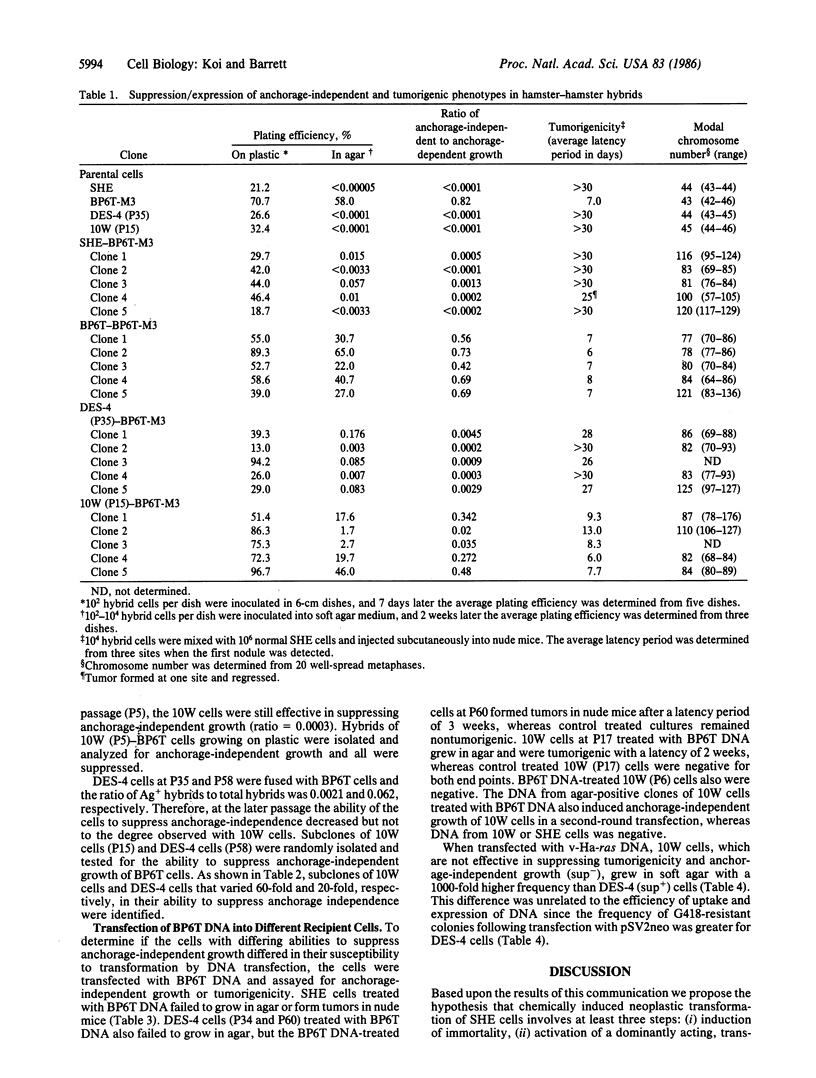
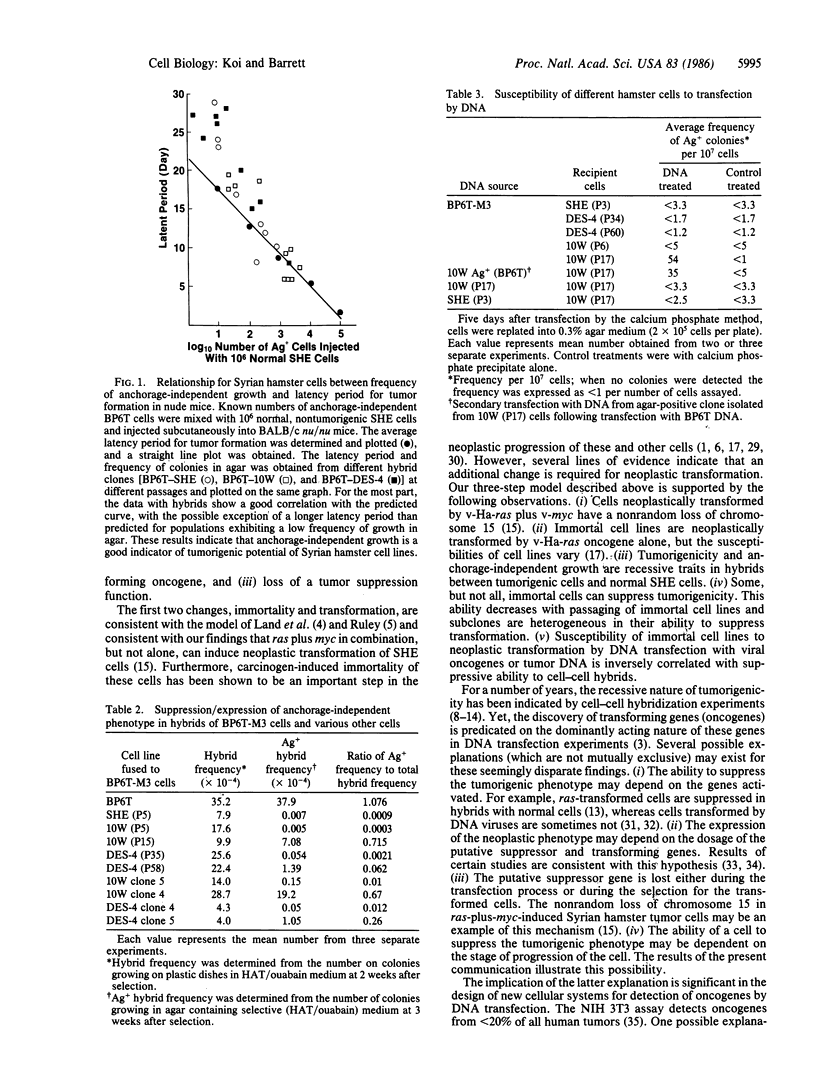
Abstract
Cell hybrids between normal, early-passage Syrian hamster embryo cells and a highly tumorigenic, chemically transformed hamster cell line, BP6T, were formed, selected, and analyzed. Tumorigenicity and anchorage-independent growth were suppressed in the hybrid cells compared to the tumorigenic BP6T cells. These two phenotypes segregated coordinately in these cells. To determine at what stage in the neoplastic process this tumor-suppressive function was lost, two chemically induced immortal cell lines were examined at different passages for the ability to suppress the tumorigenic phenotype of BP6T cells following hybridization. Hybrids of BP6T cells with the immortal, nontumorigenic cell lines at early passages were suppressed for tumorigenicity and anchorage-independent growth. This tumor-suppressive ability was reduced in the same cells at later passages and in some cases nearly completely lost, prior to the neoplastic transformation of the immortal cell lines. Subclones of the cell lines were heterogeneous in their ability to suppress tumorigenicity in cell hybrids; some clones retained the tumor-suppressive ability and others lost this function. The susceptibility to neoplastic transformation of these cells following DNA transfection with the viral ras oncogene or BP6T DNA inversely correlated with the tumor-suppressive ability of the cells. These results suggest that chemically induced neoplastic progression of Syrian hamster embryo cells involves at least three steps: induction of immortality, activation of a transforming oncogene, and loss of a tumor-suppressive function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. C. A preneoplastic stage in the spontaneous neoplastic transformation of syrian hamster embryo cells in culture. Cancer Res. 1980 Jan;40(1):91–94. [PubMed] [Google Scholar]
- Barrett J. C., Crawford B. D., Mixter L. O., Schechtman L. M., Ts'o P. O., Pollack R. Correlation of in vitro growth properties and tumorigenicity of Syrian hamster cell lines. Cancer Res. 1979 May;39(5):1504–1510. [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Evidence for the progressive nature of neoplastic transformation in vitro. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3761–3765. doi: 10.1073/pnas.75.8.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict W. F., Weissman B. E., Mark C., Stanbridge E. J. Tumorigenicity of human HT1080 fibrosarcoma X normal fibroblast hybrids: chromosome dosage dependency. Cancer Res. 1984 Aug;44(8):3471–3479. [PubMed] [Google Scholar]
- Bouck N., Head M. The majority of independently transformed BHK cell clones share a single functional lesion which determines anchorage independence and influences tumorigenicity. In Vitro Cell Dev Biol. 1985 Aug;21(8):463–469. doi: 10.1007/BF02620835. [DOI] [PubMed] [Google Scholar]
- Bouck N., di Mayorca G. Chemical carcinogens transform BHK cells by inducing a recessive mutation. Mol Cell Biol. 1982 Feb;2(2):97–105. doi: 10.1128/mcb.2.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. W., Sager R. Suppression of tumorigenicity in hybrids of normal and oncogene-transformed CHEF cells. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2062–2066. doi: 10.1073/pnas.82.7.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Aden D., Koprowski H. Somatic cell hybrids between mouse peritoneal macrophages and simian-virus-40-transformed human cells: II. Presence of human chromosome 7 carrying simin virus 40 genome in cells of tumors induced by hybrid cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1397–1400. doi: 10.1073/pnas.72.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer T. M., Annab L. A., Oshimura M., Barrett J. C. Neoplastic transformation of normal and carcinogen-induced preneoplastic Syrian hamster embryo cells by the v-src oncogene. Mol Cell Biol. 1985 Jul;5(7):1707–1713. doi: 10.1128/mcb.5.7.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H., Miller O. J., Klein G., Worst P., Tachibana T. Suppression of malignancy by cell fusion. Nature. 1969 Jul 26;223(5204):363–368. doi: 10.1038/223363a0. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Dependence of asbestos- and mineral dust-induced transformation of mammalian cells in culture on fiber dimension. Cancer Res. 1984 May;44(5):2170–2180. [PubMed] [Google Scholar]
- Kaplan P. L., Ozanne B. Cellular responsiveness to growth factors correlates with a cell's ability to express the transformed phenotype. Cell. 1983 Jul;33(3):931–938. doi: 10.1016/0092-8674(83)90036-3. [DOI] [PubMed] [Google Scholar]
- Klinger H. P. Suppression of tumorigenicity in somatic cell hybrids. I. Suppression and reexpression of tumorigenicity in diploid human X D98AH2 hybrids and independent segregation of tumorigenicity from other cell phenotypes. Cytogenet Cell Genet. 1980;27(4):254–266. doi: 10.1159/000131494. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lester S. C., LeVan S. K., Steglich C., DeMars R. Expression of human genes for adenine phosphoribosyltransferase and hypoxanthine-guanine phosphoribosyltransferase after genetic transformation of mouse cells with purified human DNA. Somatic Cell Genet. 1980 Mar;6(2):241–259. doi: 10.1007/BF01538799. [DOI] [PubMed] [Google Scholar]
- McLachlan J. A., Wong A., Degen G. H., Barrett J. C. Morphologic and neoplastic transformation of Syrian hamster embryo fibroblasts by diethylstilbestrol and its analogs. Cancer Res. 1982 Aug;42(8):3040–3045. [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W., Connell J. R. Induction of immortality is an early event in malignant transformation of mammalian cells by carcinogens. Nature. 1982 Oct 14;299(5884):633–635. doi: 10.1038/299633a0. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983 Aug 18;304(5927):648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- Oshimura M., Gilmer T. M., Barrett J. C. Nonrandom loss of chromosome 15 in Syrian hamster tumours induced by v-Ha-ras plus v-myc oncogenes. Nature. 1985 Aug 15;316(6029):636–639. doi: 10.1038/316636a0. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Evidence for the recessive nature of cellular immortality. Science. 1983 Sep 2;221(4614):964–966. doi: 10.1126/science.6879195. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation: a new frontier in cancer research. Cancer Res. 1986 Apr;46(4 Pt 1):1573–1580. [PubMed] [Google Scholar]
- Santos E., Martin-Zanca D., Reddy E. P., Pierotti M. A., Della Porta G., Barbacid M. Malignant activation of a K-ras oncogene in lung carcinoma but not in normal tissue of the same patient. Science. 1984 Feb 17;223(4637):661–664. doi: 10.1126/science.6695174. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J. Suppression of malignancy in human cells. Nature. 1976 Mar 4;260(5546):17–20. doi: 10.1038/260017a0. [DOI] [PubMed] [Google Scholar]
- Thomassen D. G., Gilmer T. M., Annab L. A., Barrett J. C. Evidence for multiple steps in neoplastic transformation of normal and preneoplastic Syrian hamster embryo cells following transfection with Harvey murine sarcoma virus oncogene (v-Ha-ras). Cancer Res. 1985 Feb;45(2):726–732. [PubMed] [Google Scholar]
- Tsunokawa Y., Esumi H., Sasaki M. S., Mori M., Sakamoto H., Terada M., Sugimura T. Integration of v-rasH does not necessarily transform an immortalized murine cell line. Gan. 1984 Sep;75(9):732–736. [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Weissman B. E., Stanbridge E. J. Complexity of control of tumorigenic expression in intraspecies hybrids of human SV40-transformed fibroblasts and normal human fibroblast cell lines. Cytogenet Cell Genet. 1983;35(4):263–268. doi: 10.1159/000131878. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Rabinowitz Z., Sachs L. Identification of the chromosomes that control malignancy. Nat New Biol. 1973 Jun 20;243(129):247–250. doi: 10.1038/newbio243247a0. [DOI] [PubMed] [Google Scholar]



