Abstract
Purpose: The aim of this study was to characterize and understand the therapy-induced changes in diffusion parameters in rectal carcinoma under chemoradiotherapy (CRT). The current literature shows conflicting results in this regard. We applied the intravoxel incoherent motion model, which allows for the differentiation between diffusion (D) and perfusion (f) effects, to further elucidate potential underlying causes for these divergent reports. Materials and methods: Eighteen patients with primary rectal carcinoma undergoing preoperative CRT were examined before, during, and after neoadjuvant CRT using diffusion-weighted imaging. Using the intravoxel incoherent motion approach, f and D were extracted and compared with postoperative tumor downstaging and volume. Results: Initial diffusion-derived parameters were within a narrow range (D1 = 0.94 ± 0.12 × 10−3 mm2/s). At follow-up, D rose significantly (D2 = 1.18 ± 0.13 × 10−3 mm2/s; P < 0.0001) and continued to increase significantly after CRT (D3 = 1.24 ± 0.14 × 10−3 mm2/s; P < 0.0001). The perfusion fraction f did not change significantly (f1 = 9.4 ± 2.0%, f2 = 9.4 ± 1.7%, f3 = 9.5 ± 2.7%). Mean volume (V) decreased significantly (V1 = 16,992 ± 13,083 mm3; V2 = 12,793 ± 8317 mm3, V3 = 9718 ± 6154 mm3). T-downstaging (10:18 patients) showed no significant correlation with diffusion-derived parameters. Conclusions: Conflicting results in the literature considering apparent diffusion coefficient (ADC) changes in rectal carcinoma under CRT for patients showing T-downstaging are unlikely to be due to perfusion effects. Our data support the view that under effective therapy, an increase in D/ADC can be observed.
Keywords: Intravoxel incoherent motion model, rectal carcinoma, tumor response, diffusion-weighted imaging, chemoradiotherapy, monitoring
Introduction
According to treatment guidelines[1], higher-stage rectal cancer (T ≥3) is treated neoadjuvantly before surgical resection to reduce tumor size. This allows for less aggressive surgical procedures such as a total mesorectal excision (TME). Because preoperative chemoradiotherapy (CRT) shows a significant individual variation in treatment response[2–4], early detection and assessment of response is the object of many studies[5–9].
To assess therapy response early, functional magnetic resonance (MR) imaging studies are increasingly applied to add information on changes in tumor pathophysiology, e.g., tumor cellularity or changes in microcirculation during CRT, and thus may be sensitive to early CRT-induced changes. On the other hand, functional imaging such as diffusion-weighted (DW) imaging, because of its limited resolution, is less suitable for the depiction of tumor decline beyond anatomic margins required to monitor T-downstaging in rectal carcinoma. The definition of T stage in rectal cancer refers not to the tumor volume but only to the anatomic wall layers of the rectum, e.g., a T3-stage rectal cancer corresponds to a transgression of the muscularis propria[10,11]. A decrease in tumor volume, therefore, does not necessarily result in T-downstaging. Furthermore, tumor involvement of the radial circumferential resection margins (so-called CRM) has been shown to be an important prognostic factor. The aim of chemoradiation treatment is to render tumor regression so as to achieve a tumor-free resection margin[1]. However, in recent studies the response to treatment was defined as a reduction of anatomic T stage[12,13], and this downstaging correlated with the diffusion-derived parameters. In these studies, the application of DW imaging to evaluate the early response of the primary rectal carcinoma to CRT showed conflicting results on considering the correlation of the diffusion values and tumor response.
Because radiation leads to necrosis[14] as well as higher vascular permeability, an increase in the apparent diffusion coefficient (ADC)[15] may be expected within 1 week of the first fraction of radiation therapy. This effect has been shown in previous studies of rectal cancer[13,16]. Sun et al.[13] showed a significant increase of the ADC under treatment in T-downstaged responders, with no significant ADC increase in the non-downstaged group. Furthermore, they postulate a lower initial ADC in rectal downstaged carcinomas. By contrast (different b values being used in these studies), Dzik-Jurasz et al.[17] reported a decrease in ADC correlating with percentage decrease in size of tumors, and Hein et al.[6] showed a significant decrease of ADC for downstaged and non-downstaged tumors after neoadjuvant CRT.
The reason for these opposing findings is currently unclear. One possible explanation may be that a stronger inflammatory reaction in tumors being T-downstaged leads to increased perfusion, which in turn may lead to an increase in ADC values, since in the presence of perfusion effects the ADC reflects a combination of perfusion and tissue microstructure. The intravoxel incoherent motion (IVIM) model derives from a combined measure of the molecular movement of water (diffusion) and circulation of blood in the capillaries (perfusion) on DW imaging[18]. Of the derived perfusion-related parameters f and D*, f is the more stable parameter, as shown both in numerical simulations[19] and clinical studies,[20] and is used in this study. Here we apply this technique to obtain a better understanding of the behavior of diffusion-derived parameters in patients with rectal carcinoma under CRT. We compare these parameters with the commonly used morphologic measure of treatment response, the decrease in volume, and the T-downstaging.
Materials and methods
Patients
This prospective study was approved by our institutional review board, and written informed consent was obtained from all patients. Patients with primary histologically proven T3 or T4 adenocarcinoma of the rectum, with any nodal stage without metastatic spread and who were scheduled to undergo preoperative CRT, were included in this study. The initial tumor spread (T stage) was assessed using pelvic MR imaging and/or intrarectal ultrasonography. Infiltration of the perirectal fat was present in all patients. The clinical and histopathologic classification and stage grouping were in accordance with TNM classification after surgery. Patients with previous chemotherapy, abdominal surgery, or irradiation were excluded.
A total of 38 consecutive patients were recruited between May 2009 and July 2011. Twenty patients were excluded because of discontinued imaging during therapy, discontinued preoperative CRT, or delayed or canceled surgery. Eighteen patients with at least one follow-up under therapy (mean age, 61.7 years; age range, 40–83 years; 12 men and 6 women) were included (Table 1) using DW MR imaging at 3 time points: before CRT (within a week), at the end of the second week of CRT, and 1–4 days before surgery. Diffusion data from 13 patients were obtained at all 3 time points: In 5 patients no complete data set was obtained, in 2 cases data points were excluded because of technical measurement errors, and in 3 cases imaging was discontinued during follow-up.
Table 1.
Summary of clinical findings
| Patient no./age (years) | Pre-/post-CRT T stage | Circumferential resection margin pre-CRT (mm) |
|---|---|---|
| 1/40 | 3/2 | 0 |
| 2/62 | 3/2 | 0 |
| 3/63 | 3/3 | 0 |
| 4/71 | 3/2 | 0 |
| 5/83 | 3/3 | 0 |
| 6/61 | 4/2 | 0 |
| 7/50 | 4/4 | 0 |
| 8/73 | 3/3 | 0 |
| 9/78 | 3/2 | 0 |
| 10/53 | 3/2 | 0 |
| 11/63 | 3/3 | 0 |
| 12/70 | 4/3 | 0 |
| 13/31 | 3/3 | 5 |
| 14/49 | 3/2 | 0 |
| 15/52 | 4/3 | 3 |
| 16/72 | 3/3 | 0 |
| 17/67 | 3/3 | 0 |
| 18/53 | 3/2 | 0 |
Rectal MR imaging
All patients were examined with a 3-T MR scanner (Siemens Medical Solutions, Erlangen, Germany) with a gradient strength of 45 mT/m and using a 6-channel phased-array body coil. Imaging protocols included morphologic images as well as DW imaging according to the following parameters: T2-weighted turbo spin-echo in the sagittal and transverse plane, perpendicular to the long axis of the rectum covering the whole tumor (repetition time/echo time (TR/TE) = 4290/108 ms, slice thickness 3 mm) as well as T2-weighted SPACE (Sampling Perfection with Application optimized contrast using different flip-angle Evolutions) imaging in the sagittal plane (enabling reconstruction in any plane) (TR/TE = 1500/118 ms, slice thickness 3 mm). Axial DW images were acquired using a single-shot echo-planar imaging pulse sequence with the following imaging parameters: TR = 4100 ms, TE = 50 ms, field of view read 280-mm phase 75%, slice thickness = 3 mm, 4 averages, bandwidth = 2170 Hz/pixel, base resolution 128, k-space based parallel imaging technique with 24 reference lines (GRAPPA) acceleration factor of 2, b values = 0, 50, 100, 150, 200, 500, and 800 s/mm2. Forty milligrams of butylscopolammonium bromide (Buscopan; Boehringer Ingelheim Vetmedica, Ingelheim, Germany) was injected intravenously to reduce intestinal motion artifacts when contraindications had been excluded.
MR imaging analysis
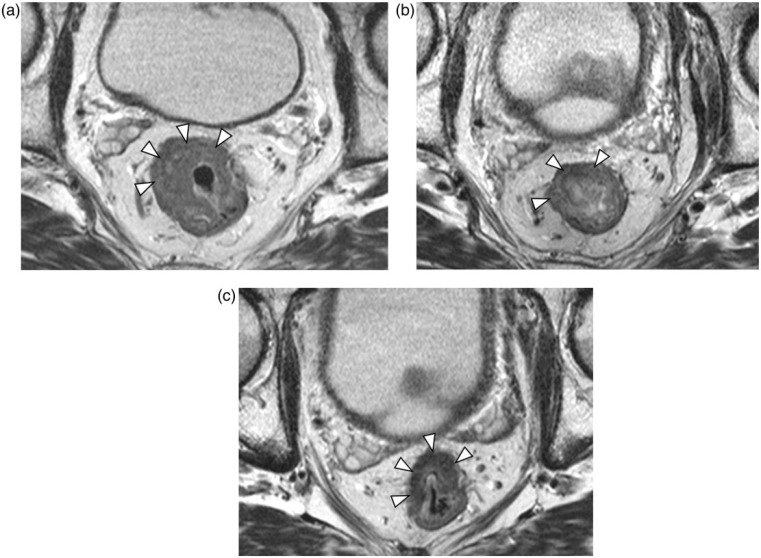
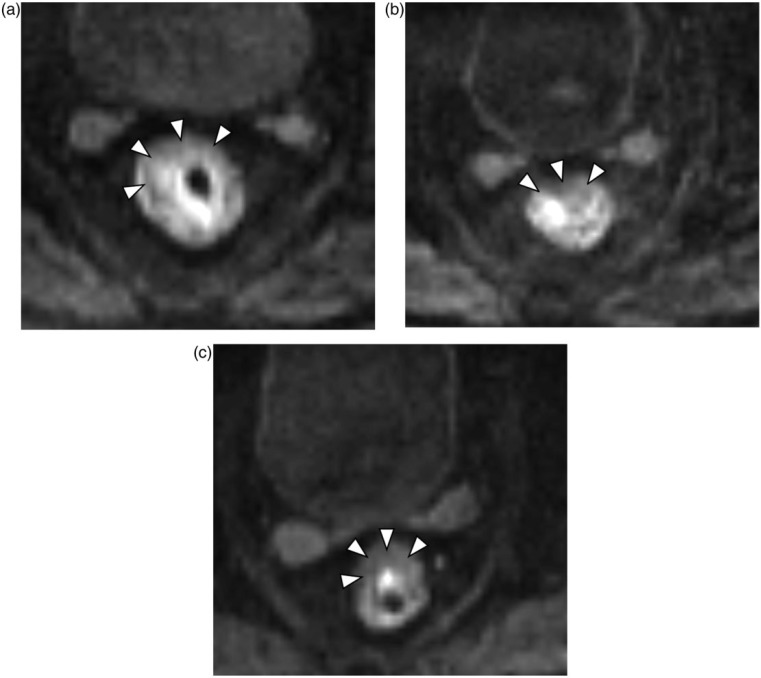
Representative T2-weighted MR images and DW MR images during treatment are shown in Figs. 1a–c and 2a–c. The image quality of DW MR imaging was sufficient to identify the tumor region in all patients.
Figure 1.
Images of 59-year-old man with rectal cancer. Axial fast spin-echo T2-weighted magnetic resonance (MR) imaging (a) pretherapy, (b) at the end of the second week of chemoradiotherapy (CRT), and (c) after CRT and before surgery, showing a volume reduction of 45%. Arrowheads indicate the tumor outline.
Figure 2.
Axial diffusion-weighted (DW) MR images with b = 800 s/mm2, D values showing a narrow range: (a) pretherapy (D = 0.92 × 10−3 mm2/s), (b) at the end of the second week of CRT (D = 1.21 × 10−3 mm2/s), and (c) after CRT and before surgery (D = 1.20 × 10−3 mm2/s). Arrowheads indicate the tumor outline.
MR images were analyzed by 2 radiologists in consensus who were blinded to the therapeutic response. DW imaging values were generated across the entire segmented tumor volume. Therefore, the complete tumor volume was manually segmented (macroscopic necrosis was excluded) on DW-derived b = 800 s/mm2 images and contoured along the edge of the tumor in axial, coronal, and sagittal reconstruction (Fig. 3a–c), section by section using software developed in-house (MITK Diffusion, Version 2011; DKFZ, Heidelberg, Germany).
Figure 3.
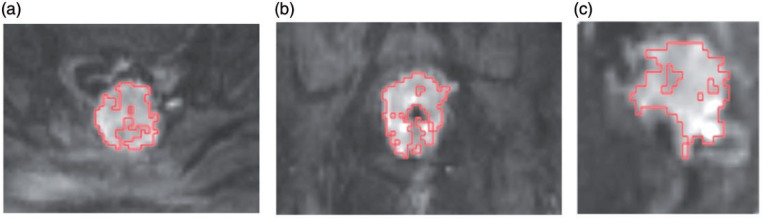
Depiction of the region of interest (ROI) outline. ROI were drawn on DW MR images with a b value of 800 s/mm2. We manually contoured along the edge of the tumor as an ROI, section by section, in thicknesses of 3 mm, in sagittal (a), coronal (b), and transversal (c) orientation.
The thus derived segmented volume of the tumor was compared and adapted with the morphologic T2 data (see rectal MR data) to avoid erroneous tumor segmentation, and the tumor volume was derived from this segmentation. Mean volume, D value, and f value derived across the volume of interest of each patient were used for comparison with the tumor volume and the T-downstaging.
DW imaging-derived parameters were evaluated separately based on the IVIM model,[21] yielding the parameters perfusion fraction f and diffusion constant D, using open-source software developed in-house (MITK Diffusion, Version 2011)[22]. The parameter estimation was based on the assumption that the diffusion measurement is influenced mainly by 2 effects, a perfusion-related effect introduced by the molecules moving in the capillary network (pseudodiffusion coefficient, D*) and extravascular effects of passive diffusion (D). Since a simultaneous nonlinear fit for all parameters D, D*, and the weighting coefficient f can be instable, measurement at b values greater than 170 s/mm2 were used in a first step to estimate f and D as proposed previously[23]. D* was then calculated in a second step by using an exhaustive search. The procedure was implemented on the average measurements inside the manually encompassed tumor volume.
Histopathologic evaluation
After surgery, the resected specimens were staged according to the International Union Against Cancer TNM staging system[24].
Treatment technique
All patients underwent preoperative CRT. Radiotherapy to the pelvis (rectal canal and pelvic lymph nodes) was given in daily doses of 1.8 Gy per fraction to 45 Gy followed by a boost to the tumor bed to a total dose of 50.4 Gy, also in 1.8 Gy per fraction. In addition, chemotherapy was administered as continuous infusion of 5-fluorouracil (300 mg/m2 body surface) over 5 days from Monday to Friday throughout radiation therapy[25]. Four weeks after completion of CRT, patients underwent restaging including local imaging and rectoscopy, followed by TME 2–4 weeks thereafter.
Statistical analysis
To analyze the correlation between the morphologic parameters and the diffusion values, all patients were assigned to one of two groups, the tumor downstaged or the tumor non-downstaged group. Tumor downstaging was assessed by comparing the pre-CRT clinical stage (cT stage) with the postoperative histopathologic stage (ypT stage). T-downstaging was defined when ypT was lower than cT. Values are reported as mean ± standard deviation. Pre- and post-treatment D and f values, and volume, were compared between the downstaged and non-downstaged groups using a t test.
Correlation between volume change and pretherapy D and f values and their changes from pretherapy to the end of the second week were investigated. Temporal changes of volume, D value, and f value were analyzed by evaluating the log of the ratio of the 2 values and performing one-sample t test.
Correlation between volume change and pretherapy D and f values and their changes from pretherapy to the end of the second week were investigated. Correlations were assessed by calculating the Spearman rank coefficient with 95% confidence interval. All statistical analyses were performed using SAS (version 9.2; SAS Institute, Cary, NC, USA). Two-tailed P values were used, and P values less than 0.05 were considered statistically significant.
Results
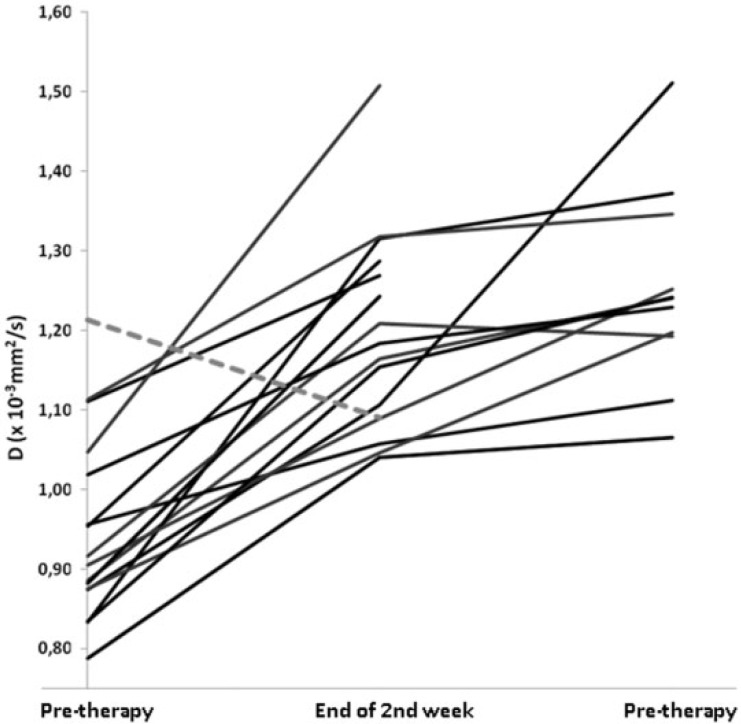
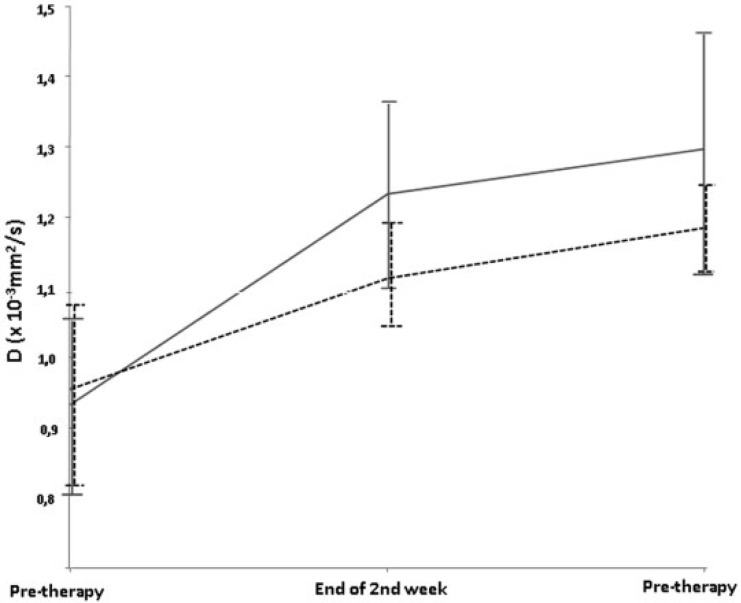
Mean initial diffusion-derived parameters were located in a narrow range (initial D1 = 0.94 ± 0.12 × 10−3 mm2/s). At follow-up, D rose significantly (D2 = 1.18 ± 0.13 × 10−3 mm2/s; P < 0.0001), and continued to increase significantly after CRT (D3 = 1.24 ± 0.14 × 10−3 mm2/s; P < 0.0001 compared with D1). Following surgery, the histopathologic tumor downstaging/non-downstaging ratio was 10:8 patients. There was no complete response among the downstaged tumors. During CRT, the difference in mean tumor D value (Fig. 4) was not significant for all time points (downstaged vs. non-downstaged D1 = 0.93 × 10−3 mm2/s vs. 0.95 × 10−3 mm2/s, P = 0.705; D2 = 1.23 × 10−3 mm2/s vs. 1.11 × 10−3 mm2/s, P = 0.0484; D3 = 1.30 × 10−3 mm2/s vs. 1.18 × 10−3 mm2/s, P = 0.204). No significant different behavior was detected for downstaged versus non-downstaged in increase of D values (P = 0.133 for the increase from initial to under therapy, P = 0.913 for the increase from initial to post-CRT). D values increased early in all but one patient (Fig. 5). This patient did not show other aberrant parameters (e.g., histology, volume response to therapy).
Figure 4.
Time course of D under therapy. The graph shows 18 lines that correspond to the mean tumor D value for each patient’s time course during CRT. All patients but one (dashed line) showed an initial increase in D value.
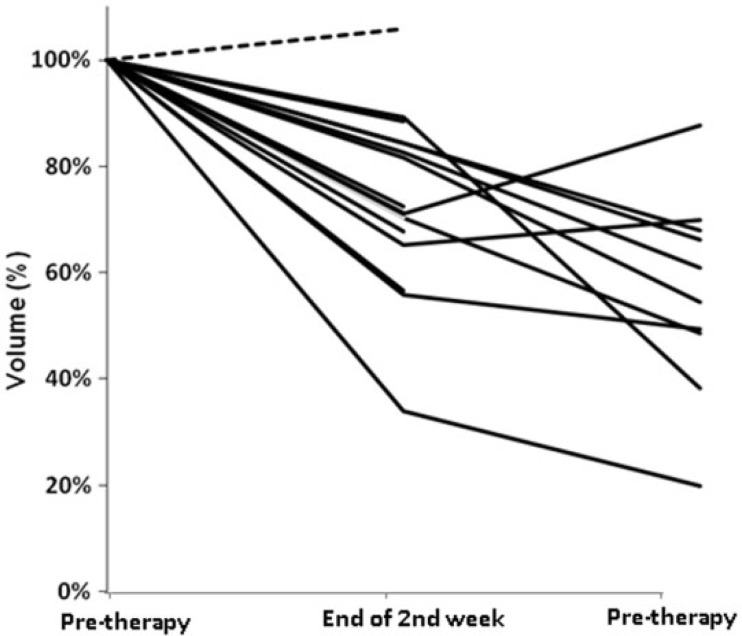
Figure 5.
Time course of volume under therapy. The graph shows 18 lines that correspond to the tumor volume time course during CRT for each patient. All patients but one (dashed line) showed a decrease in tumor volume.
f Values did not show differences over the time course (f1 = 9.4 ± 2.0%, f2 = 9.4 ± 1.7%, f3 = 9.5 ± 2.7%) nor any differences between downstaged and non-downstaged tumors (downstaged vs. non-downstaged f1 = 9.2% vs. 9.4%, P = 0.872; f2 = 9.4% vs. 9.3%, P = 0.840; f3 = 8.8% vs. 10.1%, P = 0.461).
Morphologic imaging-based volumetry showed a decrease in tumor volume in all patients but one (Fig. 6). Mean volume (V) decreased during therapy (V1 = 16,992 ± 13,083 mm3; V2 = 12,793 ± 8317 mm3; V3 = 9718 ± 6154 mm3). There was a significant volume reduction under treatment for downstaged (P = 0.007) and non-downstaged (P = 0.015) tumors. Volume reduction, however, was not significantly different for the 2 groups (P = 0.724). There was no significant difference between the mean tumor volume of the 2 groups before and under treatment (P values for comparison at each time point 0.977, 0.932, and 0.102, respectively). Mean pretreatment tumor volume in the T-downstaged (13,374 mm3) group was lower than that in the non-downstaged group (21,301 mm3), (P = 0.98). The evolution of tumor volume in the 2 groups was not significantly different. There was no correlation between the D and f values and changes in volume (Table 2). The confidence intervals were large, covering relevant correlation values. Hence, it is possible that a larger study would show significant and relevant correlation.
Figure 6.
Mean tumor D value for T-downstaged (gray line) and non-downstaged (dashed black line) during CRT. There was no statistical difference between the 2 groups.
Table 2.
Volume changes correlated with the pretherapy D/f value, and with the early D/f value change at the end of the second week
| Volume correlation parameter | r | 95% CI | P value |
|---|---|---|---|
| Pretherapy D value | −0.38 | −0.81–0.35 | 0.292 |
| Pretherapy f value | 0.02 | −0.62–0.64 | 0.96 |
| Early D value change end of 2nd week | −0.25 | −0.78–0.51 | 0.53 |
| Early f value change end of 2nd week | −0.23 | −0.77–0.52 | 0.56 |
CI, confidence interval.
Discussion
DW MR imaging is a promising tool for the early detection of changes in tumors induced by CRT. However, the current literature shows conflicting results in changes of ADC values under CRT (decreasing vs. increasing values). We applied the IVIM model to further elucidate whether the underlying reason for these divergent reports can be attributed to microcirculation effects, e.g., increased perfusion caused by more pronounced inflammatory reactions. Decreases in diffusion-derived parameters were not observed, and the influence of microperfusion on diffusion values is negligible, since f did not change under therapy. Our data support the view that under effective therapy an increase in ADC is observed, potentially reflecting the loss of cell density resulting from CRT[26] and micronecrosis (as macroscopic necrosis was largely excluded).
In our study we found significantly increasing D values in rectal carcinoma under chemotherapy and, simultaneously, a significant decrease in tumor volume. The evolution of diffusion values under CRT are in line with the results published by Sun et al.[13]. The conflicting reports of decreasing diffusion values as published by de Vries et al.[5] and Dzik-Jurasz et al.[17] could not be confirmed by the present study. DW imaging is sensitive to microscopic translational motion of water molecules that occur in each voxel whereby signal attenuation is influenced not only by diffusion processes but also by perfusion and other kinds of motion, summarized as IVIM. Therefore, when only one b value is used and the ADC is calculated, high ADCs under therapy may reflect a combination of increased perfusion and reduction of microstructure[27,28]. Thus, we investigated whether the contradictory results in ADC values could be due to strong radiation-induced inflammatory reactions and a resulting increase in f. We generally found a low perfusion fraction and, furthermore, f did not change significantly during therapy. Therefore the decrease in ADC values published by the groups of Hein, de Vries, and Dzik-Jurasz are unlikely to be related to microperfusion changes in the tumors, but it remains unclear as to why these early reports published results different from those in both our present study and another[13]. We found a relatively low perfusion fraction at baseline and no changes during follow-up examinations. In a previous report using dynamic T1-weighted contrast-enhanced maps of rectal carcinoma under CRT[29], the perfusion also remained constant at 2 and 3 weeks after therapy. However, in this study a slight but significant increase in perfusion was found after 1 week. We did not acquire such an early time point, but our current data indicate that a similar change in f at such a time point is unlikely. Nonetheless, perfusion data derived from DW imaging has to be addressed with a certain care. A recent report shows that the perfusion-related parameters especially suffer from poor reproducibility[30]. In contrast to this study that performed a voxel-based fit of the data, we performed a fit based on the total volume of interest (VOI), thus increasing the fit stability.
A significant increase of D values for T-downstaged as well as non-downstaged tumors was found. As shown in animal studies and in human tumors[26,31,32], this response occurs within days of initiating therapy and appears to be a relatively common response to therapy, regardless of pathologic categorization of the tumor.
Tumor downstaging in rectal carcinoma is not defined by volume decrease but by transgression of the tumor beyond an anatomic margin. Therefore, tumors that do show a volume reduction under CRT may not be T-downstaged. The T stage thus may be influenced predominantly by larger initial size rather than a different biological behavior; therefore, differences in changes in diffusion values under therapy between downstaged and non-downstaged tumors may not be expected. Contrary to our results, in the study by Sun et al.[13] a significant increase in ADC was found only for the T-downstaged group, although similar volume reduction rates were reported for both groups. They postulated that sensitivity to chemoradiotherapy was higher in the downstaged group; however, we feel that their volumetric data seem to be discordant with the diffusion-derived response rate, since the volume reduction seems to be constant and similar for both groups after the first follow-up.
Comparable with the results of Sun et al.[13], a significant volume reduction rate for T-downstaged and non-downstaged patients was shown in our study. A difference in initial ADC as found by Sun et al. could not be reproduced. Their findings are interpreted as resulting from stronger inflammatory reactions in the T-downstaged group, but again we feel that if there would have been a different biological reaction to therapy, the expected reduction rates of tumor volume should also have been different. Furthermore, in the study by Sun et al., non-downstaged patients presented with a higher initial volume than the downstaged group, what could have led to a bias in data analysis.
Hence, our results indicate that quantitative diffusion imaging may not be useful to differentiate between responders and nonresponders with rectal carcinoma after neoadjuvant CRT when response is defined as T-downstaging. When response is defined as volume reduction, DW imaging-derived measures are concordant with volumetric measurements.
Tumor volume measurement is widely used for antitumor therapy response, including rectal carcinoma[33]. However, its applicability in clinical routine is limited, as the rectum is a hollow organ with irregular morphology. The correlation between rectal tumor histopathologic downstaging after CRT and tumor volume reduction remains controversial[12,34,35]. The influence of T-downstaging on surgical treatment techniques is important, but the validation of T-downstaging as surrogate end point for long-term clinical outcome (overall survival and local control) in preoperative T3/T4 rectal cancer trials did not show any correlation[36]. Therefore, the T-downstaging may not have a strong relationship with the success of surgery or patient outcome. Curvo-Semedo et al.[4] analyzed the relationship between tumoral ADC and histologic prognostic parameters, and found an aggressive histologic tumor profile to correlate with lower initial ADC values. Using the ADC, several groups showed promising results for better differentiation between post-therapeutic scar tissue (representing complete remission) and residual tumor in patients 1 month or more after completion of neoadjuvant CRT[9,37].
This study has several limitations. First, the small sample size could have influenced the statistical results. However, if significant differences in diffusion values are not reproducible in smaller collectives, the additional value of diffusion data in clinical routine is limited. Although our VOI-based approach reduces the instability and, thus, the variance of parameter estimation, there is still a possibility that results may have been affected by the fact that MR images were analyzed by 2 radiologists in consensus without statistical tests of reproducibility. The aim of CRT is to render tumor regression so as to achieve a tumor-free resection margin[1]. Patients included in our study showed mostly advanced-stage disease with tumor involvement of the radial circumferential resection margins, and often not showing tumor regression after CRT from the mesorectal fascia, which could also proffer some bias. We tried to avoid slice-selection bias by segmenting the complete tumor volume, including only viable tumor, and excluding macroscopic necrosis. The identification of tumor downstaging in this study was based on a comparison between initial clinical T-staging and postoperative pathohistologic T-staging. This could have induced an inadvertent bias, because the clinical tumor stage pre-CRT would have been underestimated or overestimated in view of the inherent limitations of MR imaging and ultrasonography[38,39].
Conclusion
Applying the IVIM model, we were able to show that divergent results in the literature considering ADC changes in rectal carcinoma under CRT for patients showing T-downstaging are unlikely to be attributed to microperfusion effects. The microperfusion fraction f in rectal carcinoma under CRT is rather low and remains constant over time. Our data support the view that under effective therapy, an increase in D is observed.
Acknowledgements
The authors thank the DFG (Deutsche Forschungsgesellschaft) for their support (GA 765/2-1).
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Schmiegel W, Reinacher-Schick A, Arnold D, et al. [Update S3-guideline “colorectal cancer” 2008] Z Gastroenterol. 2008;46:799–840. doi: 10.1055/s-2008-1027726. [DOI] [PubMed] [Google Scholar]
- 2.Berger C, de Muret A, Garaud P, et al. Preoperative radiotherapy (RT) for rectal cancer: predictive factors of tumor downstaging and residual tumor cell density (RTCD): prognostic implications. Int J Radiat Oncol Biol Phys. 1997;37:619–627. doi: 10.1016/s0360-3016(96)00577-9. [DOI] [PubMed] [Google Scholar]
- 3.Janjan NA, Crane CN, Feig BW, et al. Prospective trial of preoperative concomitant boost radiotherapy with continuous infusion 5-fluorouracil for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2000;47:713–718. doi: 10.1016/s0360-3016(00)00418-1. [DOI] [PubMed] [Google Scholar]
- 4.Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG. Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging. 2012;35:1365–1371. doi: 10.1002/jmri.23589. [DOI] [PubMed] [Google Scholar]
- 5.DeVries AF, Kremser C, Hein PA, et al. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:958–965. doi: 10.1016/s0360-3016(03)00208-6. [DOI] [PubMed] [Google Scholar]
- 6.Hein PA, Kremser C, Judmaier W, et al. Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study. Eur J Radiol. 2003;45:214–222. doi: 10.1016/s0720-048x(02)00231-0. [DOI] [PubMed] [Google Scholar]
- 7.Hein PA, Kremser C, Judmaier W, et al. [Diffusion-weighted MRI—a new parameter for advanced rectal carcinoma?] Rofo. 2003;175:381–386. doi: 10.1055/s-2003-37836. [DOI] [PubMed] [Google Scholar]
- 8.Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology. 2004;232:335–346. doi: 10.1148/radiol.2322021326. [DOI] [PubMed] [Google Scholar]
- 9.Lambregts DM, Vandecaveye V, Barbaro B, et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011;18:2224–2231. doi: 10.1245/s10434-011-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371–377. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 11.Brown G, Richards CJ, Newcombe RG, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211:215–222. doi: 10.1148/radiology.211.1.r99ap35215. [DOI] [PubMed] [Google Scholar]
- 12.Barbaro B, Fiorucci C, Tebala C, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250:730–739. doi: 10.1148/radiol.2503080310. [DOI] [PubMed] [Google Scholar]
- 13.Sun YS, Zhang XP, Tang L, et al. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology. 2010;254:170–178. doi: 10.1148/radiol.2541082230. [DOI] [PubMed] [Google Scholar]
- 14.Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3:1457–1466. [PubMed] [Google Scholar]
- 15.Thoeny HC, De Keyzer F, Chen F, et al. Diffusion-weighted MR imaging in monitoring the effect of a vascular targeting agent on rhabdomyosarcoma in rats. Radiology. 2005;234:756–764. doi: 10.1148/radiol.2343031721. [DOI] [PubMed] [Google Scholar]
- 16.Kremser C, Judmaier W, Hein P, Griebel J, Lukas P, de Vries A. Preliminary results on the influence of chemoradiation on apparent diffusion coefficients of primary rectal carcinoma measured by magnetic resonance imaging. Strahlenther Onkol. 2003;179:641–649. doi: 10.1007/s00066-003-1045-9. [DOI] [PubMed] [Google Scholar]
- 17.Dzik-Jurasz A, Domenig C, George M, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307–308. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 18.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 19.Lemke A, Stieltjes B, Schad LR, Laun FB. Toward an optimal distribution of b values for intravoxel incoherent motion imaging. Magn Reson Imaging. 2011;29:766–776. doi: 10.1016/j.mri.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Luciani A, Vignaud A, Cavet M, et al. Liver cirrhosis: intravoxel incoherent motion MR imaging—pilot study. Radiology. 2008;249:891–899. doi: 10.1148/radiol.2493080080. [DOI] [PubMed] [Google Scholar]
- 21.Lemke A, Laun FB, Simon D, Stieltjes B, Schad LR. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn Reson Med. 2010;64:1580–1585. doi: 10.1002/mrm.22565. [DOI] [PubMed] [Google Scholar]
- 22.Fritzsche KH, Neher PF, Reicht I, et al. MITK diffusion imaging. Methods Inf Med. 2012;51:441–448. doi: 10.3414/ME11-02-0031. [DOI] [PubMed] [Google Scholar]
- 23.Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. J Magn Reson Imaging. 2010;31:589–600. doi: 10.1002/jmri.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood DA. Clinical staging and end results classification: TNM system of clinical classification as applicable to carcinoma of the colon and rectum. Cancer. 1971;28:109–114. doi: 10.1002/1097-0142(197107)28:1<109::aid-cncr2820280120>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg R, Nekarda H, Zimmermann F, et al. Histopathological response after preoperative radiochemotherapy in rectal carcinoma is associated with improved overall survival. J Surg Oncol. 2008;97:8–13. doi: 10.1002/jso.20844. [DOI] [PubMed] [Google Scholar]
- 26.Theilmann RJ, Borders R, Trouard TP, et al. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6:831–837. doi: 10.1593/neo.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klauss M, Lemke A, Grunberg K, et al. Intravoxel incoherent motion MRI for the differentiation between mass forming chronic pancreatitis and pancreatic carcinoma. Invest Radiol. 2011;46:57–63. doi: 10.1097/RLI.0b013e3181fb3bf2. [DOI] [PubMed] [Google Scholar]
- 28.Pahernik S, Griebel J, Botzlar A, et al. Quantitative imaging of tumour blood flow by contrast-enhanced magnetic resonance imaging. Br J Cancer. 2001;85:1655–1663. doi: 10.1054/bjoc.2001.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vries A, Griebel J, Kremser C, et al. Monitoring of tumor microcirculation during fractionated radiation therapy in patients with rectal carcinoma: preliminary results and implications for therapy. Radiology. 2000;217:385–391. doi: 10.1148/radiology.217.2.r00nv02385. [DOI] [PubMed] [Google Scholar]
- 30.Andreou A, Koh DM, Collins DJ, et al. Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. Eur Radiol. 2013;23:428–434. doi: 10.1007/s00330-012-2604-1. [DOI] [PubMed] [Google Scholar]
- 31.Galons JP, Altbach MI, Paine-Murrieta GD, Taylor CW, Gillies RJ. Early increases in breast tumor xenograft water mobility in response to paclitaxel therapy detected by non-invasive diffusion magnetic resonance imaging. Neoplasia. 1999;1:113–117. doi: 10.1038/sj.neo.7900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jennings D, Hatton BN, Guo J, et al. Early response of prostate carcinoma xenografts to docetaxel chemotherapy monitored with diffusion MRI. Neoplasia. 2002;4:255–262. doi: 10.1038/sj.neo.7900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carbone SF, Pirtoli L, Ricci V, et al. Diffusion-weighted MR volumetry for assessing the response of rectal cancer to combined radiation therapy with chemotherapy. Radiology. 2012;263:311. doi: 10.1148/radiol.12112454. [DOI] [PubMed] [Google Scholar]
- 34.Kim YH, Kim DY, Kim TH, et al. Usefulness of magnetic resonance volumetric evaluation in predicting response to preoperative concurrent chemoradiotherapy in patients with resectable rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:761–768. doi: 10.1016/j.ijrobp.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Kim NK, Baik SH, Min BS, et al. A comparative study of volumetric analysis, histopathologic downstaging, and tumor regression grade in evaluating tumor response in locally advanced rectal cancer following preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2007;67:204–210. doi: 10.1016/j.ijrobp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Methy N, Bedenne L, Conroy T, et al. Surrogate end points for overall survival and local control in neoadjuvant rectal cancer trials: statistical evaluation based on the FFCD 9203 trial. Ann Oncol. 2010;21:518–524. doi: 10.1093/annonc/mdp340. [DOI] [PubMed] [Google Scholar]
- 37.Jang KM, Kim SH, Choi D, Lee SJ, Park MJ, Min K. Pathological correlation with diffusion restriction on diffusion-weighted imaging in patients with pathological complete response after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: preliminary results. Br J Radiol. 2012;85:e566–e572. doi: 10.1259/bjr/24557556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh DM, Smith NJ, Swift RI, Brown G. The relationship between MR demonstration of extramural venous invasion and nodal disease in rectal cancer. Clin Med Oncol. 2008;2:267–273. doi: 10.4137/cmo.s370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moran B, Brown G, Cunningham D, et al. Clarifying the TNM staging of rectal cancer in the context of modern imaging and neo-adjuvant treatment: ‘y’ ‘u’ and ‘p’ need ‘mr’ and ‘ct’. Colorectal Dis. 2008;10:242–243. doi: 10.1111/j.1463-1318.2007.01260.x. [DOI] [PubMed] [Google Scholar]