp.2006.099044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holsen LM, Zarcone JR, Chambers R, Butler MG, Bittel DC, et al. Genetic subtype differences in neural circuitry of food motivation in Prader-Willi syndrome. Int J Obes (Lond) 2009;33:273–83. doi: 10.1038/ijo.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, et al. Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. Int J Obes (Lond) 2012;36:638–47. doi: 10.1038/ijo.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bizzarri C, Rigamonti AE, Luce A, Cappa M, Cella SG, et al. Children with Prader-Willi syndrome exhibit more evident meal-induced responses in plasma ghrelin and peptide YY levels than obese and lean children. Eur J Endocrinol. 2010;162:499–505. doi: 10.1530/EJE-09-1033. [DOI] [PubMed] [Google Scholar]
- 11.Haqq AM, Grambow SC, Muehlbauer M, Newgard CB, Svetkey LP, et al. Ghrelin concentrations in Prader-Willi syndrome (PWS) infants and children: changes during development. Clin Endocrinol (Oxf) 2008;69:911–20. doi: 10.1111/j.1365-2265.2008.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy L, Bittel DC, Kibiryeva N, Kalra SP, Torto R, et al. Circulating adiponectin levels, body composition and obesity-related variables in Prader-Willi syndrome: comparison with obese subjects. Int J Obes (Lond) 2006;30:382–7. doi: 10.1038/sj.ijo.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proto C, Romualdi D, Cento RM, Romano C, Campagna G, et al. Free and total leptin serum levels and soluble leptin receptors levels in two models of genetic obesity: the Prader-Willi and the Down syndromes. Metabolism. 2007;56:1076–80. doi: 10.1016/j.metabol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest. 2010;120:2931–41. doi: 10.1172/JCI41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth CL, Bongiovanni KD, Gohlke B, Woelfle J. Changes in dynamic insulin and gastrointestinal hormone secretion in obese children. J Pediatr Endocrinol Metab. 2010;23:1299–309. doi: 10.1515/jpem.2010.204. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JA, Regnault TR. In utero origins of adult insulin resistance and vascular dysfunction. Semin Reprod Med. 2011;29:211–24. doi: 10.1055/s-0031-1275522. [DOI] [PubMed] [Google Scholar]
- 17.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–8. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130:e856–64. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller J, Kranzler J, Liu Y, Schmalfuss I, Theriaque DW, et al. Neurocognitive findings in Prader-Willi syndrome and early-onset morbid obesity. J Pediatr. 2006;149:192–8. doi: 10.1016/j.jpeds.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.McNeilly AD, Williamson R, Sutherland C, Balfour DJ, Stewart CA. High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and non-matching to position task. Behav Brain Res. 2011;217:134–41. doi: 10.1016/j.bbr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Debette S, Beiser A, Hoffmann U, Decarli C, O’Donnell CJ, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68:136–44. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yau PL, Javier DC, Ryan CM, Tsui WH, Ardekani BA, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53:2298–306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segura B, Jurado MA, Freixenet N, Bargalló N, Junqué C, et al. White matter fractional anisotropy is related to processing speed in metabolic syndrome patients: a case-control study. BMC Neurol. 2010;10:64. doi: 10.1186/1471-2377-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, et al. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59:2171–7. doi: 10.2337/db09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis. 2010;20:723–36. doi: 10.3233/JAD-2010-091687. [DOI] [PubMed] [Google Scholar]
- 26.Chan NN, Feher MD, Bridges NA. Metformin therapy for diabetes in Prader-Willi syndrome. J R Soc Med. 1998;91:598. doi: 10.1177/014107689809101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch HJ, Eldar-Geva T, Benarroch F, Rubinstein O, Gross-Tsur V. Primary testicular dysfunction is a major contributor to abnormal pubertal development in males with Prader-Willi syndrome. J Clin Endocrinol Metab. 2009;94:2262–8. doi: 10.1210/jc.2008-2760. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt F, Kapellen TM, Wiegand S, Herbst A, Wolf J, et al. DPV-Wiss Study Group BMBF Competence Network Diabetes. Diabetes mellitus in children and adolescents with genetic syndromes. Exp Clin Endocrinol Diabetes. 2012;120:579–85. doi: 10.1055/s-0032-1306330. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Deng J, Sheng W, Zuo Z. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav. 2012;101:564–74. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:E55. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 31.Dykens EM, Maxwell MA, Pantino E, Kossler R, Roof E. Assessment of hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring) 2007;15:1816–26. doi: 10.1038/oby.2007.216. [DOI] [PubMed] [Google Scholar]
- 32.Adler-Wailes DC, Periwal V, Ali AH, Brady SM, McDuffie JR, et al. Sex-associated differences in free fatty acid flux of obese adolescents. J Clin Endocrinol Metab. 2013;98:1676–8. doi: 10.1210/jc.2012-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newal H, Myles N, Ward PB, Samaras C, Shiers D, et al. Efficacy of metformin for prevention of weight gain in psychiatric populations: a review. Int Clin Psychopharmacol. 2012;27:69–75. doi: 10.1097/YIC.0b013e32834d0a5b. [DOI] [PubMed] [Google Scholar]
- 34.Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology. 2012;153:4111–9. doi: 10.1210/en.2012-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adam CL, Findlay PA. Decreased blood-brain leptin transfer in an ovine model of obesity and weight loss: resolving the cause of leptin resistance. Int J Obes (Lond) 2010;34:980–8. doi: 10.1038/ijo.2010.28. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Wang Y, Bao C, Xu Y, Shen H, et al. Metformin interacts with AMPK through binding to γ subunit. Mol Cell Biochem. 2012;368:69–76. doi: 10.1007/s11010-012-1344-5. [DOI] [PubMed] [Google Scholar]
- 37.Viollet B, Andreelli F. AMP-activated protein kinase and metabolic control. Handb Exp Pharmacol. 2011;(203):303–30. doi: 10.1007/978-3-642-17214-4_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lv WS, Wen JP, Li L, Sun RX, Wang J, et al. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res. 2012;1444:11–9. doi: 10.1016/j.brainres.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 39.Aubert G, Mansuy V, Voirol MJ, Pellerin L, Pralong FP. The anorexigenic effects of metformin involve increases in hypothalamic leptin receptor expression. Metabolism. 2011;60:327–34. doi: 10.1016/j.metabol.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Pimentel GD, Ropelle ER, Rocha GZ, Carvalheira JB. The role of neuronal AMPK as a mediator of nutritional regulation of food intake and energy homeostasis. Metabolism. 2012;62:171–8. doi: 10.1016/j.metabol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Stark R, Ashley SE, Andrews ZB. AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol Cell Endocrinol. 2013;366:215–23. doi: 10.1016/j.mce.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Garfield AS, Patterson C, Skora S, Gribble FM, Reimann F, et al. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology. 2012;153:4600–7. doi: 10.1210/en.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haqq AM, Muehlbauer M, Svetkey LP, Newgard CB, Purnell JQ, et al. Altered distribution of adiponectin isoforms in children with Prader-Willi syndrome (PWS): association with insulin sensitivity and circulating satiety peptide hormones. Clin Endocrinol (Oxf) 2007;67:944–51. doi: 10.1111/j.1365-2265.2007.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haqq AM, Muehlbauer MJ, Newgard CB, Grambow S, Freemark M. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J Clin Endocrinol Metab. 2011;96:E225–32. doi: 10.1210/jc.2010-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]