Abstract
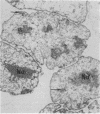
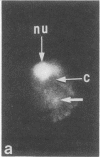
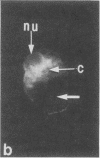
Quantitative flow microfluorometry of neoglycoprotein (bovine serum albumin coupled to sugar and to fluorescein) binding demonstrated the existence of sugar-binding sites (i.e., lectin-like molecules) in isolated BHK cell nuclei. The very similar labeling intensities obtained with nuclei isolated by cell lysis and with permeabilized karyoplasts obtained by enucleation strengthened the idea that the binding sites are borne by actual nuclear structures and not by cytoplasmic or membrane-derived contaminants. With both nuclei-isolation procedures, neoglycoproteins (containing similar numbers of sugar residues) used as markers can be similarly classified. Fluorescence microscopy further indicated that in both nuclear preparations, the neoglycoprotein binding sites were associated with the nucleoli as well as with nucleoplasmic ribonucleoprotein elements. Nuclei from exponentially growing cells bound much greater amounts of neoglycoprotein than did nuclei from contact-inhibited cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Barondes S. H. Chicken tissue binding sites for a purified chicken lectin. J Supramol Struct. 1980;13(2):219–227. doi: 10.1002/jss.400130210. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P., Basu S. Probable involvement of a glycoconjugate in IMR-32 DNA synthesis: decrease of DNA polymerase alpha 2 activity after tunicamycin treatment. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1488–1491. doi: 10.1073/pnas.79.5.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Simet I., Basu S. Inhibition of human neuroblastoma DNA polymerase activities by plant lectins and toxins. Proc Natl Acad Sci U S A. 1979 May;76(5):2218–2221. doi: 10.1073/pnas.76.5.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hubert J., Seve A. P., Bouvier D., Masson C., Bouteille M., Monsigny M. In situ ultrastructural localization of sugar-binding sites in lizard granulosa cell nuclei. Biol Cell. 1985;55(1-2):15–20. doi: 10.1111/j.1768-322x.1985.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. Lectins as molecules and as tools. Annu Rev Biochem. 1986;55:35–67. doi: 10.1146/annurev.bi.55.070186.000343. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bhorjee J. S. Isolation and characterization of nuclei and purification of chromatin from differentiating cultures of rat skeletal muscle. Exp Cell Res. 1982 Jan;137(1):141–154. doi: 10.1016/0014-4827(82)90016-7. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Midoux P. Uptake of neoglycoproteins via membrane lectin(s) of L1210 cells evidenced by quantitative flow cytofluorometry and drug targeting. Biol Cell. 1984;51(2):187–196. doi: 10.1111/j.1768-322x.1984.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Sene C., Maget-Dana R., Delmotte F. Sugar-lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur J Biochem. 1980 Feb;104(1):147–153. doi: 10.1111/j.1432-1033.1980.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E. Role of monosaccharides in the interaction of two lymphocyte mediators with their target cells. J Immunol. 1976 Mar;116(3):816–820. [PubMed] [Google Scholar]
- Seve A. P., Hubert J., Bouvier D., Bouteille M., Maintier C., Monsigny M. Detection of sugar-binding proteins in membrane-depleted nuclei. Exp Cell Res. 1985 Apr;157(2):533–538. doi: 10.1016/0014-4827(85)90138-7. [DOI] [PubMed] [Google Scholar]
- Seve A. P., Hubert J., Bouvier D., Masson C., Geraud G., Bouteille M. In situ distribution in different cell types of nuclear glycoconjugates detected by two lectins. J Submicrosc Cytol. 1984 Oct;16(4):631–641. [PubMed] [Google Scholar]
- Townsend R., Stahl P. Isolation and characterization of a mannose/N-acetylglucosamine/fucose-binding protein from rat liver. Biochem J. 1981 Jan 15;194(1):209–214. doi: 10.1042/bj1940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M. H., Weinstein I. B. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):669–674. doi: 10.1016/s0006-291x(75)80436-0. [DOI] [PubMed] [Google Scholar]