Abstract
Chronic constipation is a common health problem that significantly affects the quality of life of patients and impacts in terms of costs; current treatments based on fiber and laxatives cause dissatisfaction to doctors and patients in more than half of the cases. New drugs are now available or in very advanced stages of research, with different and innovative mechanisms of action as prucalopride, lubiprostone, and linaclotide. Prucalopride an enterokinetic, is a selective high-affinity 5-hydroxytryptamine (5-HT)4 receptor agonist of serotonin that increases the peristaltic reflex and the colonic contractions; lubiprostone, a type 2 chlorine channel activator, or linaclotide, a guanylate cyclase-C agonist of enterocytes, both prosecretory agents, stimulate the secretion of fluid within the intestinal lumen. In general, these promising drugs have proven efficacy and safety as a specific therapeutic option in patients with chronic constipation. Yet the solution might not be sufficient for everybody and still without the ideal drug that might be useful in all cases, the pharmacological revolution for colonic motility disorders has arrived.
Key Words: chronic constipation, treatment, laxatives, prucalopride, linaclotide
Constipation is a symptom particularly subjective; whereas for some patients it means infrequent stools, others express difficulty in defecation or hard stools.1 For years it has been trying to standardize the meaning of chronic constipation (CC); the American College of Gastroenterology and the Latin-American Consensus on Chronic Constipation similarly define it as “a functional disorder with an evolution of at least 3-6 months, characterized by infrequent stools, difficulty passing and long time to achieve deposition.”2,3 Formerly an international group of experts in gastrointestinal motility met to define a consensus document on functional gastrointestinal disorders known as the Rome criteria. The last update was in 2006, as Rome III; according to these criteria, it is considered CC when it meets at least 2 of the following: <3 bowel movements per week (and in ≥25% of the times), hard stools, or sensation of incomplete evacuation or anorectal obstruction or use of manual maneuvers to defecate, plus insufficient criteria for irritable bowel syndrome. These symptoms must be present during the previous 3 months and having been started at least 6 months before diagnosis4 (Fig. 1).
FIGURE 1.
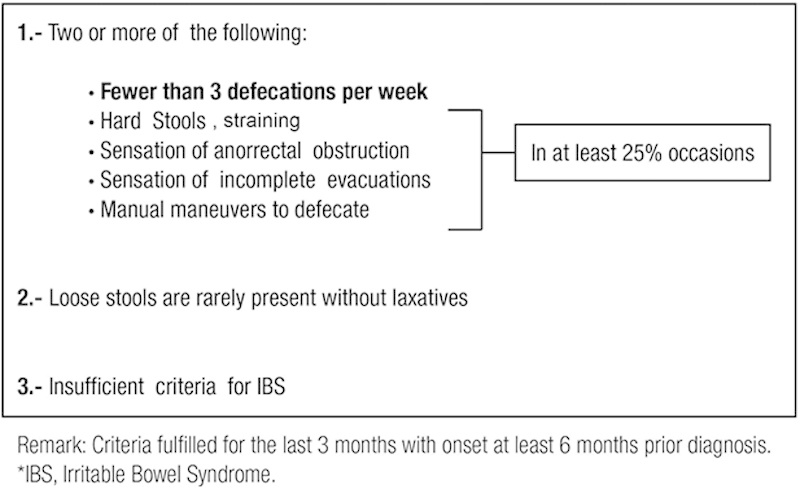
ROME III diagnostic criteria for functional (chronic) constipation.4
Although to most physicians the main point is the frequency of bowel movements (<3 bowel movements a week), for patients the main point is the accompanying symptoms (hard stools, infrequent bowel movements, straining, and incomplete evacuation), as demonstrated by studies in Canada and Mexico where subjects defined themselves as constipated. Because 55% to 80% had straining or difficult evacuation and 25% to 65% reported hard stools in the survey, the number of bowel movements occupied only between the third and sixth place of the predominant symptoms.5,6
PREVALENCE
The worldwide prevalence of constipation is estimated between 4% and 27%.2,7 A recent meta-analysis in community studies, performed in several continents, found a prevalence of chronic idiopathic constipation between 12% and 17%.8 In Mexico, based on 3 studies conducted between 2006 and 2009 (1 in volunteers and 2 in open population) using Rome II and Rome III criteria, the estimated prevalence was between 14% and 23% (Schmulson et al9), 5% and 10% (Lopez-Colombo et al10), and 16% and 25% (Remes-Troche et al6), respectively. Through meta-analysis, a 14.4% prevalence of CC was identified in Mexico.11 Subjects in the fourth decade of life (productive age) are most frequently affected, predominantly among young women at a ratio of 3:1, and this condition increases exponentially with age.2,11 In people over 65 years of age, the prevalence of CC rises up to 50% and as high as 74% in the elderly living in nursing homes.12,13 The prevalence of constipation is comparable to other common diseases and even higher than migraine, asthma, diabetes, or coronary disease, but it is perceived as less frequent and severe.14
ECONOMIC IMPACT AND QUALITY OF LIFE
Annually CC is responsible for more than 2.5 million/visits and more than 80,000 hospitalizations in the United States; the estimated annual expenditure on laxatives prescribed by doctors is $500 to $800 million dollars, plus an expense of >$200 million in over the counter products. The evaluation of quality of life is a tool that lets us know directly and indirectly the impact of diseases on physical and emotional scales; in CC, despite being a functional disorder, it has been shown with several assessment instruments (SF-36, SCL-90, GSRS, PGWB, among others) that subjects have a lower quality of life, more scholar/job absenteeism, and loss of work productivity.7,14,15
Sun et al,15 through a national survey of health and well-being (SF-12), compared the quality of life of 1430 patients with constipation versus a control group without constipation. Constipated patients reported significantly lower quality of life (physical and mental function) plus higher levels of lost work productivity and disability (absenteeism, presenteeism) than did controls (P<0.01 for all parameters). In a meta-analysis of quality of life in CC performed by Belsey et al,16 it was found that patients with constipation have lower quality of life when compared with healthy controls in all areas of evaluation (function and physical health, mental health, general pain, social function, vitality, and overall emotional scales).
CC TREATMENT SATISFACTION
There have been some studies that evaluate the level of satisfaction of CC treatment. Johanson et al17 conducted a study on 557 subjects with CC and applied a 45-item questionnaire and identified that half of the respondents (47%) reported ineffective relief in frequency and accompanying symptoms of CC with the use of fiber and laxatives (either prescribed or over the counter). Wald et al18 conducted a multinational study (USA, UK, Germany, France, Italy, Brazil, and South Korea) including more than 13,879 questionnaires from subjects who defined themselves as constipated, and identified in 20% to 40% of the patients the persistence of constipation symptoms, despite adequate use of laxatives.
TREATMENT OF CC
The treatment of CC includes changes in lifestyle, aerobic exercise, increased water intake, and diet improvement including more fiber-rich foods such as cereals, vegetables, and the use of laxatives.19
FIBER AND BULK FORMING AGENTS
The fiber and bulking agents are the most similar agents to the physiological mechanisms of evacuation; fiber decreases colonic transit time, increases stool volume, decreases intracolonic pressure especially at rectal sigmoid that reduces patient discomfort, reduces bile salt concentration that implies a decrease of gut contractile activity, and produces changes in the colonic microbial mass favoring growth of the bacterial population.20
There are different types of fiber, natural and synthetic; the most common among natural ones is wheat bran (20 g/d) and synthetic methylcellulose (4 g/d) through commercial preparations based on seeds, but the most used are psyllium derivatives (10 to 20 g/d). The appropriate dose should be achieved gradually, always taking care to ensure a plentiful water intake. The positive response to fiber may take 4 to 6 weeks, which is why it is advisable to maintain dietary fiber treatment for 1 to 2 months before considering treatment failure.21
Some patients cannot tolerate fiber because of excessive bloating and gas production. Psyllium acts like a dietary fiber and undergoes the action of colonic bacteria, with the consequent development of meteorism, bloating, and flatulence.22 The synthetic agents such as methylcellulose and polycarbophil are not degraded by bacterial enzymes of the intestinal flora, thus avoiding the formation of gas.
A great advantage of these agents is the lack of systemic side effects and can be used for a long time. Contraindications for their use are intestinal obstruction and hypersensitivity and they should be avoided in acute abdominal pain, nausea or vomiting, flatulence, bloating, and severe meteorism.23
The few clinical trials evaluating the efficacy of psyllium have shown to be superior to placebo in improving the consistency and frequency of stools.24 Psyllium is recommended for the treatment of constipation, with level II evidence and grade B recommendation.21
LAXATIVES
Laxatives can be classified as: lubricant, emollients, stimulants, osmotic, and rectal laxatives (Table 1). The osmotic laxatives are generally the first choice and include nonabsorbable sugars (lactulose), saline (magnesium hydroxide), and polyethylene glycol.
TABLE 1.
Main Types of Laxatives
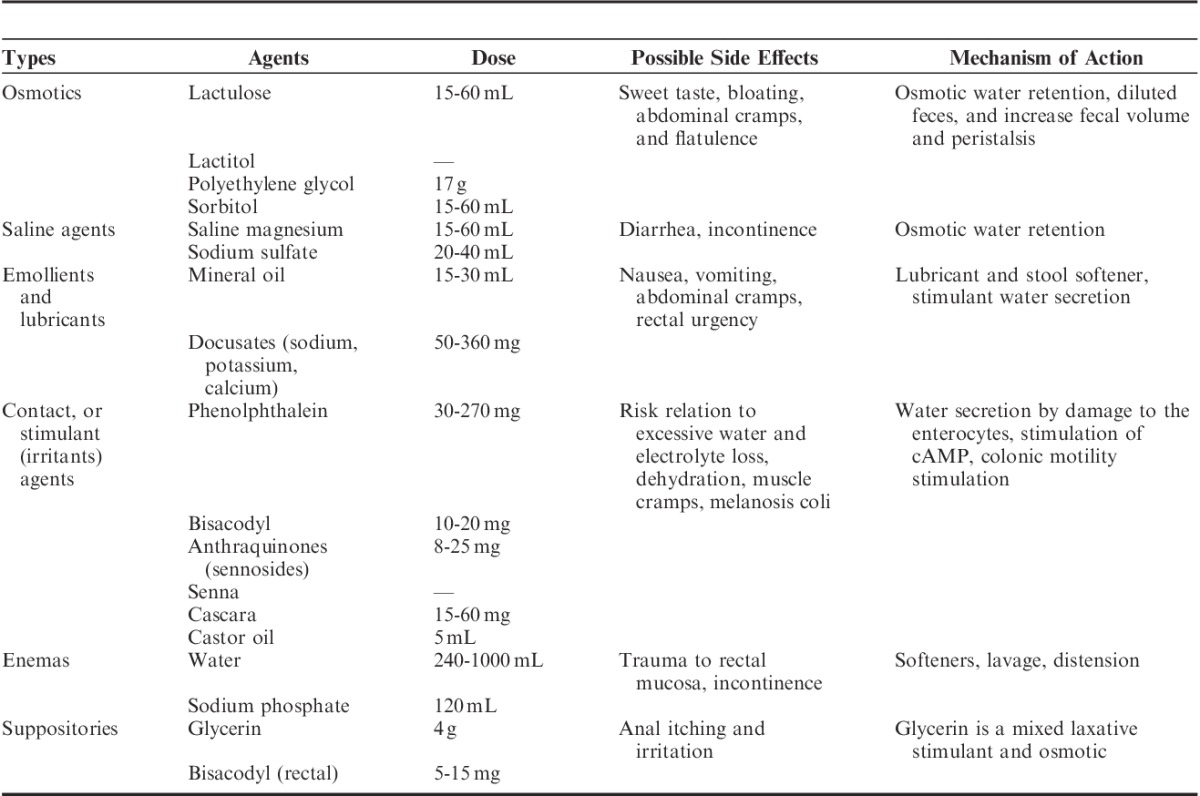
A recent review based on evidence evaluated the efficacy and safety of laxatives in CC, and only the solutions of polyethylene glycol and lactulose showed a high level of evidence and were assigned: grade A recommendation to polyethylene glycol and lactulose solutions, grade B to methylcellulose and sorbitol, and grade C for magnesium hydroxide. Paraffin and glycerin suppositories showed no scientific evidence.21,25
Osmotic laxatives are indicated in patients with slow colonic transit, when other measures outlined above are not sufficient, and in patients with anismus. An adequate water intake is recommended during their use and monitoring the electrolyte levels; similarly, caution in patients with renal insufficiency should be taken, as magnesium salts may cause hypermagnesemia.26
Lubricants are indicated less frequently because of their adverse effects. Mineral oil may inhibit the absorption of liposoluble vitamins and calcium. In children and elderly, it has been reported that mineral oil increases the risk for aspiration and lipoid pneumonia.27
Stimulant or irritant laxatives can be aggressive and are rarely indicated as long-term treatment; they should be used in cases of transient constipation. Prolonged use of stimulant laxatives may cause melanosis coli, electrolyte, and acid-base balance disturbances; some intrinsic colonic nerve plexus injuries have been described, but there is no convincing evidence in humans.28 Their use is justified in palliative care and for occasional use, because of the development of tolerance. The efficacy and long-term safety is unclear, because studies that support it are of low scientific evidence.27
Cascara (Rhamnus purshiana) has been used as laxative since a long time; the anthraquinone glycosides are responsible for most of its mechanism of action. It acts in the large bowel increasing peristalsis. It is available in several forms: dried, natural extract, liquid, or solid extract. Another commonly used product is Senna leaves (Cassia senna) for their cathartic properties.29 Patients with fecal impaction should require manual disimpaction and subsequently hypertonic enemas 1 to 2 per day until the left colon is cleared, along with increasing intake of fluids and fiber.21
TEGASEROD
Serotonin or 5-hydroxytryptamine (5-HT) plays a key role in mediating peristalsis and stimulating intestinal secretion by 5-HT4 receptors in the intestinal wall. Tegaserod is a partial agonist of the serotonin subtype 4 receptor; initial studies on animals, healthy volunteers, and patients with CC have shown promotility activity of tegaserod with the augmentation of the peristaltic reflex, enhanced intestinal secretion, and reduced visceral hypersensitivity.14 Two large randomized placebo-controlled trials with >2600 patients with CC demonstrated that tegaserod (2 and 6 mg twice daily for 12 wk) produced significant improvements. In both studies, tegaserod dose of 6 mg response rate [increase of ≥1 complete spontaneous bowel movement (CSBM)/wk during weeks 1 to 4] was significantly better (43.2% and 40.2%) than placebo (25.1% and 26.7%, respectively, P<0.0001).30,31 In addition, in both trials, statistically significant improvements were observed in the majority of secondary endpoints (number of bowel movements, stool form, abdominal bloating, straining, and abdominal discomfort) over the 12-week period. Diarrhea was the most common adverse event and in general was safe and well tolerated.30,31
In March 2007, tegaserod was withdrawn from the market as a result of the findings of suggested association with an increase in serious cardiovascular effects (angina, myocardial infarction, and cerebrovascular events) from trials and reports by the manufacturer to the FDA. The frequency of these events was very low (0.1%); also an observational study of tegaserod did not find an increase in the risk for cardiovascular ischemic events, but in general it was considered that the risk surpasses the benefit.32,33
PRUCALOPRIDE
Prucalopride belongs to a chemical class of dihydrobenzofuran carboxamide derivatives with potent enterokinetic activity (a prokinetic selective for the large intestine). It is a high-affinity 5-HT4 serotonin receptor agonist (over 150-fold); therefore, it does not activate any of the other 7 serotonin receptors (eg, 5-HT1,2, or the human ether a-go-go potassium channel receptor gene), with a decreased risk for cardiovascular adverse events such as ischemic type or serious arrhythmias (as could potentially happen with tegaserod or cisapride, respectively).34–36
The highest amount of serotonin receptors is found in the intestine (>90%), mainly in the enterochromaffin cells of the intestinal mucosa. Stimulation of the intestinal mucosa induces the release of serotonin from the enterochromaffin cells activating intrinsic primary afferent neurons, which then release another neurotransmitter, acetylcholine. Early studies in vivo and in vitro revealed that prucalopride improves the peristaltic reflex and propulsive motor patterns in the gastrointestinal tract. In dogs, it modified motility patterns by the stimulation of serotonin 5-HT4 receptor in the proximal colon, enhancing gastroduodenal motility, accelerating gastric emptying, and inducing huge peristaltic contractions that provide the main propulsion force for defecation.35–38 The mechanism of action of prucalopride is to activate 5-HT4 receptors in the neurons of the myenteric plexus that increase the intestinal muscle contraction, which in turn stimulates colonic motility and produces high-amplitude propagated colon contractions.34
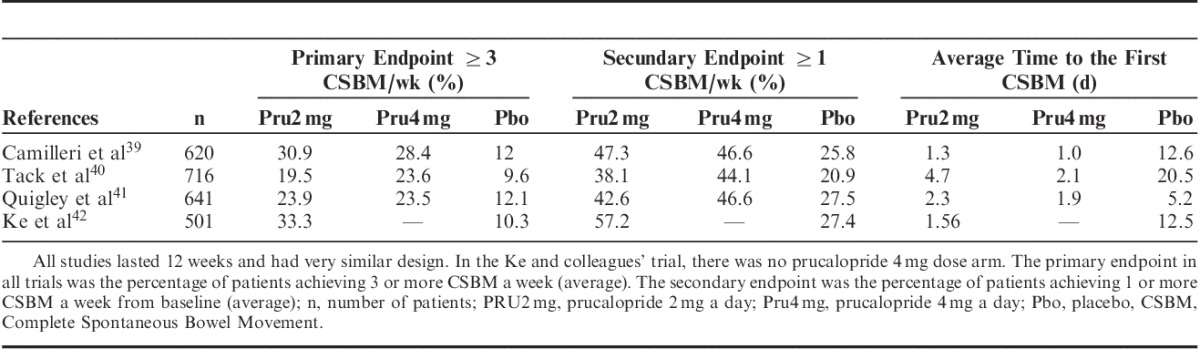
The efficacy of prucalopride once daily was established by 3 phase III clinical trials (randomized, double-blind, placebo-controlled for 12 wk) in patients with chronic idiopathic constipation. These studies were multicenter and multinational (USA, Canada, UK, Belgium, Norway, Sweden, the Netherlands, Australia, and South Africa) that evaluated more than 1977 patients.39,40,41 The primary endpoint was the presence of ≥3 CSBM per week during the 12 weeks of treatment. The secondary endpoints assessed were: increase in ≥1 spontaneous bowel movement (SBM) per week; improvement in symptom assessment: consistency and straining; and improvement in the quality of life (PAC-QoL) scale39–41 (Fig. 2).
FIGURE 2.

Definitions of bowel movements used as endpoints in majority of chronic constipation trials.
Data from the pivotal studies show that the number of subjects who reached the primary endpoint or normalization of bowel movements was higher in the groups of prucalopride 2 and 4 mg versus placebo (23.6% and 24.7%, respectively vs. 11.3%) with statistically significant difference (P<0.001), without any increased benefit with the 4 mg dose over 2 mg of prucalopride.39–41 In addition, significant improvement in the frequency of CSBM and SBM per week (week 12) was reported in the 2 mg prucalopride group versus placebo (48.1% vs. 23.4%). Regarding the time (in days) to present the first satisfactory CSBM was significantly lower with prucalopride 2 and 4 mg versus placebo (2.7 and 1.9 d vs. 12.7 d respectively, P≤0.001).39–41 In all 3 studies, the prucalopride group reported improvement in the assessment of disease symptoms including abdominal discomfort, stool characteristics, and rectal symptoms, and a significant benefit in the quality of life evaluation such as treatment satisfaction, bowel habits, physical, and psychosocial distress.39–41 A recent study of the Asian-Pacific region with more than 500 patients found similar results regarding the safety and effectiveness of prucalopride 2 mg versus placebo (33% vs. 10.3% for ≥3 CSBM/wk, P<0.00142; Table 2).
TABLE 2.
Summary of Primary and Secondary Endpoints of Prucalopride Trials
Safety
The most commonly reported adverse events with prucalopride 2 and 4 mg were headache, nausea, abdominal pain, and diarrhea, which occurred mainly between the first and second day after the administration. Quigley et al,41 in a subanalysis, excluded the adverse events of day 1 of treatment and identified that headache, nausea, and abdominal pain were statistically similar to placebo. Serious adverse events were similar in both groups. Only treatment discontinuation because of the adverse events was higher in the prucalopride 4 mg group than in the placebo group.39–41
Given the caution of cardiovascular adverse effects on the background of similar class drugs, Camilleri et al43 in the United States and Muller-Lissner et al44 in Europe studied the safety and efficacy of prucalopride in a subset of adults over 65 years old. These randomized placebo-controlled trials with different doses of prucalopride for 4 weeks included over 388 patients (aged up to 95 y and most with a history of cardiovascular disease). All patients were evaluated by electrocardiogram, QTc interval, cardiac Holter, and recorded all adverse events. The findings were that prucalopride was safe and well tolerated, with no difference in vital signs, electrocardiogram, QTc interval, or incidence of supraventricular and ventricular arrhythmias. In the latter study, no greater benefit was demonstrated by increasing the dose beyond 1 mg in elderly.
Finally an open-label follow-up study was conducted on 1455 patients from the pivotal studies under long-term treatment for 2 years (average 18 mo) with prucalopride. In this study, it was found that 40% to 50% of patients stopped requiring chronic use of laxatives and satisfaction with bowel function remained for at least 18 months. Discontinuation because of adverse events was only 1% to 3%.45
LUBIPROSTONE
Lubiprostone selectively activates type 2 chlorine channels (CIC-2), promoting intestinal fluid secretion, although some of their mechanisms of action are still unknown. In the GI tract, there are different chlorine channels, with a critical role as fluid transportation, depolarization of the smooth muscle cells, postsynaptic transmission, and maintenance of pH and intracellular volume. One of the most important chlorine channels is the cystic fibrosis transmembrane regulator conductance protein. The CIC-2s are located on the apical membrane of the stomach, small intestine, and colon.46,47 The CIC-2 channel is a transmembrane protein that is highly selective for chlorine; its role includes fluid regulation, transportation and secretion, volume, pH, and cell membrane potential maintenance. CIC-2s are within the bowel toward the apical cell membrane. Activation of channels by CIC-2 phosphorylation causes a flow of chlorine and sodium ions and an influx of water into the intestinal lumen.47 Lubiprostone is classified as a prostone, a bicyclic fatty-acid compound derived from prostaglandin E1 metabolite, which by selectively activating type 2 chlorine channels of the apical membrane of the gastrointestinal epithelium, increases the intestinal secretion of fluid into the lumen, and possibly this promotes an increase in small bowel and distal colon transit, probably by the stimulation of local receptors sensitive to distension.46–48
Lubiprostone has demonstrated its efficacy and safety in the treatment of CC in different trials. A phase II, double-blind placebo-controlled study evaluated the efficacy and safety of multiple doses (24, 48, or 72 mcg/d) of lubiprostone in 129 patients with CC for 3 weeks. The frequency of SBMs was higher for lubiprostone groups (5.1 to 6.1) versus placebo (3.8) being statistically significant (P=0.046). SBM frequencies at week 1 and 2 were significantly higher in patients with lubiprostone 48 and 72 mcg/d versus placebo (P≤0.020).49
Another randomized double-blind phase II study of escalating doses of lubiprostone (16, 32, or 48 mcg daily for 2 wk) versus placebo was conducted in 14 centers in Japan on 128 patients with chronic idiopathic constipation. In the lubiprostone groups, they identified a significant dose-dependent increase compared with baseline in the average SBM by week 1, except for the dose of 16 mcg.50 Subsequently, Johanson et al51 conducted a multicenter phase III placebo-controlled trial of lubiprostone (24 mcg bid for 4 wk), including 242 patients from 20 US centers with a diagnosis of CC, according to Rome II criteria. Patients receiving lubiprostone reported a higher number of SBMs in the first week than the placebo group (5.6 vs. 3.4, P=0.0001) and a higher frequency of SBM also at weeks 2, 3, and 4 (P≤0.002). Stool consistency, straining, severity of constipation, and treatment effectiveness, evaluated by the patients, significantly improved with lubiprostone compared with placebo at all weeks (P≤0.0003). Another similar multicenter randomized parallel-group trial on 237 patients evaluated the efficacy and safety of lubiprostone 24 mcg twice daily versus placebo. Lubiprostone produced higher number of SBM from week 1 and at all assessment points (as complete responders ≥4 SBM/wk, P≤0.017) stool consistency, straining, and severity of constipation.52
To evaluate the long-term safety and efficacy of lubiprostone in chronic idiopathic constipation, a multicenter open-labeled trial was conducted on 248 patients taking 24 mcg twice daily with a 48 weeks follow-up. Dose reduction was permitted according to the response; the average drug dose throughout the study was 40.8 mcg/d. As for the long-term efficacy, there was a significant improvement (P<0.0001) in the severity of constipation, abdominal bloating, and discomfort, through 48 weeks as compared with baseline.53
Safety
The most common adverse effects in most phase II clinical trials were nausea, headache, and diarrhea. In phase III studies, the adverse effects that occurred more frequently were nausea in 31.7% and headache in 11.7%.49,51 In the long-term study (1 year), the most common adverse events were nausea (19.8%), diarrhea (9.7%), abdominal distension (6.9%), headache (6.9%), and abdominal pain (5.2%). There was no difference in electrolytes, CBC, nitrogenous, or electrocardiogram. There were no deaths and only 1 serious adverse event was considered possibly related to the drug.47,53 In a postmarketing analysis, an allergic reaction and dyspnea was reported in some patients within 1 hour after the first dose, but with resolution within 2 to 3 hours with no long-term adverse effects.47
LINACLOTIDE
Linaclotide is a guanylate cyclase-C (GC-C) agonist that acts locally in the intestinal epithelium luminal surface as a secretagogue. Activation of the GC-C causes an increase in intracellular and extracellular concentrations of cyclic guanosine monophosphate, stimulating the secretion of chlorine and bicarbonate into the intestinal lumen mainly by the activation of cystic fibrosis transmembrane regulator channel. Linaclotide is a 14 amino-acid peptide minimally absorbed and that mimics the action of the endogenous guanylin and uroguanylin activating the GC-C receptor; it is minimally absorbed and with a low bioavailability.54,55
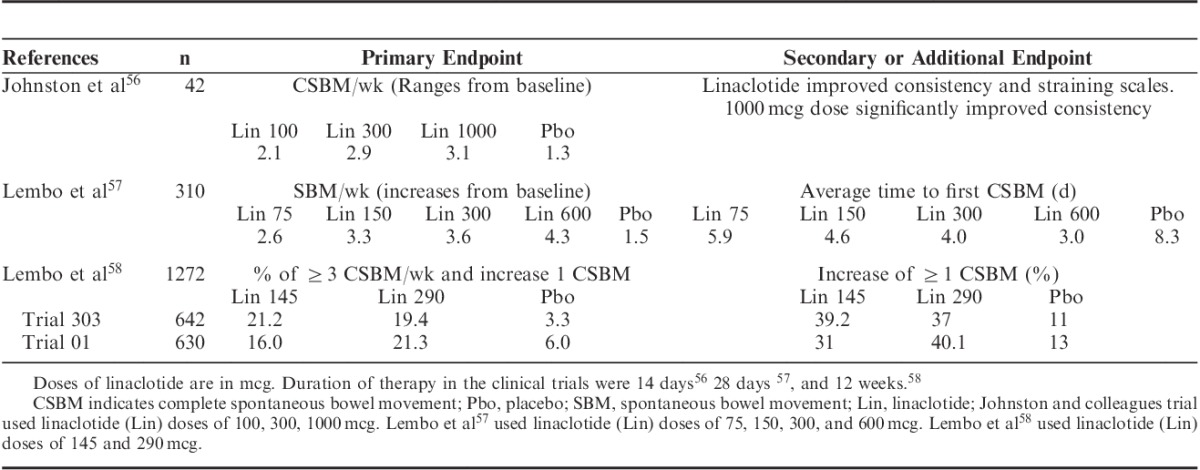
Johnston et al56 in a pilot study on 42 patients evaluated the safety, tolerability, and efficacy of linaclotide at different doses (100, 300 or 1000 mcg) versus placebo for 2 weeks. Linaclotide produced a dose-dependent increase in CSBMs and improved stool consistency score (P<0.05), straining, and general relief. Subsequently, safety and efficacy were evaluated in a 2-week double-blind parallel group, placebo-controlled trial in 310 patients with CC assigned to linaclotide 75, 150, 300, or 600 mcg. All doses improved the primary efficacy endpoint that was SBM per week compared with pretreatment baseline versus placebo (increase of 2.6, 3.3, 3.6, and 4.3 vs. 1.5, respectively, P<0.05). Furthermore, linaclotide significantly improved stool consistency, straining, abdominal discomfort, bloating, and quality of life.57
Lembo et al58 conducted 2 multicenter (208 centers in the United States and 8 Canadian centers) randomized, double-blind placebo-controlled with parallel group, phase III studies (trial 303 and 01) involving 1276 patients with CC. Patients received 145 or 290 mcg of linaclotide once daily for 12 weeks versus placebo. The primary endpoint was ≥3 CSBMs per week and an increase of one or more CSBM from baseline lasting at least 9 of the 12 weeks. The primary endpoint was achieved in 21.2% and 16% with a dose of 145 mcg and 19.4% and 21.3% with dose of 290 mcg of linaclotide compared with 3.3% and 6.0% for placebo (P<0.01). In addition, improvements in all secondary endpoints were found [frequency, stool consistency scales, straining, abdominal discomfort, bloating, constipation severity, and quality of life assessment (PAC-QoL)] with both doses of linaclotide than placebo58 (Table 3). Trial 303 also included at the end of the treatment a period of 4 weeks of double-blind randomized crossover therapy with 290 mcg linaclotide or placebo (patients initially assigned to any dose of linaclotide continued with the same dose of linaclotide or placebo, according to the randomization, and patients who initially received placebo were randomized to linaclotide 290 mcg). Interestingly, patients who continued taking linaclotide and those who were switched from placebo to linaclotide had a sustained increase in CSBMs during such period of time, and in addition patients who were switched from linaclotide to placebo showed a decrease in the range of CSBM similar to that of the placebo group during the initial period of treatment. Long-term use studies of linaclotide in CC are pending.
TABLE 3.
Summary of Primary and Secondary or Relevant Endpoints of Linaclotide Trials
Safety
In all studies conducted at 2, 4, and 12 weeks with linaclotide, the most significant adverse event was diarrhea (14% to 16% in trials 301 and 01) that was usually present within the first 2 weeks of treatment. Other events reported included flatulence and abdominal pain, but only diarrhea had a higher incidence as compared with the placebo group. The rate of treatment discontinuation in trials 303 and 01 was 4.2%.58
INVESTIGATIONAL COMPOUNDS
A variable number of new compounds of different drug classes for the treatment of CC are at various phases of investigation, such as velusetrag (5-HT4 agonist), plecanatide (agonist of GC-C), and elobixibat (inhibitor of the ileal bile acid transporter).
Velusetrag, formerly TD-5108, is a dihydroxyquinoline-carboxamide and is a potent 5-HT4 receptor agonist with a high affinity (≥500-fold selectivity) and has no effect on human ether a-go-go -potassium channels. Velusetrag in studies on dogs produces increased motility in the gastrointestinal tract, including the gastric antrum, duodenum, jejunum, and colon.59 In a randomized double-blind trial, 60 healthy subjects received velusetrag (5, 15, 30, and 50 mg single doses and continuous doses for 6 d) or placebo. Velusetrag significantly increased the colonic transit and bowel emptying time of the descending colon after single doses (mainly 15 and 30 mg dose) and multiple dose accelerated the gastric emptying.60 A phase II double-blind randomized study on 401 patients evaluated the efficacy, safety, and tolerability of velusetrag (15, 30, or 50 mg/d) for 4 weeks in CC versus placebo. Patients receiving velusetrag showed a significant increase in SBMs (average increase of 3.6, 3.3, and 3.5 SBM/wk with 15, 30, and 50 mg, respectively, compared with 1.4 with placebo, P<0.0001). They also found significant improvement in CSBM, stool consistency, and time to achieve the first bowel movement.61 Common drug-related adverse events were diarrhea (11% to 15%), headache, nausea, and vomiting; 5% of the patients discontinued because of adverse effects. Undesired effects occurred mainly on day 1 or 2 of treatment, with an average duration of 1 to 7 days. No adverse cardiac effects were reported in the treatment group.61 A systematic review of the cardiovascular safety profile of 5-HT4 agonists concludes that, on the basis of the available evidence, the highly selective 5-HT4 agonists offer greater safety for the treatment of GI motility disorders without cardiovascular safety concerns.59
Plecanatide is also a member of the rapidly emerging class of GC-C agonists that mimic the effects of uroguanylin a natriuretic peptide that activates the GC-C receptor expected to lead to secretion of fluid into the intestinal lumen, facilitating bowel movements.62 Phase I study assesses the safety, tolerability, and pharmacokinetics of a single dose (0.1 to 48 mg) of oral plecanatide in 79 healthy volunteers. Plecanatide was safe and well tolerated at all doses. The number of adverse events were comparable and without dose-related increases.62 A multicenter randomized 12-week study of plecanatide (0.3, 1, or 3 mg daily) or placebo in 946 patients with CC was recently reported. There were significantly more responders (>3 CSBM/wk and >1 CSBM from baseline) in the group of plecanatide 3 mg (21.5%) versus placebo (11.5%; P=0.003). Also a significant trend for plecanatide was achieved for weekly responder rate, median time to first SBM, stool consistency, straining, and PAQ-QoL scores.63
Finally, elobixibat, an enantiomer of 1,5-benzothiazepine, acts locally in the lumen of the gastrointestinal tract, binding and inhibiting the ileal bile acid transporter, with enhanced delivery of bile acid to the colon. Elobixibat, in a randomized phase II placebo-controlled study with 3 different doses, increased the number of SBM progressively with drug dosage versus placebo, and also improved straining and bloating; the main adverse events were abdominal pain and diarrhea.64
CONCLUSIONS
CC is a common health problem that, despite being considered a functional disorder, significantly affects the quality of life of patients and has a negative impact on health systems in terms of cost; current treatments based on fiber and laxatives cause dissatisfaction to the physicians and patients in more than half of the cases. In the last decade, research has focused on a better understanding of gastrointestinal motility disorders and as a result, effective drugs have been developed. An example of this situation is CC where such treatments can face the problem from different points of pathophysiology, affecting a large number of patients. New drugs are available or in different phases of research, yet without being able to have the perfect drug or the one that is useful in all cases; definitively now is the turn of the pharmacological revolution for colonic motility disorders.
Footnotes
J.M.-N. is an employee of Janssen Pharmaceuticals, Mexico City, Mexico. M.A.G.-M. and N.X.O.-O. declare that they have nothing to disclose.
Reprints: Nayeli X. Ortiz-Olvera, MD, MSc, Departamento de Gastroenterología, Unidad Médica de Alta Especialidad (UMAE), Hospital de Especialidades, Centro Médico Nacional Siglo XXI, IMSS, Av. Cuauhtémoc 330, Colonia Doctores, México DF, CP 06725, Mexico (e-mail: nayelixoortiz@yahoo.com.mx).
REFERENCES
- 1.Lacima G, Pera M, Amador A, et al. Long-term results of biofeedback treatment for faecal incontinence: a comparative study with untreated controls.Colorectal Dis. 2010;12:742–749 [DOI] [PubMed] [Google Scholar]
- 2.Brandt LJ, Prather CM, Quigley EM, et al. Systematic review on the management of chronic constipation in North America.Am J Gastroenterol. 2005;100:S5–S22 [DOI] [PubMed] [Google Scholar]
- 3.Schmulson-Wasserman M, Francisconi C, Olden K, et al. The Latin-American consensus on chronic constipation.Gastroenterol Hepatol. 2008;31:59–74 [DOI] [PubMed] [Google Scholar]
- 4.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders.Gastroenterology. 2006;130:1480–1491 [DOI] [PubMed] [Google Scholar]
- 5.Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking.Am J Gastroenterol. 2001;96:3130–3137 [DOI] [PubMed] [Google Scholar]
- 6.Remes-Troche JM, Carmona-Sánchez R, González-Gutiérrez M, et al. What people mean by constipation? A general population based-study.Rev Gastroenterol Mex. 2009;74:321–328 [PubMed] [Google Scholar]
- 7.Pinto-Sanchez MI, Bercik P.Epidemiology and burden of chronic constipation.Can J Gastroenterol. 2011;25:11B–15B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suares NC, Ford AC.Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis.Am J Gastroenterol. 2011;106:1582–1591 [DOI] [PubMed] [Google Scholar]
- 9.Schmulson M, Ortiz O, Santiago-Lomeli M, et al. Frequency of functional bowel disorders among healthy volunteers in Mexico city.Dig Dis. 2006;24:342–347 [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Colombo A, Morgan D, Bravo-González D, et al. The epidemiology of functional gastrointestinal disorders in Mexico: a population-based study.Gastroenterol Res Pract. 2012;2012:606174.doi:10.1155/2012/606174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remes-Troche JM, Tamayo-dela Cuesta JL, Raña-Garibay R, et al. Guidelines for diagnosis and treatment of constipation in Mexico. A) Epidemiology (meta-analysis of the prevalence), pathophysiology and classification.Rev Gastroenterol Mex. 2011;76:126–132 [PubMed] [Google Scholar]
- 12.Fosnes GS, Lydersen S, Farup PG.Drugs and constipation in elderly in nursing homes: what is the relation?Gastroenterol Res Pract. 2012;2012:290231.doi:10.1155/2012/290231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao S.Update on the management of constipation in the elderly: new treatment options.Clin Interv Aging. 2010;5:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eoff JC, Lembo AJ.Optimal treatment of chronic constipation in managed care: review and roundtable discussion.J Manag Care Pharm. 2008;14:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun SX, DiBonaventura M, Purayidathil FW, et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey.Dig Dis Sci. 2011;56:2688–2695 [DOI] [PubMed] [Google Scholar]
- 16.Belsey J, Greenfield S, Candy D, et al. Systematic review: impact of constipation on quality of life in adults and children.Aliment Pharmacol Ther. 2010;31:938–949 [DOI] [PubMed] [Google Scholar]
- 17.Johanson JF, Kralstein J.Chronic constipation: a survey of the patient perspective.Aliment Pharmacol Ther. 2007;25:599–608 [DOI] [PubMed] [Google Scholar]
- 18.Wald A, Scarpignato C, Mueller-Lissner S, et al. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation.Aliment Pharmacol Ther. 2008;28:917–930 [DOI] [PubMed] [Google Scholar]
- 19.Liu LW.Chronic constipation: current treatment options.Can J Gastroenterol. 2011;25suppl B22B–28B [PMC free article] [PubMed] [Google Scholar]
- 20.Tramonte SM, Brand MB, Mulrow CD, et al. The treatment of chronic constipation in adults. A systematic review.J Gen Intern Med. 1997;12:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bove A, Bellini M, Battaglia E, et al. Consensus statement AIGO/SICCR diagnosis and treatment of chronic constipation and obstructed defecation (part II: treatment).World J Gastroenterol. 2012;18:4994–5013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashraf W, Park F, Lof J, et al. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation.Aliment Pharmacol Ther. 1995;9:639–647 [DOI] [PubMed] [Google Scholar]
- 23.Jones MP, Talley NJ, Nuyts G, et al. Lack of objective evidence of efficacy of laxatives in chronic constipation.Dig Dis Sci. 2002;47:2222–2230 [DOI] [PubMed] [Google Scholar]
- 24.Xing JH, Soffer EE.Adverse effects of laxatives.Dis Colon Rectum. 2001;44:1201–1209 [DOI] [PubMed] [Google Scholar]
- 25.Attar A, Lémann M, Fergursonn A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation.Gut. 1999;44:226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation.Gut. 2000;46:522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laung L, Riutta T, Kotecha J, et al. Chronic constipation: an evidence-based review.J Am Board Fam Med. 2011;24:436–451 [DOI] [PubMed] [Google Scholar]
- 28.Ford AC, Talley NJ.Laxatives for chronic constipation in adults.BMJ. 2012;345:e6168. [DOI] [PubMed] [Google Scholar]
- 29.Saz-Peiro P, Ortiz-Lucas M, Saz-Tejero S.General care for constipation.Medicina Naturista. 2010;4:66–71 [Google Scholar]
- 30.Johanson JF, Wald A, Tougas G, et al. Effect of tegaserod in chronic constipation: a randomized double-blind, controlled trial.Clin Gastroenterol Hepatol. 2004;2:796–805 [DOI] [PubMed] [Google Scholar]
- 31.Kamm MA, Muller-Lissner S, Talley NJ, et al. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study.Am J Gastroenterol. 2005;100:362–372 [DOI] [PubMed] [Google Scholar]
- 32.Wood P.Tegaserod in the treatment of constipation-predominant irritable bowel syndrome. Do the risks outweigh the benefits?Naunyn-Schmiedeberg’s Arch Pharmacol. 2012;385:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loughlin J, Quinn S, Rivero E, et al. Tegaserod and the risk of cardiovascular ischemic events: an observational cohort study.J Cardiovasc Pharmacol Ther. 2010;15:151–157 [DOI] [PubMed] [Google Scholar]
- 34.De Maeyer JH, Lefebvre RA, Schuurkes JA.5-HT4 receptor agonists: similar but not the same.Neurogastroenterol Motil. 2008;20:99–112 [DOI] [PubMed] [Google Scholar]
- 35.Gershon D, Tack J.The serotonin signaling system: from basic understanding to drug development for functional GI disorders.Gastroenterology. 2007;132:397–414 [DOI] [PubMed] [Google Scholar]
- 36.Briejers MR, Bosmans JP, Van Daele P, et al. The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound.Eur J Pharmacol. 2001;423:71–83 [DOI] [PubMed] [Google Scholar]
- 37.Briejer MR, Prins NH, Schuurkes JA.Effects of the enterokinetic prucalopride (R093877) on colonic motility in fasted dogs.Neurogastroenterol Motil. 2001;13:465–472 [DOI] [PubMed] [Google Scholar]
- 38.Prins NH, Van Haselen JF, Lefebvre RA, et al. Pharmacological characterization of 5-HT4 receptors mediating relaxation of canine isolated rectum circular smooth muscle.Br J Pharmacol. 1999;127:1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camilleri M, Kerstens R, Rykx A, et al. A placebo-controlled trial of prucalopride for severe chronic constipation.N Engl J Med. 2008;358:2344–2354 [DOI] [PubMed] [Google Scholar]
- 40.Tack J, Van Outryve M, Beyens G, et al. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives.Gut. 2009;58:357–365 [DOI] [PubMed] [Google Scholar]
- 41.Quigley EM, Vandeplassche L, Kerstens R, et al. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation- a 12-week, randomized, double-blind, placebo-controlled study.Aliment Pharmacol Ther. 2009;29:315–328 [DOI] [PubMed] [Google Scholar]
- 42.Ke M, Zou D, Yuan Y, et al. Prucalopride in the treatment of chronic constipation in patients from the Asia-Pacific región: a randomized, doublé-blind, placebo-controlled study.Neurogastroenterol Motil. 2012;24:999–e541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camilleri M, Beyens G, Kerstens R, et al. Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study.Neurogastroenterol Motil. 2009;21:1256–e117 [DOI] [PubMed] [Google Scholar]
- 44.Müller-Lissner S, Rykx A, Kerstens R, et al. A double-blind, placebo-controlled study of prucalopride in elderly patients with chronic constipation.Neurogastroenterol Motil. 2010;22:991–998e255 [DOI] [PubMed] [Google Scholar]
- 45.Camilleri M, Van Outryve MJ, Beyens G, et al. Clinical trial: the efficacy of open-label prucalopride treatment in patients with chronic constipation-follow-up of patients from the pivotal studies.Aliment Pharmacol Ther. 2010;32:1113–1123 [DOI] [PubMed] [Google Scholar]
- 46.Brandt LJ, Chey W, Foxx-Orenstein AE, et al. And evidence-based systematic review on the management of irritable bowel syndrome.Am J Gastroenterol. 2009;104suppl 1S1–S35 [DOI] [PubMed] [Google Scholar]
- 47.Lacy BE, Chey WD.Lubiprostone: chronic constipation and irritable bowel syndrome with constipation.Expert Opin Pharmacother. 2009;10:143–152 [DOI] [PubMed] [Google Scholar]
- 48.Ginzburg R, Ambizas EM.Clinical pharmacology of lubiprostone, a chloride channel activator in defecation disorders.Expert Opin Drug Metab Toxicol. 2008;4:1091–1097 [DOI] [PubMed] [Google Scholar]
- 49.Johanson JF, Lubiprostone Ueno R.A locally acting chloride channel activator, in adult patients with chronic constipation, a double-blind placebo-controlled, dose-ranging study to evaluate efficacy and safety.Aliment Pharmacol Ther. 2007;25:1351–1361 [DOI] [PubMed] [Google Scholar]
- 50.Fukudo S, Hongo M, Kaneko H, et al. Efficacy and safety of oral lubiprostone in constipated patients with or without irritable bowel syndrome: a randomized, placebo-controlled and dose-finding study.Neurogastroenterol Motil. 2011;23:544–e205 [DOI] [PubMed] [Google Scholar]
- 51.Johanson JF, Morton D, Greenan J.Multicenter 4-week, double blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation.Am J Gastroenterol. 2008;103:170–177 [DOI] [PubMed] [Google Scholar]
- 52.Barish CF, Drossman D, Johanson JF, et al. Efficacy and safety of lubiprostone in patients with chronic constipation.Dig Dis Sci. 2010;55:1090–1097 [DOI] [PubMed] [Google Scholar]
- 53.Lembo AJ, Johanson JF, Parkman HP, et al. Long-term safety and effectiveness of lubiprostone, a chloride channel (CIC-2) activator, in patients with chronic idiopathic constipation.Dig Dis Sci. 2011;56:2639–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busby RW, Bryant AP, Bartolini WP, et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit.Eur J Pharmacol. 2010;649:328–335 [DOI] [PubMed] [Google Scholar]
- 55.Eutamene H, Bradesi S, Larauche M, et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain.Neurogastroenterol Motility. 2010;22:312–e84 [DOI] [PubMed] [Google Scholar]
- 56.Johnston JM, Kurtz CB, Drossman DA, et al. Pilot study on the effect of linaclotide in patients with chronic constipation.Am J Gastroenterol. 2009;104:125–132 [DOI] [PubMed] [Google Scholar]
- 57.Lembo AJ, Kurtz CB, MacDougall JE, et al. Efficacy of linaclotide for patients with chronic constipation.Gastroenterology. 2010;138:886–895 [DOI] [PubMed] [Google Scholar]
- 58.Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation.N Engl J Med. 2011;365:527–536 [DOI] [PubMed] [Google Scholar]
- 59.Tack J, Camilleri M, Chang L, et al. Systematic review: cardiovascular safety profile of 5-HT4 agonists developed for gastrointestinal disorders.Aliment Pharmacol Ther. 2012;35:745–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manini ML, Camilleri M, Goldberg M, et al. Effects of Velusetrag (TD-5108) on gastrointestinal transit and bowel function in health and pharmacokinetics in health and pharmacokinetics in health and constipation.Neurogastroenterol Motil. 2010;22:42–49e7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldberg M, Li YP, Johanson JF, et al. Clinical trial: the efficacy and tolerability of velusetrag, a selective 5-Ht4 agonist with high intrinsic activity, in chronic idiopathic constipation-a 4-week randomized, double-blind, placebo-controlled, dose-response study.Aliment Pharmacol Ther. 2010;32:1102–1112 [DOI] [PubMed] [Google Scholar]
- 62.Shailubhai K, Comiskey S, Foss JA, et al. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses.Dig Dis Sci. 2013;58:2580–2586 [DOI] [PubMed] [Google Scholar]
- 63.Miner PB, Surowitz R, Ron Fogel R, et al. Plecanatide, a novel guanylate cyclase-C (GC-C) receptor agonist, is efficacious and safe in patients with chronic idiopathic constipation (CIC): results from a 951 patient, 12 week, multi-center trial. DDW AGA Abstracts.Gastroenterology. 2013;144suppl 1S-163 [Google Scholar]
- 64.Chey WD, Camilleri M, Chang L, et al. A randomized placebo-controlled phase IIb trial of A3309, a bile acid transporter inhibitor, for chronic idiopathic constipation.Am J Gastroenterol. 2011;106:1803–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]


