Abstract
Purpose: To describe the clinical, imaging, and pathologic characteristics and diagnostic methods of telangiectatic osteosarcoma (TOS) for improving the diagnostic level. Materials and methods: The authors retrospectively reviewed patient demographics, serum alkaline phosphatase (AKP) levels, preoperative biopsy pathologic reports, pathologic materials, imaging findings, and treatment outcomes from 26 patients with TOS. Patient images from radiography (26 cases) and magnetic resonance (MR) imaging (22 cases) were evaluated by 3 authors in consensus for intrinsic characteristics. There were 15 male and 11 female patients in the study, with an age of 9–32 years (mean age 15.9 years). Results: Eighteen of 26 patients died of lung metastases within 5 years of follow-up. The distal femur was affected more commonly (14 cases, 53.8%). Regarding serum AKP, normal (8 cases) or mildly elevated (18 cases) levels were found before preoperative chemotherapy. Radiographs showed geographic bone lysis without sclerotic margin (26 cases), cortical destruction (26 cases), periosteal new bone formation (24 cases), soft-tissue mass (23 cases), and matrix mineralization (4 cases). The aggressive radiographic features of TOS simulated the appearance of conventional high-grade intramedullary osteosarcoma, though different from aneurysmal bone cyst. MR images demonstrated multiple big (16 cases) or small (6 cases) cystic spaces, fluid-fluid levels (14 cases), soft-tissue mass (22 cases), and thick peripheral and septal enhancement (22 cases). Nine of 26 cases were misdiagnosed as aneurysmal bone cysts by preoperative core-needle biopsy, owing to the absence of viable high-grade sarcomatous cells in the small tissue samples. Conclusion: The aggressive growth pattern with occasional matrix mineralization, and multiple big or small fluid-filled cavities with thick peripheral, septal, and nodular tissue surrounding the fluid-filled cavities are characteristic imaging features of TOS, and these features are helpful in making the correct preoperative diagnosis of TOS.
Keywords: Telangiectatic osteosarcoma, magnetic resonance imaging, radiography, pathology, biopsy
Introduction
Osteosarcoma is the most common primary malignant bone tumor in children and adolescents. Telangiectatic osteosarcoma (TOS) is a rare subtype that represents from 2% to 12% of all cases of osteosarcoma[1–5]. TOS is characterized by multiple, aneurysmally dilated, blood-filled cavities with high-grade sarcomatous cells in the peripheral rim and septae[6]. Identification of the unique histologic and radiographic features of TOS is crucial for correct diagnosis. Nevertheless, in many cases TOS is misdiagnosed. For example, TOS can be easily confused with aneurysmal bone cyst[7], both radiologically and pathologically. Misdiagnosis always results in delay in initiation of proper therapy and potentially affects the prognosis[8–11]. In previous studies of TOS, the clinical significance of the serum alkaline phosphatase (AKP) and preoperative biopsy pathology has been unclear. The prognosis for patients with TOS has been debated in the literature for many decades[12–14]. Moreover, to date detailed evaluation of imaging characteristics is limited[6,9].
In the current study, we retrospectively reviewed a series of TOS in our hospital over a period of 10 years. The clinical, imaging, and pathologic characteristics for the diagnosis of TOS are described and evaluated.
Materials and methods
Patients
We conducted a search of a bone tumor database to identify all patients with osteosarcoma and TOS proved by postoperative pathology who were treated in our hospital from January 2001 to December 2010. Patients who were included in this study fully meet the histologic diagnostic criteria for TOS as defined in the World Health Organization Classification[15]. Postoperative pathology showed hemorrhagic cavities with viable high-grade sarcomatous cells and small amounts of osteoid mineralization around the periphery and septations of these spaces. Information regarding the clinical characteristics and treatment regimen of TOS patients was collected by a review of the medical records. All available pathology reports and materials, and images for these patients were reviewed retrospectively. Pathologic records of core-needle biopsy before preoperative chemotherapy were obtained. Images that were used to evaluate primary tumors included a combination of radiography and magnetic resonance (MR) imaging. Laboratory results of the serum AKP level before preoperative chemotherapy were carefully evaluated. All patients were followed up by telephone.
This retrospective study was approved by the Institutional Review Board of the First Affiliated Hospital, Sun Yat-sen University. Informed patient consent was not required by either institution.
Image evaluation
Images were reviewed in consensus by 3 experienced musculoskeletal radiologists. Radiography (26 cases) and MR images (22 cases) were obtained prior to therapy. Evaluation of the anatomic site included side involved, specific bone affected, and lesion range such as epiphysis, metaphysis, or diaphysis. Tumor size was determined in largest dimension by MR imaging modality that best depicted the lesion range.
Radiographic analysis
Radiography images were analyzed according to the characteristics as described by Murphey et al.[6], including patterns of bone destruction (geographic, moth-eaten, or permeative), width of the zone of transition with or without sclerotic margin, absence or presence and severity (mild, moderate or marked) of expansile remodeling of bone, absence or presence and continuity of periosteal new bone formation, presence or absence of cortical destruction and soft-tissue mass, presence or absence of intraosseous or extraosseous matrix mineralization, presence or absence of pathologic fracture, and presence or absence of radiolucent striations in or around the lesion.
MR imaging protocol and analysis
MR imaging was performed using a 1.5-T or 3.0-T superconductive MR unit (Magnetom Vision, Magnetom Trio Tim; Siemens Medical Systems, Erlangen, Germany). MR images available for review included spin-echo (SE) T1-weighted images, fast SE T2-weighted images, or T2-weighted fat-saturated images, as well as SE contrast-enhanced T1-weighted images with or without fat suppression. The contrast agent Magnevist (Bayer Schering Pharma, Berlin-Wedding, Germany) was used at a dose of 0.1 mmol/kg. The following imaging parameters were used: T1-weighted sequence (repetition time (TR)/echo time (TE) 400–600 ms/9–40 ms), T2-weighted sequence (TR/TE 3000–4000 ms/80–100 ms); slice thickness 4 mm, gap 0.4 mm; field of view (FOV) 230 × 230 to 360 × 360 mm; matrix 256 × 256; number of excitations (NEX) 2.
MR images were evaluated for tissue homogeneity or heterogeneity, and predominant signal intensity of the lesion on both T1-weighted and T2-weighted MR images. The presence or absence of cortical destruction, soft-tissue mass, fluid-fluid level, hemorrhage, and the size of cystic spaces were also determined. The predominant patterns of enhancement (thin or thick peripheral and/or septal, with or without nodularity) on postcontrast enhanced T1-weighted images were evaluated with the same criteria used by Murphey et al.[6].
Results
Clinical characteristics
Of all 512 patients with osteosarcoma who were treated in our institution from January 2001 to December 2010, 26 patients (5.1%) were identified with TOS. The clinical characteristics recorded are summarized in Table 1. In our study, 26 patients (15 male and 11 female) ranged in age from 9 to 32 years with the mean age of 15.9 years. The maximum size of lesion ranged from 6 to 26 cm. Lesions were located in unilateral long bones, and 21 (80.8%) lesions involved the metaphysis. Lesions were around the knee in 76.9% of cases, and the distal femur was affected more commonly (14 cases, 53.8%).
Table 1.
Clinical characteristics of the 26 patients
| Clinical characteristics | No. of patients (%) |
|---|---|
| Sex | |
| Male | 15 (57.7) |
| Female | 11 (42.3) |
| Side involved | |
| Left | 16 (61.5) |
| Right | 10 (38.5) |
| Clinical symptoms | |
| Pain | 26 (100) |
| Swelling | 7 (26.9) |
| Mass | 12 (46.2) |
| Tumor site | |
| Distal femur | 14 (53.8) |
| Femoral neck | 2 (7.7) |
| Proximal humerus | 4 (15.4) |
| Proximal tibia | 5 (19.2) |
| Proximal fibula | 1 (3.9) |
| Lesion range | |
| Metaphysis | 21 (80.8) |
| Metaphysis–diaphysis junction area | 5 (19.2) |
| Disease stage at diagnosis | |
| Localized | 23 (88.5) |
| Metastatic | 3 (11.5) |
| Within 5-year follow-up | |
| Died of lung metastasis | 18 (69.2) |
| Alive with lung metastasis | 5 (19.3) |
| Alive without lung metastasis | 3 (11.5) |
Lung multiple metastases were found in 3 patients (11.5%) at diagnosis. Eighteen patients (69.2%) died of lung metastases within 5 years. The remaining 8 patients survived at less than 3 years of follow-up.
Laboratory results
Before the initiation of preoperative chemotherapy, the average serum AKP level was normal in 8 patients and was elevated by 1.2-times normal value in 18 patients (69.2%).
Radiographic findings
Radiographic features are summarized in Table 2. Radiographs (26 cases) showed bone lysis with a wide transition (without sclerotic margin) in all cases (Figs. 1a, 2a, and 3a), including geographic bone destruction (92.3%) and moth-eaten destruction (7.7%). Expansile remodeling of bone was also found, including mild bone expansion (19.2%) (Fig. 2a) and marked bone expansion (3.9%) (Fig. 3a). Cortical destruction appeared in all 26 patients, with associated soft-tissue mass in 23 cases (88.5%). Discontinuous periosteal new bone formation was common (92.3%). No calcification or ossification was found within all soft-tissue masses. Pathologic fractures occurred in the femur (4 cases) and tibia (1 case). There was no radiolucent striation in all cases.
Table 2.
Radiographic features of the 26 patients
| Radiographic features | No. of patients (%) |
|---|---|
| Bone destruction | |
| Geographic | 24 (92.3) |
| Moth-eaten | 2 (7.7) |
| Sclerotic margin around bone lysis | |
| Absence | 26 (100) |
| Presence | 0 |
| Expansile remodeling of bone | |
| Absence | 20 (76.9) |
| Mild | 5 (19.2) |
| Marked | 1 (3.9) |
| Cortical destruction | |
| Absence | 0 |
| Presence | 26 (100) |
| Periosteal new bone formation | |
| Absence | 2 (7.7) |
| Presence but discontinuity | 24 (92.3) |
| Soft-tissue mass | |
| Absence | 3 (11.5) |
| Presence | 23 (88.5) |
| Matrix mineralization | |
| Intraosseous | 4 (15.4) |
| Extraosseous | 0 |
| Pathologic fracture | |
| Absence | 21 (80.8) |
| Presence | 5 (19.2) |
| Radiolucent striation | |
| Absence | 26 (100) |
| Presence | 0 |
Figure 1.
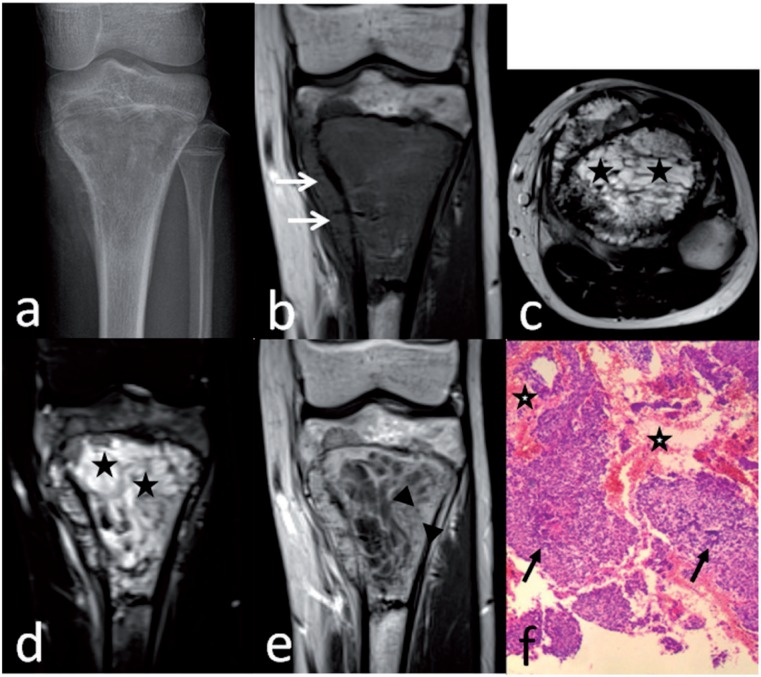
Telangiectatic osteosarcoma of the proximal tibia in a 14-year-old boy. Anteroposterior radiograph (a) shows a geographic destruction with a wide transition. Coronal T1-weighted image (b) shows marrow replacement by heterogeneous tissue with cortical destruction and associated soft-tissue mass (arrows). Axial T2-weighted image (c) and coronal T2-weighted fat-suppressed image (d) shows predominately multiple small cystic spaces (stars). Coronal T1-weighted enhanced image (e) shows a thick rim and septa with nodularity (between arrowheads). Preoperative biopsy pathology (f) shows some blood spaces (stars), anaplastic malignant tumor cells (arrows), and neoplastic osteoid tissue, suggesting the diagnosis of telangiectatic osteosarcoma (hematoxylin–eosin stain; original magnification ×40).
Figure 2.
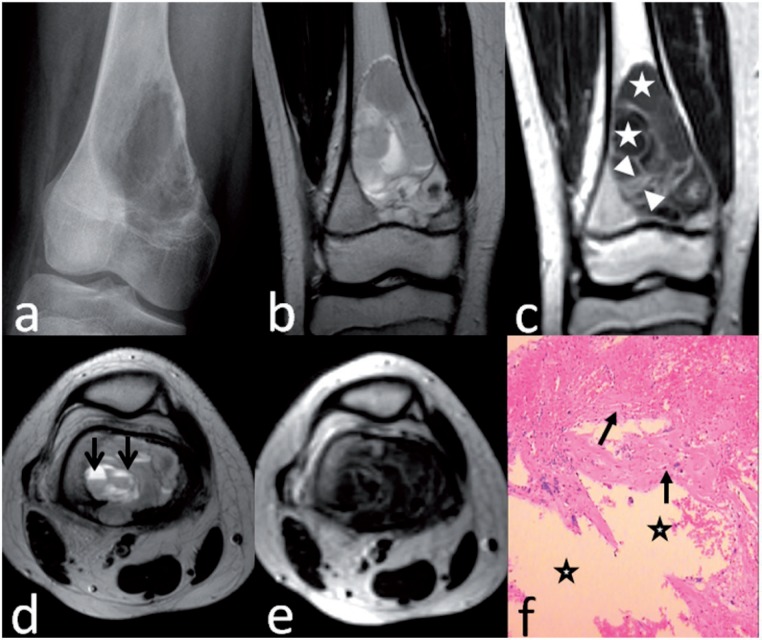
Telangiectatic osteosarcoma of the distal femur in a 12-year-old boy. Anteroposterior radiograph (a) shows a geographic lytic lesion with mild expansile remodeling of bone. Coronal T2-weighted image (b) shows marrow replacement by heterogeneous high signal intensity. Coronal T1-weighted enhanced image (c) shows a thick septa (between arrowheads) and big cystic spaces (stars). Axial T2-weighted image (d) shows fluid levels (arrows) in the cystic spaces. T1-weighted enhanced image (e) shows honeycomb-like appearance. Preoperative biopsy pathology (f) shows some blood spaces (stars) and surrounding fibroconnective tissues (arrows) without high-grade sarcomatous cells, suggesting the diagnosis of aneurysmal bone cyst (hematoxylin–eosin stain; original magnification ×40).
Figure 3.
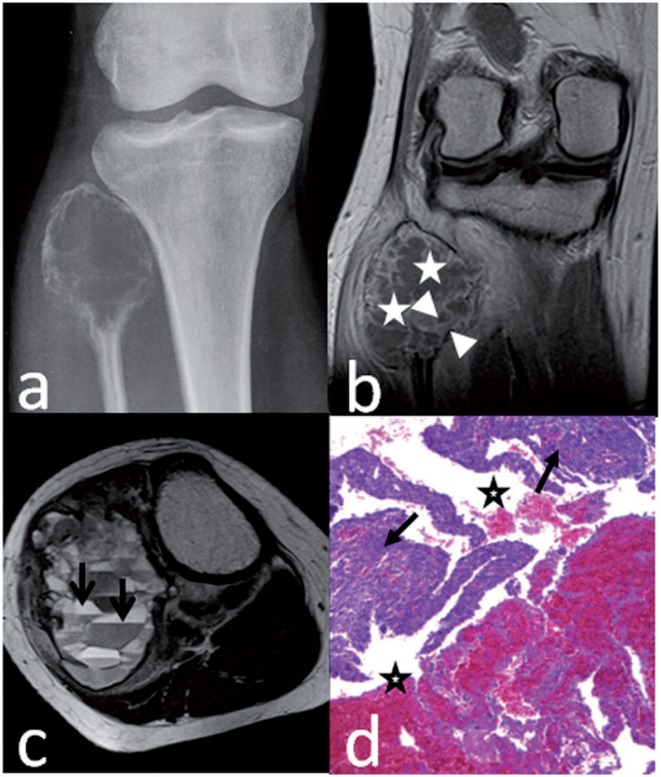
Telangiectatic osteosarcoma of the proximal fibula in a 26-year-old woman. Anteroposterior radiograph (a) shows a geographic lytic lesion with marked expansile remodeling of bone. Coronal T1-weighted enhanced MR image (b) shows a thick rim (between arrowheads) around the cystic spaces (stars). Axial T2-weighted MR image (c) shows fluid levels (arrows) in big cystic spaces. Preoperative biopsy pathology (d) shows some blood spaces (stars) and surrounding high-grade sarcomatous tissue (arrows), suggesting the diagnosis of telangiectatic osteosarcoma (hematoxylin–eosin stain; original magnification ×40).
MR imaging findings
MR imaging of all 22 cases showed marrow replacement by heterogeneous tissue with signal intensity predominantly similar to or lower than that of muscle on T1-weighted images (Fig. 1b), and with predominantly high signal intensity (much higher than that of muscle) on T2-weighted images (Figs. 1c and 2b) or fat-suppressed T2-weighted images (Fig. 1d). Cortical destruction with associated soft-tissue mass was seen in all patients (100%) (Fig. 1c). Areas of hemorrhage with high signal intensity depicted on all MR pulse sequences were seen in 9 patients (40.9%). Multiple cystic spaces with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images were seen in all patients (100%). There were predominately big cystic spaces with diameter no less than 5 mm in 16 patients (72.7%) (Figs. 2d and 3c), and predominately small cystic spaces with diameter less than 5 mm in 6 patients (27.3%) (Fig. 1c). Fluid levels were seen in 14 patients (63.6%) on axial T2-weighted images (Figs. 2d and 3c). Postcontrast images in all patients showed honeycomb-like appearance, which has characteristics of predominantly thick peripheral and septal patterns of enhancement with or without nodularity (rim, septa, and nodularity thicker than 2 mm) (Figs. 1e, 2c, 2e, and 3b).
Preoperative biopsy: pathologic findings and diagnoses
The size of tumor samples obtained by core-needle biopsy was 10–20 mm long and 3–5 mm in diameter. The pathologic reports and findings of preoperative biopsy in 26 patients were as follows: (1) negative diagnostic results (3 cases), showing a large number of blood clots and few solid components; (2) aneurysmal bone cyst (9 cases), showing large blood spaces and surrounding fibroconnective tissues without high-grade sarcomatous cells (Fig. 2f); (3) conventional osteosarcoma (10 cases), showing some anaplastic malignant tumor cells and osteoid mineralization without obvious blood spaces; (4) TOS (4 cases), showing hemorrhagic cavities with viable high-grade sarcomatous cells and small amounts of osteoid mineralization around the periphery and septations of these spaces (Figs. 1f and 3d).
Discussion
TOS is a rare variant of osteosarcoma with well-defined histologic characteristics that clearly differentiate it from other osteosarcoma subtypes. However, similar patient demographics, clinical symptoms, and tumor locations often confuse the differential diagnosis between TOS and conventional intramedullary osteosarcoma[6,16]. TOS represents approximately 5.1% of all osteosarcomas reviewed in our study. Tumors affected adolescents or younger adults with mean age of 15.9 years, showed a slight male predilection, and commonly involved the distal femur (53.8%) and metaphysis (80.8%). One possible explanation for the high rate of pathologic fractures (19.2%) in our patients with TOS is the extensively lytic, cystic nature of the tumor and expansile remodeling of bone, which decreases the bone biomechanical strength in terms of fracture resistance[3,9,10]. The normal or slight increase in the serum AKP level in our patients may reflect small amounts of neoplastic bone pathologically, and seldom visible matrix mineralization radiographically, in TOS. Implementation of neoadjuvant chemotherapy has been suggested to improve the prognosis of TOS[4]. In our study, prevalence of lung metastasis at diagnosis was 11.5%, and 69.2% of TOS patients died of lung metastases within 5 years of follow-up with the implementation of neoadjuvant chemotherapy, suggesting a poor prognosis for patients with TOS.
In our study, nonspecific bone lysis with the expansile remodeling of bone (23.1%) and the absence of matrix mineralization (84.6%) on radiographs may simulate the features of aneurysmal bone cyst. However, these more aggressively radiographic appearances, including cortical destruction (100%), discontinuous periosteal new bone formation (92.3%), and associated soft-tissue mass (88.5%), are different from the features of aneurysmal bone cyst. These findings are consistent with previous reports[6,17,18]. However, radiolucent striation in TOS suggestive of hypertrophy of normal veins as described by Vanel et al.[9] or extraosseous matrix mineralization reflecting osteoid-producing tumor[6,9] was not detected on radiologic images in our study.
TOS has been reported to be mainly composed of aneurysmally dilated blood-filled cavities. However, the MR imaging features of TOS, including multiple large fluid-filled spaces, fluid levels, and areas of hemorrhage, simulate those of aneurysmal bone cysts[19–22]. Two additional common imaging features supporting the diagnosis of TOS were recently suggested by Murphey et al.[6], the first of which is thick peripheral, septal, and nodular tissue surrounding the fluid-filled spaces on MR imaging. The second feature is the aggressive growth of TOS, including cortical destruction with an associated soft-tissue mass. According to our MR imaging observations, these 2 imaging features were detected in all 22 cases (100%), supporting Murphey’s suggestions regarding the differential diagnosis between TOS and aneurysmal bone cyst.
Although blood spaces in TOS are pathologically similar to aneurysmal bone cyst, the viable high-grade sarcomatous cells around the periphery and septations of these blood spaces in TOS do not appear in aneurysmal bone cyst. Thus, the aneurysmal bone cyst-like blood cavities and the high-grade malignant tumor cells are characteristics of the pathologic diagnosis of TOS. However, in our reviewed preoperative pathologic reports several cases of TOS were misdiagnosed as conventional osteosarcoma or aneurysmal bone cyst, the reason for which may be that some of the characteristic histologic findings were overlooked because of the limited sample volume obtained by core-needle biopsy.
Interestingly, in our study blood spaces in TOS were predominately composed of big cystic spaces (72.7%) or small cystic spaces (27.3%). In our opinion, small cystic spaces are seldom seen in aneurysmal bone cyst in comparison with TOS. However, because relatively few cases are reviewed in our study, the clinical significance of the size of cystic space in the differential diagnosis of TOS and aneurysmal bone cyst needs further investigation.
Conclusion
TOS, a rare variant of osteosarcoma with normal or slight increase in the serum AKP level and poor prognosis, can be misdiagnosed as aneurysmal bone cyst or conventional osteosarcoma by core-needle biopsy. The aggressive growth pattern with occasional matrix mineralization, and fluid-filled cavities in variable size with thick peripheral, septal, and nodular tissue surrounding the fluid-filled cavities, are characteristic imaging features of TOS. A combined-modality approach of preoperative core-needle biopsy and imaging is helpful in making a diagnosis of TOS.
Acknowledgements
We would like to thank Dr Ye-bin Jiang from Osteoporosis and Arthritis Laboratory, University of Michigan, for his help in revising our paper. This study was supported in part by a grant from the Science and Technology Planning Project of Guangdong Province, China (Grant No. 2011B031800096).
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Farr GH, Huvos AG, Marcove RC, Higinbotham NL, Foote FW., Jr Telangiectatic osteogenic sarcoma: a review of twenty-eight cases. Cancer. 1974;34:1150–1158. doi: 10.1002/1097-0142(197410)34:4<1150::aid-cncr2820340426>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Matsuno T, Unni KK, McLeod RA, Dahlin DC. Telangiectatic osteogenic sarcoma. Cancer. 1976;38:2538–2547. doi: 10.1002/1097-0142(197612)38:6<2538::aid-cncr2820380643>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Rosen G, Huvos AG, Marcove R, Nirenberg A. Telangiectatic osteogenic sarcoma: improved survival with combination chemotherapy. Clin Orthop Relat Res. 1986;207:164–173. [PubMed] [Google Scholar]
- 4.Bacci G, Ferrari S, Ruggieri P, et al. Telangiectatic osteosarcoma of the extremity: neoadjuvant chemotherapy in 24 cases. Acta Orthop Scand. 2001;72:167–172. doi: 10.1080/000164701317323426. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 6.Murphey MD, wan Jaovisidha S, Temple HT, Gannon FH, Jelinek JS, Malawer MM. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229:545–553. doi: 10.1148/radiol.2292021130. [DOI] [PubMed] [Google Scholar]
- 7.Huvos AG, Rosen G, Bretsky SS, Butler A. Telangiectatic osteogenic sarcoma: a clinicopathologic study of 124 patients. Cancer. 1982;49:1679–1689. doi: 10.1002/1097-0142(19820415)49:8<1679::aid-cncr2820490824>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Chawla SP, Benjamin RS. Effectiveness of chemotherapy in the management of metastatic telangiectatic osteosarcoma. Am J Clin Oncol. 1988;11:177–180. doi: 10.1097/00000421-198804000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Vanel D, Tcheng S, Contesso G, et al. The radiological appearances of telangiectatic osteosarcoma: a study of 14 cases. Skeletal Radiol. 1987;16:196–200. doi: 10.1007/BF00356952. [DOI] [PubMed] [Google Scholar]
- 10.Gomes H, Menanteau B, Gaillard D, Behar C. Telangiectatic osteosarcoma. Pediatr Radiol. 1986;16:140–143. doi: 10.1007/BF02386639. [DOI] [PubMed] [Google Scholar]
- 11.Suh YL, Chi JG. Telangiectatic osteosarcoma-a case report. J Korean Med Sci. 1989;4:97–101. doi: 10.3346/jkms.1989.4.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mervak TR, Unni KK, Pritchard DJ, McLeod RA. Telangiectatic osteosarcoma. Clin Orthop Relat Res. 1991;270:135–139. [PubMed] [Google Scholar]
- 13.Bacci G, Bertoni F, Longhi A, et al. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity: histologic response to preoperative chemotherapy correlates with histologic subtype of the tumor. Cancer. 2003;97:3068–3075. doi: 10.1002/cncr.11456. [DOI] [PubMed] [Google Scholar]
- 14.Bacci G, Picci P, Ferrari S, Sangiorgi L, Zanone A, Brach del Prever A. Primary chemotherapy and delayed surgery for non-metastatic telangiectatic osteosarcoma of the extremities: results in 28 patients. Eur J Cancer. 1994;30A:620–626. doi: 10.1016/0959-8049(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 15.Matsuno T, Okada K, Knuutila S. Telangiectatic osteosarcoma. In: Fletcher C, Unni K, Mertens F, editors. Tumors of soft tissue and bone. Lyon, France: IARC Press; 2000. pp. p. 271–272. [Google Scholar]
- 16.Murphey MD, Robbin MR, McRae GA, Flemming DJ, Temple HT, Krandsdorf MJ. The many faces of osteosarcoma. Radiographics. 1997;17:1205–1231. doi: 10.1148/radiographics.17.5.9308111. [DOI] [PubMed] [Google Scholar]
- 17.Adler CP. Case report 111: diagnosis telangiectatic osteosarcoma of the femur with features of an aggressive aneurysmal bone cyst. Skeletal Radiol. 1980;5:56–60. doi: 10.1007/BF00347101. [DOI] [PubMed] [Google Scholar]
- 18.Brown MJ, Logan PM, O’Connell JX, Janzen DL, Connell DG. Diaphyseal telangiectatic osteosarcoma as a second tumor after bilateral retinoblastomas. Skeletal Radiol. 1996;25:685–688. doi: 10.1007/s002560050160. [DOI] [PubMed] [Google Scholar]
- 19.Ruiter DJ, Cornelisse CJ, van Rijssel TG, van der Velde EA. Aneurysmal bone cyst and telangiectatic osteosarcoma: a histopathological and morphometric study. Virchows Arch A Pathol Anat Histol. 1977;373:311–325. doi: 10.1007/BF00432531. [DOI] [PubMed] [Google Scholar]
- 20.Martinez V, Sissons HA. Aneurysmal bone cyst: a review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 1988;61:2291–2304. doi: 10.1002/1097-0142(19880601)61:11<2291::aid-cncr2820611125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversies, patient presentation, and imaging. AJR Am J Roentgenol. 1995;164:573–580. doi: 10.2214/ajr.164.3.7863874. [DOI] [PubMed] [Google Scholar]
- 22.Vergel De Dios AM, Bond JR, Shrives TC, McLeod RA, Unni KK. Aneurysmal bone cyst: a clinicopathologic study of 238 cases. Cancer. 1992;69:2921–2931. doi: 10.1002/1097-0142(19920615)69:12<2921::aid-cncr2820691210>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]