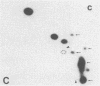
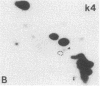
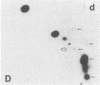
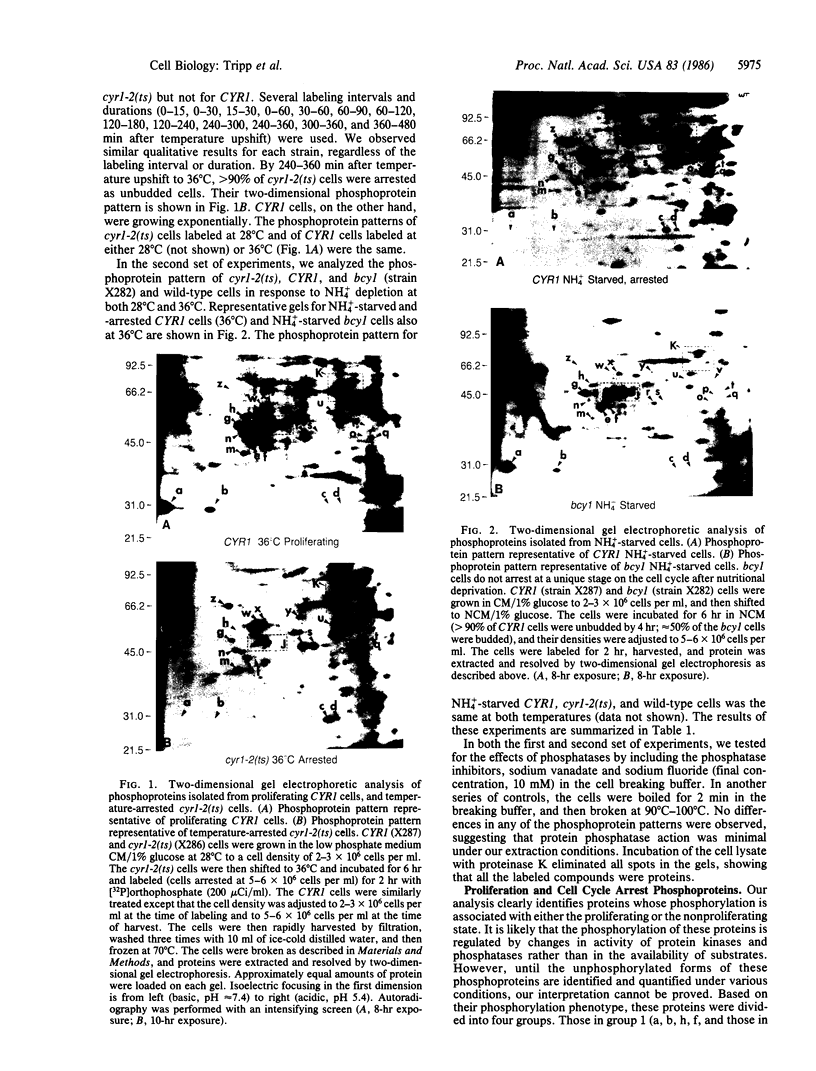
Abstract
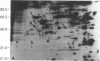
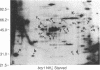
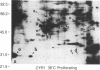
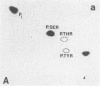
Recent genetic and biochemical studies of two mutants of the cAMP pathway in yeast, cyr1 and bcy1, have demonstrated that cAMP-dependent protein phosphorylation plays a major regulatory role in the control of proliferation and differentiation. As a first step in examining this regulatory system in more detail and in identifying the protein substrates of cAMP-dependent protein kinase, we have analyzed phosphoprotein patterns in the mutants cyr1-2(ts) and bcy1 by two-dimensional polyacrylamide gel electrophoresis. Our analysis has revealed several proteins whose phosphorylation is controlled positively or negatively by the cAMP pathway in yeast. The presence of some of these phosphoproteins was directly associated with proliferation (positive regulation), while that of others was correlated with cell cycle arrest (negative regulation). The phosphoprotein patterns of cyr1-2(ts) temperature-arrested cells, and nitrogen (NH+4)-starved cells, were strikingly similar, suggesting that response to NH+4 is mediated in part by adenylate cyclase. Phosphoproteins whose presence correlated with cell cycle arrest were found to be phosphorylated on serine and threonine residues, while the major phosphoproteins present predominantly in proliferating cells were phosphorylated only on serine residues. None of the greater than 20 phosphoproteins we examined contained phosphotyrosine under either growth condition.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castellanos R. M., Mazón M. J. Identification of phosphotyrosine in yeast proteins and of a protein tyrosine kinase associated with the plasma membrane. J Biol Chem. 1985 Jul 15;260(14):8240–8242. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Hindley J., Phear G. A. Sequence of the cell division gene CDC2 from Schizosaccharomyces pombe; patterns of splicing and homology to protein kinases. Gene. 1984 Nov;31(1-3):129–134. doi: 10.1016/0378-1119(84)90203-8. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Control of cell division in Saccharomyces cerevisiae mutants defective in adenylate cyclase and cAMP-dependent protein kinase. Exp Cell Res. 1983 Jun;146(1):151–161. doi: 10.1016/0014-4827(83)90333-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Initiation of meiosis in yeast mutants defective in adenylate cyclase and cyclic AMP-dependent protein kinase. Cell. 1983 Feb;32(2):417–423. doi: 10.1016/0092-8674(83)90461-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Oshima Y., Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Piñon R. Effects of ammonium ions on sporulation of Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 15;105(2):367–378. doi: 10.1016/0014-4827(77)90134-3. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieven G., Thorner J., Martin G. S. Protein-tyrosine kinase activity in Saccharomyces cerevisiae. Science. 1986 Jan 24;231(4736):390–393. doi: 10.1126/science.2417318. [DOI] [PubMed] [Google Scholar]
- Wright J. F., Ajam N., Dawes I. W. Nature and timing of some sporulation-specific protein changes in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Oct;1(10):910–918. doi: 10.1128/mcb.1.10.910. [DOI] [PMC free article] [PubMed] [Google Scholar]