Abstract
Pharmaceuticals in the environment have received increased attention over the past decade, as they are ubiquitous in rivers and waterways. Concentrations are in sub-ng to low μg/L, well below acute toxic levels, but there are uncertainties regarding the effects of chronic exposures and there is a need to prioritise which pharmaceuticals may be of concern. The read-across hypothesis stipulates that a drug will have an effect in non-target organisms only if the molecular targets such as receptors and enzymes have been conserved, resulting in a (specific) pharmacological effect only if plasma concentrations are similar to human therapeutic concentrations. If this holds true for different classes of pharmaceuticals, it should be possible to predict the potential environmental impact from information obtained during the drug development process. This paper critically reviews the evidence for read-across, and finds that few studies include plasma concentrations and mode of action based effects. Thus, despite a large number of apparently relevant papers and a general acceptance of the hypothesis, there is an absence of documented evidence. There is a need for large-scale studies to generate robust data for testing the read-across hypothesis and developing predictive models, the only feasible approach to protecting the environment.
The Read-Across Hypothesis
The issue of pharmaceuticals in the environment has come to the fore in recent years with the realization that these drugs, designed to act at specific mammalian targets, may have effects in non-target organisms provided that the molecular target (usually a receptor or enzyme) is conserved. This so-called “Read-Across Hypothesis” was first articulated by Huggett et al.,1 but the concept of target conservation between vertebrate groups had been recognized previously.2 The hypothesis also presumes that a specific interaction between a drug and a target will result in a pharmacological response before a toxicological one, and will require a plasma concentration similar to that necessary to see a pharmacological effect in humans. If this hypothesis is correct, it should be possible to use information derived from the drug development process, such as molecular target, mode of action, effective dose and physicochemical properties, together with genomic and physiological knowledge of the non-target organism, to predict the likelihood of an effect occurring, assuming relevant exposure.3 This concept is not new; the well-established use of rodents in the drug development process relies on the similarity of drug targets and physiology with humans. The use of model organisms such as Danio rerio (zebrafish) in drug screening4−6 indicates that it is possible to “read up” from a lower vertebrate such as a fish to humans, and our increased knowledge of the genomes and physiology of fish has confirmed that they are in many respects very similar to mammals, including the presence of drug targets and similar responses.7,8 It should be noted that this hypothesis relates to biological read-across, as opposed to the chemical read-across approach used to predict toxicity from untested chemicals of the same class,9,10 or indeed addressing general toxicity from a battery of tests.11
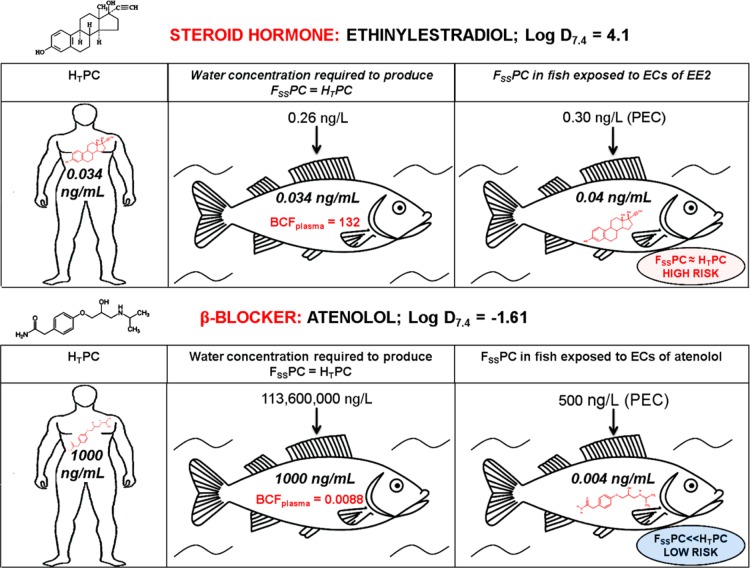
The theoretical Fish Plasma Model presented by Huggett et al.1 aims to assess whether a pharmaceutical present in the environment is likely to have an effect on fish (Figure 1). The model compares the human therapeutic plasma concentration (Cmax) of a drug to a predicted steady state concentration in fish, calculated using Log Kow (octanol:water partition coefficient, a measure of lipophilicity) and environmental concentration (measured or predicted). The closer the predicted fish plasma concentration is to Cmax, the greater the likelihood that a pharmacological effect will be seen, although it should be noted that Log Kow is affected by the pKa and may therefore not give an accurate estimate for ionogenic compounds. There are over 3000 human pharmaceuticals in general use, and many of these have been detected in surface waters and sewage treatment work effluents.12−14 Thus, aquatic organisms may be continually exposed to a cocktail of drugs, in contrast to the transient nature of human drug exposure. It is unrealistic to assess each drug experimentally,15 and although environmental concentrations are low (in the ng/L to low μg/L range), it cannot be presumed that no effects will occur (Figure 1). Experimental validation of the Fish Plasma Model will provide a framework for identifying the drugs that are of most concern.15 The past decade has seen a proliferation of studies relating to effects of pharmaceuticals on fish and other organisms, although few of these have explicitly tested the read-across hypothesis.
Figure 1.
Application of the Fish Plasma Model1 to two pharmaceuticals, ethinylestradiol (EE2) and atenolol. The model compares the “Human Therapeutic Plasma Concentration” (HTPC) and the predicted “Fish Steady-State Plasma Concentration” (FSSPC). If HTPC ≈ FSSPC, the risk of a pharmaceutical having pharmacological effects in fish is high. EE2 at environmentally relevant concentrations (i.e., PEC) will produce FSSPC ≈ HTPC, indicating a high risk of pharmacological effects occurring. Atenolol, which is highly hydrophilic, does not bioaccumulate to a significant extent, resulting in FSSPC ≪ HTPC and therefore no effect is expected. EC = environmental concentration.
This review will provide a critical commentary of the extent to which these studies, mostly concerned with human pharmaceuticals, have validated the read-across hypothesis in general, and the Fish Plasma Model in particular. It will also highlight many of the factors that influence the applicability of the hypothesis. We will focus mainly on fish, as these are recognized as obvious indicators of aquatic contamination, but the increasing evidence for extending the read-across concept to invertebrates, and even plants, will also be considered. It is beyond the scope of this review to discuss test substance concentrations in tissues other than plasma, as this would not add to the evidence-base for the read-across hypothesis, given that tissue concentrations are not reported in relation to pharmacological effects in humans.
Target Conservation
The evolutionary conservation of the primary drug target is a prerequisite for the read-across hypothesis, and the key paper by Gunnarsson et al.16 demonstrated the power of in silico analysis in this regard. They looked at 1318 human drug targets across 16 species, and found 86% to be conserved in zebrafish, 61% in Daphnia pulex (water flea) and 35% in Chlamydomonas reinhardtii (green algae), indicating the potential for lower vertebrates, and even invertebrates and plants, to respond to pharmaceuticals present in the environment. Interestingly, enzymes were especially well conserved across all species, whereas receptors were not.16 This suggests that drugs targeting enzymes may potentially affect a greater number of species than a drug targeting a receptor, although there are insufficient studies to date to provide convincing evidence for this in practice.
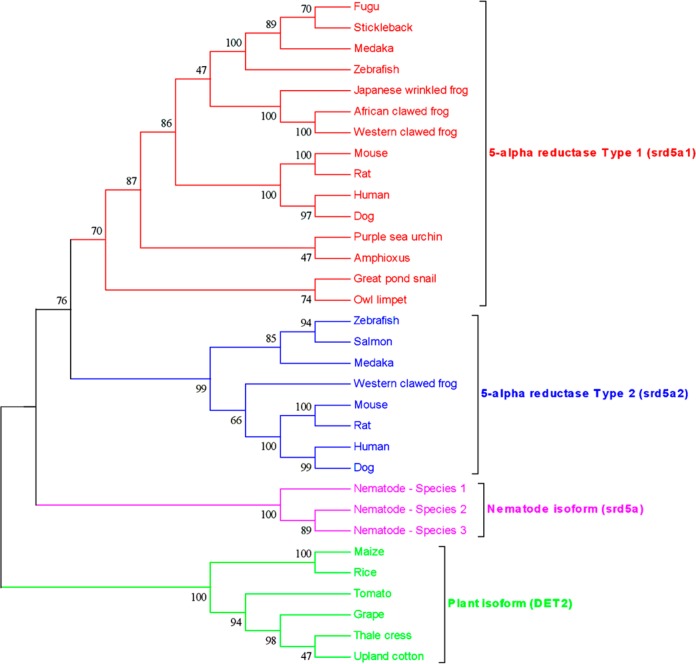
The increasing knowledge of gene sequences from a range of species should facilitate our predictive capacity for assessing target conservation. For example, 5α-reductase, the enzyme converting testosterone to its more potent metabolite dihydrotestosterone, has only recently been identified and characterized in fish (Pimephales promelas, fathead minnow),17 and phylogenetic analysis suggests homologues to be present in molluscs and nematodes, as well as plants (e.g., Arabidopsis) (Figure 2). The Arabidopsis homologue, DET2, is particularly interesting, as it is known to play a role in light-regulated development, and is inhibited by 4-MA, one of the 4-azasteroids which are potent inhibitors of mammalian 5α-reductase.20,21 Thus, 5α-reductase inhibitors used to treat benign prostatic hyperplasia and male-pattern baldness, if present in the aquatic environment, could potentially affect not just fish,22 but a range of aquatic organisms, including plants.
Figure 2.
Phylogenetic tree of the enzymes 5-alpha reductase Type 1 (srd5a1) and Type 2 (srd5a2) from 25 species ranging from human to plants. The two enzymes represent the primary targets of the pharmaceutical dutasteride. The phylogenetic tree was inferred using the Neighbor-Joining method. Numbers at nodes represent bootstrap values (%, 1000 replicates). The evolutionary distances were computed using the Jones–Thornton–Taylor (JTT) matrix-based method. Amino acid sequences were aligned using MUSCLE18 and the evolutionary analyses were conducted in MEGA5.19 The sequences and their accession numbers are detailed in Supporting Information Table S1.
However, the potential complexity in crossing species boundaries should not be underestimated; for example, many receptors exist in multiple subtypes. Our understanding of the differential regulation and functional roles of receptor subtypes is often incomplete, and therefore the possibility that a drug may interact with any subtype present, irrespective of the specificity displayed in humans, cannot be discounted. Thus, the apparent absence of a particular subtype in a species does not mean that a specifically targeted drug will have no mode of action effect. It is also possible that a single receptor in a lower vertebrate fulfils the roles of several receptor subtypes in humans.
Little attention is usually paid to the conservation of secondary targets, such as proteins in the pathway downstream of the primary target, which is an implicit requirement for the read-across hypothesis to be fully valid. However, the importance of establishing this may be questionable, as generally an absence of the primary therapeutic effect would indicate that the particular pathway has not been conserved. A greater issue, therefore, is a lack of knowledge regarding the physiology of organisms such as fish at the level of, for example, receptor signaling. If we do not understand the particular role of a receptor in the normal physiology of an organism, that is, the consequences of its activation or inhibition, it becomes unclear whether effects seen following exposure to a particular pharmaceutical are due to specific effects caused at a particular target, a side-effect, or indeed a toxic effect. Incidentally, the biochemical, metabolic and cell signaling pathways are often better understood in invertebrates such as Drosophila and Caenorhabditis elegans, but the sequence conservation of drug targets is generally lower (38–42%).16
An understanding of what is a side effect and what is a secondary consequence of the primary drug action is also required; thus, the cardiovascular risk associated with cyclo-oxygenase (Cox) enzyme inhibition is a direct pharmacological consequence of inhibiting Cox 2, and not due to a side effect of nonsteroidal anti-inflammatory drugs (NSAIDs).23 Given our lack of knowledge of how physiological responses are linked to specific targets in many nonmammalian organisms, this adds to the complexity in interpreting exposure data. Our (in)ability to recognize effects, let alone side-effects, in non-target organisms will also compromise our ability to validate the read-across hypothesis. We currently do not have the validated techniques to assess if many drugs are affecting fish, let alone lower vertebrates, invertebrates or plants. Further, high variability in specific physiological endpoints often makes realistic study design impossible, or analysis prohibitively expensive.
Studies That Support the Read-Across Hypothesis
Conservation of the drug target alone does not guarantee a functional interaction, and therefore experimental studies are required. Most studies have been carried out in fish, and in terms of assessing the impact of pharmaceuticals present in the environment, fish are one of the most likely (but not always the most sensitive) classes of organisms to be affected. Despite the large number of publications where effects of drugs have been studied, there are relatively few (see Supporting Information Table S2) in which effects have been correlated to the internal exposure concentration (i.e., amount of substance in the body), usually indicated by plasma concentration as opposed to exposure concentration (amount of substance in the water)). Table 1 proposes a classification of experimental studies according to how well they address the read-across hypothesis. A level 1 study provides little or no relevant information, whereas a level 4 study, incorporating measurements of drug exposure and internal concentrations, as well as a mode of action endpoint, will directly address all aspects of the hypothesis. Currently, no level 4 study has been published, although one study by Valenti et al.24 gets reasonably close, as discussed below, to this highest level of support for the hypothesis.
Table 1. Classification of Studies According to How Well They Address the Read-Across Hypothesis.
| Level | Exposure concentration | Endpoints | Internal concentration | Specific Pharmacological Effects | Comments |
|---|---|---|---|---|---|
| 4 | Measured | Mode of action related | Measured | Seen only at HTPCa | Experimental design has integrated information from mammalian data |
| 3 | Measured | Mode of action related | X | Cannot be related to HTPC | |
| 2 | Measured | X | X | X | Experimental design is independent from mammalian data |
| 1 | X | X | X | X |
HTPC, human therapeutic plasma concentration. Only studies at Level 4 address all aspects of the read-across hypothesis, and can relate effects to the human therapeutic plasma concentration. Level 3 studies may be able to confirm that similar effects to those seen in humans occur in aquatic organisms, but without a measure of the internal concentration it is not possible to relate these effects to the human therapeutic plasma concentration, and therefore determine if an aquatic organism is more, or less, sensitive to a particular drug. Studies at Levels 1 and 2 provide little or no relevant information.
The importance of having a measure of internal exposure is that some pharmaceuticals can bioconcentrate,25−28 perhaps resulting in an internal concentration several hundred times higher than the external concentration. For example, Fick et al.13 demonstrated >50-fold bioconcentration for 10 out of 16 pharmaceuticals present in sewage effluents. A measure of the internal drug concentration is also required in order to demonstrate that the pharmaceutical is taken up; in the study by Fick et al.13 it was reported that benzafibrate and telmisartan were not detected in fish plasma despite being present in the sewage effluent, and predicted to bioconcentrate. Without knowledge of plasma concentrations, which reflect the pharmacokinetics of the drug, a comparison with human therapeutic concentrations cannot be made and incorrect conclusions could be drawn regarding the exposure concentration at which a pharmaceutical elicits a response, and thereby incorrectly estimating the threat to non-target organisms if the drug is present in the environment.
An additional requirement for validating the read-across hypothesis is that internal exposure is linked to a biological effect reflecting the primary therapeutic response, by measuring relevant endpoints in physiologically relevant tissues. Given the general acceptance of the hypothesis, it is therefore somewhat surprising that only one study to date has confirmed the administered pharmaceutical dose by measuring water concentrations, obtained the internal exposure level by measuring plasma concentrations, linked exposure to a biological effect by measuring relevant endpoints, compared the results to those expected at Cmax, and demonstrated that effects were seen only at concentrations similar to those in a human taking the drug. Thus, in a poster presented at SETAC North America, Moen et al.29 demonstrated that the beta-blocker propranolol had the expected cardiac effects in rainbow trout (Onchorynchuss mykiss) only when plasma concentrations were similar to human therapeutic plasma concentrations. As this study remains, to our knowledge, the only one that fully addresses all aspects of the read-across hypothesis and can therefore be considered a level 4 study (Table 1), it is unfortunate that further details are not available in the peer-reviewed literature.
A recent study24 observed behavioral effects of sertraline (a selective serotonin reuptake inhibitor used to modify behavior in humans) in fathead minnows exposed to water concentrations of 2.8–28.1 μg/L. The resulting plasma concentrations (305 ng/mL at the lowest exposure concentration) were close to the normal human therapeutic range (50–250 ng/mL30), thus supporting the read-across hypothesis. However, it is unfortunate that the lowest exposure concentration tested resulted in plasma levels similar to human Cmax, so we do not know if any effects would have been observed below Cmax, a requirement to fully validate the read-across hypothesis. The highest exposure concentration of sertraline used (28.1 μg/L) gave a measured plasma concentration of 2000 ng/mL, and is therefore likely to have resulted in plasma concentrations in the toxic range for humans;30,31 however, no (further) adverse effects were reported.24 A previous study, which did not measure plasma concentrations, reported that an exposure concentration of 72 μg/L sertraline was lethal to fathead minnows,32 which would suggest that the plasma concentrations reached in the most recent study24 were well below the fatal range.
The studies reported by Zeilinger et al (33) and Fick et al,13 when considered together, provide strong support for the read-across hypothesis. Levonorgestrel, a synthetic progestagen present in oral contraceptives, has been shown to inhibit reproduction of adult fathead minnows at exposure concentrations of 0.8 ng/L and higher.33 Subsequently, it was demonstrated that levonorgestrel was strongly bioconcentrated in rainbow trout exposed to sewage effluent, where a measured concentration of ≥1 ng/L resulted in a plasma concentration of 8.5–12 μg/L.13 This exceeds the human therapeutic concentration of 2.4 μg/L, and suggests that the mode of action effects observed by Zeilinger et al.33 could be attributed to the bioconcentration of levonorgestrel.
A number of studies have measured water and plasma concentrations of the pharmaceutical(s) of interest, but have used endpoints that do not relate to primary therapeutic effects (see Supporting Information Table S2 for a list of publications). These may provide partial support for the read-across hypothesis, in that non-target effects are not seen at human therapeutic plasma concentrations: for example, in fathead minnows, propranolol produced no toxic effects until the plasma concentration exceeded Cmax by 100-fold, which occurred at a water concentration of 1 mg/L.34 Similarly, Owen et al.35 observed an effect of propranolol on rainbow trout growth at plasma concentrations above Cmax and sufficiently high to be toxic to humans. These studies did not measure cardiac effects, and so it is not clear whether a modulation of heart rate would have occurred at plasma concentrations equal to human therapeutic plasma concentrations. Incidentally, Owen et al.35 also reported changes in heart size, and remodelling of the heart is a reported secondary effect of long-term beta-blocker use in humans.
There are now a reasonable number of high quality studies that, although they do not include measurement of plasma concentrations of the pharmaceuticals being studied, involve assessing one or more endpoints relevant to the mode of action. One of the first of these studies was a demonstration that ethinylestradiol, a synthetic estrogen used in oral contraceptives, stops fish reproducing, besides having other effects in fish indicative of its estrogenic nature.36 A number of other classes of steroidal pharmaceuticals have also been shown to have effects on fish appropriate to their mode of action in humans: this includes synthetic glucocorticoids37 and synthetic progestagens.33,38 Pharmaceuticals that inhibit the actions of natural steroidal hormones, either because they act as receptor antagonists or inhibit the synthesis of hormones, also have the same effects in fish as they do in humans. For example, the human estrogen receptor antagonist Tamoxifen inhibits fish reproduction,39,40 as do aromatase inhibitors.41,42 Fibrates, a lipid-regulating class of drugs used to treat cardiovascular problems in humans, also lower plasma concentrations of lipoproteins in fish.43 Furthermore, antidepressants such as fluoxetine44 and oxazapam,28 but not citalopram,45 affect various aspects of behavior in fish. In all these studies, and others, the effects of the pharmaceuticals on fish were those that would be anticipated if the drugs had the same mode of action in fish as they do in humans. However, because plasma concentrations of the pharmaceuticals were not determined, it is premature to conclude that these level 3 studies (Table 1) provide unequivocal support for the read-across hypothesis. They are undoubtedly not in conflict with the hypothesis (they do not refute it), a conclusion supported by using the Fish Plasma Model1 to predict internal concentrations (see Supporting Information Table S3 for details). However, because effective plasma concentrations of pharmaceuticals cannot be compared between humans and fish, the studies provide at best only partial support for the hypothesis.
It is more difficult to interpret studies where gene expression has been used as the endpoint of exposure. In particular, if the genes studied are not directly related to the mode of action, it needs to be remembered that gene expression can be responsive to a range of influences that may not be related to the pharmaceutical being tested. Thus, the significance of changes in global fathead minnow brain gene expression in response to 4 μg/L propranolol (measured) for 21 days,46 and increases in vitellogenin, CYP1A and p53 gene expression in medaka (Oryzia latipes) liver, gill, and intestine following 4 day exposure to 1 μg/L diclofenac,47 is unclear. Indeed, they may not be reproducible, as in the study with propranolol,46 the exposure concentration was much lower than that required to obtain therapeutic levels.34 Cuklev et al.48 demonstrated that a 14 day exposure of juvenile rainbow trout to 1.6–81.5 μg diclofenac/L, resulting in plasma concentrations 1.5–88% of Cmax, affected hepatic gene expression. These findings could be interpreted to suggest that environmentally relevant concentrations of diclofenac could alter gene expression in fish, but it is unclear whether these genes would also be induced upon exposure to any pharmaceutical, or even as a consequence of something unrelated to drug exposure. However, the authors demonstrated that at plasma diclofenac concentrations close to Cmax the majority of differentially regulated genes were functionally associated with inflammation and the immune response, and therefore consistent with the mode of action of diclofenac.48 A recent study49 has provided a cautionary note regarding gene expression studies: the lack of validation and knowledge of the complexity of gene expression, small sample size and differential biological impact on individual fish, make it difficult, perhaps even impossible presently, to relate changes in gene expression to drug exposure.50
How Far Does the Concept of Read-Across Extend?
The majority of studies concerned with pharmaceuticals in the environment have involved fish. However, Huggett et al.51 recognized the need for studies to be done in invertebrates, algae, and plants, as conceptually these organisms could also respond to pharmaceuticals if the target is conserved. There is increasing evidence that this is the case; for example, the rotifer Brachionus manjavacas contains a proposed progesterone receptor in its reproductive organs,52 and Daphnia magna an eiconsanoid biosynthesis pathway, the target for NSAIDs.53 The Schistosoma flatworm contains many potential drug targets, such as receptors and enzymes, and appears to have cellular signaling, metabolic and neuroendocrine pathways similar to those present in higher organisms.54
However, presence of a target is not sufficient on its own, and a major challenge is interpretation of responses; the response resulting from activation or inhibition of a particular target in an invertebrate or a plant may be very different, and perhaps unrecognizable, from the response seen in a human. It is likely that targets related to fundamental systems such as reproduction, metabolic pathways or detoxification are more conserved in their functions, and therefore easier to recognize as being affected by pharmaceuticals. An example of this would be the enzyme HMGR (3-hydroxy-3-methylglutaryl coenzyme A reductase), the rate-limiting enzyme in cholesterol biosynthesis and the target for statins. Orthologues were predicted in almost all eukaryotes,16 and statins have been shown to inhibit HMGR activity in plants,55 fungi56 and invertebrates.57 In particular, the work of Brain et al.55 supports the read-across hypothesis: the effects in the plant Lemna gibba was seen at low μg/L doses, and the molecular endpoint used (mevalonic acid pathway biosynthesis, which is regulated by HMGR)—directly related to the mode of action of the drugs tested—was some 2–3 times more sensitive than a gross morphological endpoint (wet weight).
Many of the studies carried out in lower eukaryotes are subject to the same criticisms as fish studies. For example, high doses of drugs have been administered, no measurement of the internal exposure concentration was made, or the endpoints used are not mode of action related.58−61 It therefore becomes unclear whether the effects observed are related to the equivalent human therapeutic dose; represent the effects of an “overdose”; or indeed occur at a lower exposure concentration than the nominal dose. However, it is probably unrealistic to expect that a full validation of the read-across hypothesis is possible in plants, fungi, and invertebrates, in a large part due to the technical difficulties in measuring internal concentrations in very small organisms.
Apparent Limitations of the Approach—Studies That Appear not to Support the Read-Across Hypothesis
The mode of action principle underpinning the read-across hypothesis does not preclude other effects being seen, but it is presumed that these will occur when the internal concentration exceeds the Cmax. Therefore, any studies where significant, and perhaps toxic, effects are seen at or below the Cmax, would suggest that the read-across hypothesis is not universally applicable, perhaps as a consequence of differences in detoxification mechanisms between humans and non-target organisms.
The most well-known example of the effect of a pharmaceutical that does not appear to support the read-across hypothesis is the death of Old World vultures (Gyps genus), due to kidney failure, following ingestion of diclofenac present in the cadavers of cattle on which they fed.62 Diclofenac, an NSAID, inhibits the COX enzyme, reducing prostaglandin synthesis. The human therapeutic dose is approximately 1.5–9 mg/kg (500–3,000 ng/mL plasma), with toxic effects, such as irreversible kidney and liver failure,63,64 occurring at 150 mg/kg (50 000 ng/mL plasma).30 Side effects, including renal damage, occur in some 1–10% of treated patients,65 indicating that the relatively low safety margin of diclofenac is a problem in sensitive individuals. So were the Gyps vultures highly sensitive to diclofenac, resulting in a toxic response at concentrations well below Cmax? Closer examination of the data appears to suggest not. Diclofenac plasma concentrations may have been as high as 40 000 ng/mL in these vultures (assuming no metabolism, based on an estimated body weight of 10 kg, an ingestion of 10 mg diclofenac, a plasma volume of 250 mL66 and a hematocrit of 0.5).67 This is very close to the human toxic dose of a drug that few veterinary surgeons would prescribe directly for birds.
Additionally, polymorphic variations in CYP enzymes that influence the metabolism of diclofenac could be important in the sensitivity of Gyps vultures to diclofenac. New World vultures, such as turkey vulture (Cathartes aura), are remarkably resistant to diclofenac, tolerating doses up to 25 mg/kg for 7 days.68 In contrast, Old World vultures ingesting approximately 1 mg diclofenac/kg died of renal failure.69 No diclofenac was detected in Cathares tissues and the estimated half-life was 6 h68 whereas, in the Gyps vultures, diclofenac residues were found in the kidney62 and the estimated half-life was 16–18 h.70
Thus, the diclofenac and vultures story, while representing the only example where impact at a population level can be attributed to the presence of a pharmaceutical in the environment, does not invalidate the read-across hypothesis. In fact, our view would be that it validates it by demonstrating the expected toxic effects seen in humans.
The literature on pharmaceuticals in the environment contains many studies reporting effects at low exposure concentrations. There is probably a need to recognize that drugs act over a range of doses, and in assessing whether a non-target organism displays greater sensitivity than a human, the “no observable therapeutic effect level” should be considered. Probably more of an issue is the fact that these studies have not generally measured the internal exposure concentration or the administered dose (for example71), or calculated the theoretical bioconcentration factor, and therefore any observed effects cannot be related to what would be expected based on mammalian drug development data. It is therefore not possible to determine whether the responses seen are due to bioconcentration of the drug, and therefore higher dose at target, or whether fish are more sensitive than humans, which could be the implication if no bioconcentration has occurred. In terms of assessing the potential impact of environmental exposures, the conclusions drawn would differ depending on which scenario was correct, and there is, therefore, a need for these studies to be replicated before too much emphasis can be placed on them.
It is also difficult to fully interpret studies where tissue, as opposed to plasma, concentrations of a drug have been measured (for example: diclofenac,72 antidepressants,73 gemfibrozil,74 oxazapam,28). Therefore, the suggestion that bluntnose minnow (Pimephales notatus) is up to an order of magnitude more sensitive to ibuprofen than humans, based on gill ibuprofen concentrations and the effect on prostaglandin E2 synthesis,75 must be viewed with caution. While species differences cannot be ruled out, the metabolism of ibuprofen in rainbow trout gill microsomes76 and liver cells77 appears to be similar to mammals.
There are a large number of studies where extremely high doses (mg/L) of pharmaceuticals have been administered, or are required, to elicit an effect. For example, De Felice et al.78 demonstrated differences in hepatic gene expression in zebrafish embryos exposed to a nominal concentration of 1.25 mg/L diclofenac for 48 h. Similarly, Saravanan et al.79 found changes in a range of blood parameters following exposure of Indian major carp (Cirrhinus mrigala) fingerlings to 14.2 mg/L (nominal) ibuprofen for 35 days. The observed changes appear to be related to toxicity rather than mode of action, and therefore may not be drug-specific. Additionally, no plasma or body concentrations of the drugs were determined in these studies.
The influence of polymorphisms on drug responses is increasingly being recognized in humans,80 forming the basis of “personalized medicine”.81 If polymorphic variation is also relevant for other organisms, we may need to be more aware of the potential side effects of drugs—could these become a major effect in some species, or perhaps more likely, some individuals within each species? It also highlights the fact that we must be aware of potential species differences (which has implications for the test species used in regulatory testing), and allow a sufficient safety margin between environmental and effect concentrations. A recent paper82 reported variation among amphibian species in their sensitivity to the insecticide endosulfan, which was linked to their phylogenetic groupings.
Environmental Relevance and a Predictive Model
Clearly, the aim of the research being carried out under the “Pharmaceuticals in the Environment” banner is to aid decision making with regards to the risks drugs may pose to non-target organisms exposed via the environment. Several recent publications provide support for the Fish Plasma Model as a tool to prioritise drugs,15,83,84 although it is recognized that the input parameters, in particular the predictor of bioconcentration, should be refined. It is unrealistic for experimental data to be obtained for all pharmaceuticals in use, or on all possible species receiving exposure, but the read-across hypothesis could be tested with a number of drugs, representing different modes of action, and the data obtained used to derive an improved model for predicting likely environmental impact of different drugs, or classes of drugs. The read-across hypothesis relies on a conserved mode of action, and it would therefore be expected that all drugs of a certain class, having the same mode of action, could contribute to an effect. Therefore, in considering whether drugs are present in the environment at concentrations likely to cause effects, the total load of a particular class of drugs must be considered. While a mixture situation is not simple—similar drugs may have different degrees of uptake, as illustrated by atenolol and propranolol35,85—a sufficiently large margin between the therapeutic concentrations, which is presumed to be below toxic concentrations, and environmental concentrations should allow for the worst-case scenario of totally additive effects to be predicted.
Few studies have attempted to assess the internal concentrations as a consequence of environmental exposure to pharmaceuticals. The studies by Brown et al.,86 Fick et al.,13 and Lahti et al.87 are the only ones that, to our knowledge, have taken a field-work approach and exposed juvenile rainbow trout to sewage treatment work effluents for 2–3 weeks. The presence of five86 or 2513 pharmaceuticals was measured in plasma and compared with the modeled uptake, and for most drugs the measured bioconcentration was in reasonably good agreement with the predicted values. Exceptions included levonorgestrel and cilazapril, where measured concentrations were 260 and 100 times higher than modeled, respectively, and telmisartan and benzafibrate, which were not detected despite being predicted to bioconcentrate.13 The exact reasons for these apparent discrepancies are not known, but may be related to the log Kow values used in the modeling. In the case of benzafibrate, there are variations in the log Kow values generated by different algorithms; the American Chemical Development (ACD) prediction gives values lower than those generated by other algorithms (2.5 compared to 4.25), and is the only one to provide a log D7.4 value, which at −1.09 would suggest that no bioaccumulation of this drug would occur. Fick et al.13 used a log Kow of 4.25, resulting in a predicted bioconcentration factor of 168 which may well have been an overestimation. In the case of telmisartan, the modeling suggested a bioconcentration factor of 178649;13 whereas using the log D7.4 would give a predicted value of 380. This is still higher than the observed bioconcentration, and consideration needs to be given to the highly lipophilic nature of this drug which may affect its bioavailability.
Lahti et al.87 did not compare measured with modeled uptake, but found that only three pharmaceuticals (diclofenac, naproxen and ibuprofen), of the 15 drugs measured, were detectable in plasma. Thus, it is clear that factors other than the log Kow (upon which the prediction of plasma concentrations was originally based in the model proposed by Huggett et al.1) influences bioconcentration of drugs, and gaining this understanding will be crucial if we are to produce a robust, predictive model (see15). The counterintuitive observation of differences in measured plasma concentrations in fish placed in three different sewage treatment work effluent sites in Sweden86 and Finland,87 may perhaps be explained by the apparent considerable variation between individual fish.87 Difficulties in reliably measuring plasma concentrations of pharmaceuticals may perhaps have contributed to this variation, but the nature of the exposure matrix, such as the presence of colloids or surfactants and diurnal variation in the exposure concentrations, may also affect uptake parameters.86 Indeed, a study of three NSAIDs (ketoprofen, ibuprofen and naproxen) present in undiluted sewage effluent or clean water showed more efficient bioconcentration from the effluent.88 Variation in pharmaceutical uptake depending on the characteristics of the exposure medium, in particular pH, has been recognized by others,32,89 and it has been suggested that any modeling must take account of this by using the pH-corrected octanol–water partition coefficient (Dow)15,85 or pH-corrected liposome-water partition coefficients (Dlipw).89 However, there remains considerable variation between individual fish exposed in the same “clean” laboratory tanks (e.g., Owen et al.35), suggesting that differences in uptake mechanisms, such as gill membrane based drug transporters, or metabolic clearance rates, may also play a significant role.
Understanding factors that affect uptake is clearly crucial; poor uptake of citalopram, a selective serotonin reuptake inhibitor, is believed to explain the lack of effects in rainbow trout and guppies (Poecilia reticulata),45 even though modeling suggests the drug could present an environmental hazard.15,84 In fish, the presence of sex-steroid-binding globulin (SSBG) in the gills26 is believed to explain the very effective uptake of steroids, resulting in plasma concentrations 1000-fold higher than the surrounding environment and exceeding the Cmax.13 However, this generalized assumption may not hold true for all steroids, as illustrated by the apparent contradictory results for two glucocorticoids, beclomethasone and dexamethasone. While exposure of fathead minnows to 1 μg/L beclomethasone dipropionate resulted in mode of action-relevant biological effects,37 an exposure concentration of 500 μg/L dexamethasone was required to produce effects on reproduction, growth and development.90 The explanation for these results is most likely related to the differing uptake of the two glucocorticoids; although the studies did not measure plasma concentrations, modeling of the bioconcentration of beclomethasone diproprionate (the formulation used by Kugathas and Sumpter37), and dexamethasone indicates that only beclomethasone diproprionate would bioconcentrate to any significant extent (Table 2). In this regard, it should be noted that due to its lower lipophilicity, dexamethasone is often administered by injection to patients. This highlights the importance of clearly stating the specific formulation of the pharmaceutical used in all exposure studies, as the absence/presence and identity of specific adjunctive chemical groups (e.g., acetate, valerate, propionate) on the parent compound may significantly affect the bioaccumulation process, and hence the potential effects on fish.
Table 2. Chemical Modifications of Three Glucocorticoids Affect the Predicted Plasma Bioconcentration Factor (BCF).
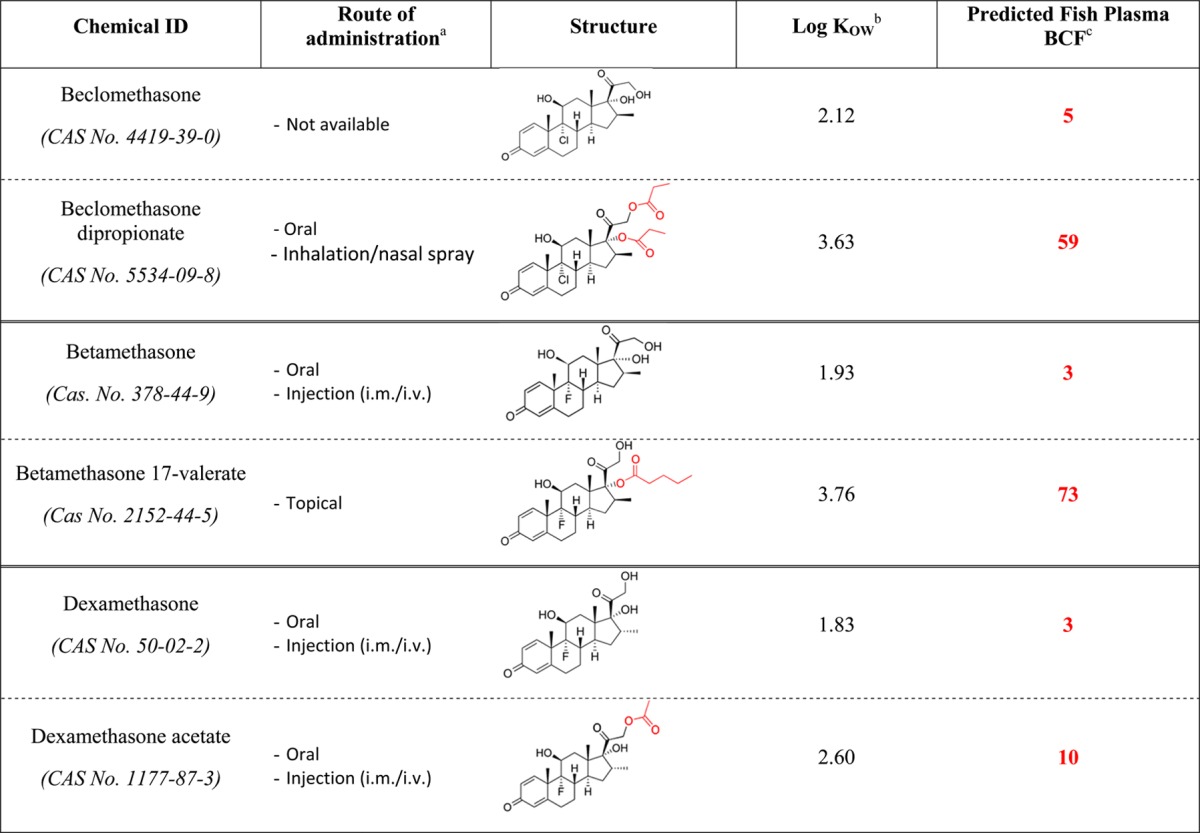
Normal route of administration to patients.
LogKOW was predicted by the software ALOGPs.
BCF calculated according to Fitzsimmons et al.91
Concluding Remarks
The read-across hypothesis can be viewed simply as what we see in one species is likely to be reflected in others, that conserve the same target, at the level of a pharmacological response (mode of action); that is, we see the same end result. Although conservation of the molecular target is fundamental to the hypothesis, we do not understand the mechanism of action for most pharmaceuticals at a sufficiently detailed level for the molecular target to be a robust endpoint. Most drugs have multiple mechanisms, multiple modes of action, and ultimately a range of effects. Some are clearly the principle mechanism and the net effect is that a disease state or symptoms are alleviated in most people. However, the secondary pharmacology contributes to the side effects that are associated with many pharmaceuticals. Any of these responses may be expected to read across to other species with similar systems. In terms of evidencing the read-across hypothesis, we should be able to test the intended therapeutic end point, or a major secondary endpoint.
In principle, read-across would be expected to hold true for most, but not all, classes of drugs (and indeed other chemicals). A notable exception would be some of the biopharmaceuticals, such as antibodies. These are exquisitely specific, and only work in one species, man, and as we have already alluded to, increased evolutionary distance from man will reduce the likelihood of read-across. Further, antibodies will not be present in the environment, as they are unlikely to be excreted by patients, and even if they were, their poor stability outside the human body and large molecular size would prevent uptake into fish or other organisms.
There is still much work to be done. For example, in writing this paper, we have made the assumption that the binding kinetics of a drug to plasma proteins and tissue are the same in a fish as in a human. However, if a drug is less tightly bound in fish plasma than in a human, there will be a greater amount of drug available to flood the tissue compartment92 at the same plasma concentration, and in terms of effect it is the unbound concentration of a drug that matters. Thus, understanding the pharmacokinetics in fish may be important, especially if the expected therapeutic end points are seen at plasma concentrations below human therapeutic concentrations. Additionally, differential pharmacokinetics may contribute to apparent species differences in no observed effect concentration (NOEC) values.
Looking Forward
Although the read-across hypothesis is generally accepted, this review has shown that there is currently scant evidence for it. This is not through a lack of research effort, as attested to by the large number of publications, but rather that most studies have been found wanting in some respects, mostly in relation to measurements of the pharmaceuticals in water and the exposed organism. Such analyses require highly specialist and inherently expensive facilities, and are beyond the reach of many research groups. In our view, fewer and better designed studies, perhaps informed by the Adverse Outcome Pathway conceptual framework,93 are required in order to provide the basis for a robust risk assessment of pharmaceuticals present in the environment. The importance of this is exemplified by a recently published study94 which makes the claim that 1 ng/L fluoxetine has significant effects on cuttlefish (Sepia officinalis). If these results, which are intuitively surprising, were repeatable (for example, we have concerns because there was no analytical chemistry confirmation of doses in the static exposures; there was high mortality; the inverse dose effect is difficult to interpret), they would have profound implications—it would suggest that current environmental concentrations of fluoxetine are sufficiently high enough to affect aquatic organisms.
In order to ensure that environmental policy and regulations are not informed by studies that are unlikely to be repeatable, a consortium-based approach, as well as work within and between experienced, well-resourced laboratories, may be a way forward, ensuring that complete studies (Table 1) that will generate robust data are undertaken. It would be beneficial to agree standard operating protocols and blind analysis of samples—presently, interpretation and comparisons of results are difficult and often impossible because of varying experimental conditions (exposure length, age of fish, different species); endpoints that are related or unrelated to mode of action; lack of information about exposure and internal concentrations; and even the particular formulation of the drug used is not always provided. A concerted, collaborative effort by the scientific community and industry can provide the resources required to fully understand the factors influencing bioconcentration (perhaps the most important in assessing risks for aquatic organisms), and allow studies to be done on a sufficiently large scale to reduce the uncertainty created by the biological variability often observed. The publication process can be used to encourage more complete studies, as it is only on the basis of robust data that we can develop predictive models, which in our view, is the only feasible approach to protecting the environment.
Acknowledgments
We are supported by BBSRC Industrial Partnership Award BB/I00646X/1 and BBSRC Industrial CASE Partnership Studentship BB/I53257X/1 with AstraZeneca Safety Health and Environment Research Programme.
Supporting Information Available
Table S1 details the accession numbers of sequences used to construct the phylogenetic tree for 5-alpha reductase. Table S2 collates published laboratory studies of 10 pharmaceuticals that also report measured water and plasma concentrations. Table S3 shows the predicted drug plasma concentration for studies28,33,36−42,44,45 which observed mode of action related effects, but where drug plasma concentrations were not measured. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Huggett D. B.; Cook J. C.; Ericson J. E.; Williams R. T. A Theoretical model for utilizing mammalian pharmacology and safety data to prioritise potential impacts of human pharmaceuticals to fish. J. Human Ecol. Risk Assess. 2003, 9, 1789–1799. [Google Scholar]
- Evans D. H.The Physiology of Fishes; CRC Press: Boca Raton, FL., 1993. [Google Scholar]
- Winter M. J.; Owen S. F.; Murray-Smith R.; Panter G. H.; Hetheridge M. J.; Kinter L. B. Using data from drug discovery and development to aid the aquatic environmental risk assessment of human pharmaceuticals: Concepts, considerations, and challenges. Integr. Environ. Assess. Manag. 2010, 6, 38–51. [DOI] [PubMed] [Google Scholar]
- Sumanas S.; Lin S. Zebrafish as a model system for drug target screening and validation. Drug Discovery Today: Targets 2004, 3, 89–96. [Google Scholar]
- Rihel J.; Prober D. A.; Arvanites A.; Lam K.; Zimmerman S.; Jang S.; Haggarty S. J.; Kokel D.; Rubin L. L.; Peterson R. T.; Schier A. F. Zebrafish behavioural profiling links drugs to biological targets and rest/wake regulation. Science 2010, 327, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara G.; Karpf J. A.; Myers J. A.; Alexander M. S.; Guyon J. R.; Kunkel L. M. Drug screening in a zebrafish model of Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. 2011, 108, 5331–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T.; Saito K.; Deguchi T.; Fujimori K.; Tadokoro M.; Yuba S.; Ohgushi H.; Kawarabayashi Y. Pharmacological characterisation of isoproterenol-treated medaka fish. Pharmacol. Res. 2008, 58, 348–355. [DOI] [PubMed] [Google Scholar]
- Menningen J. A.; Stroud P.; Zamora J. M.; Moon T. W.; Trudeau V. L. Pharmaceuticals as neuroendocrine disruptors: Lessons learned from fish on prozac. J. Toxicol. Environ. Health, Part B 2011, 14, 387–412. [DOI] [PubMed] [Google Scholar]
- Schüürmann G.; Ebert R.-U.; Kühne R. Quantitative read-across for predicting the acute fish toxicity of organic compounds. Environ. Sci. Technol. 2011, 45, 4616–4622. [DOI] [PubMed] [Google Scholar]
- Kühne R.; Ebert R.-U.; von der Ohe P. C.; Ulrich N.; Brack W.; Schüürmann G. Read-across prediction of the acute toxicity of organic compounds toward the water flea Daphnia magna. Mol. Inf. 2013, 32, 108–120. [DOI] [PubMed] [Google Scholar]
- Nendza M.; Wenzel A. Discriminating toxicant classes by mode of action - 1. (Eco)toxicity profiles. Environ. Sci. Pollut. Res. 2006, 133192–203. [DOI] [PubMed] [Google Scholar]
- Runnalls T. J.; Margiotta-Casaluci L.; Subramaniam K.; Sumpter J. P. Pharmaceuticals in the aquatic environment: Steroids and anti-steroids as high priorities for research. Hum. Ecol. Risk Assess. 2010, 16, 1318–1338. [Google Scholar]
- Fick J.; Lindberg R. H.; Parkkonen J.; Arvidsson B.; Tysklind M.; Larsson D. G. J. Therapeutic levels of levonorgestrel detected in blood plasma of fish: Results from screening rainbow trout exposed to treated sewage effluents. Environ. Sci. Technol. 2010, 44, 2661–2666. [DOI] [PubMed] [Google Scholar]
- Santos L. H.; Araujo A. N.; Fachini A.; Pena A.; Delerue-Matos C.; Montenegro M. C. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [DOI] [PubMed] [Google Scholar]
- Schreiber R.; Gundel U.; Franz S.; Kuster A.; Rechenberg B.; Altenburger R. Using the fish plasma model for comparative hazard identification for pharmaceuticals in the environment by extrapolation from human therapeutic data. Regul. Toxicol. Pharmacol. 2011, 61, 261–275. [DOI] [PubMed] [Google Scholar]
- Gunnarsson L.; Jauhiainen A.; Kristiansson E.; Nerman O.; Larsson D. G. J. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 2008, 42, 5807–5813. [DOI] [PubMed] [Google Scholar]
- Margiotta-Casaluci L.; Courant F.; Antignac J. P.; Le Bizec B.; Sumpter J. P. Identification and quantification of 5α-dihydrotestosterone in the teleost fathead minnow (Pimephales promelas) by gas chromatography-tandem mass spectrometry. Gen. Comp. Endrocrinol. 2013, 191, 202–209. [DOI] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K.; Peterson D.; Peterson N.; Stecher G.; Nei M.; Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Nagpal P.; Vitart V.; McMorris T. C.; Chory J. A role of brassino-steroids in light-dependent development of Arabidopsis. Science 1996, 272, 398–401. [DOI] [PubMed] [Google Scholar]
- Li J.; Biswas M. G.; Chao A.; Russell D. W.; Chory J. Conservation of function between mammalian and plant steroid 5a-reductase. Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 3554–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta-Casaluci L.; Hannah R. E.; Sumpter J. P. Mode of action of human pharmaceuticals in fish: The effects of the 5-alpha-reductase inhibitor, dutasteride, on reproduction as a case study. Aquat. Toxicol. 2013, 128–129, 113–123. [DOI] [PubMed] [Google Scholar]
- Cannon C. P.; Cannon P. J. Cox-2 inhibitors and cardiovascular risk. Science 2012, 336, 1386–1387. [DOI] [PubMed] [Google Scholar]
- Valenti T. W.; Gould G. G.; Berninger J. P.; Connors K. A.; Keele N. B.; Prosser K. N.; Brooks B. W. Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter seeking behaviour in adult male fathead minnows. Environ. Sci. Technol. 2012, 46, 2427–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio R. T.; Hinton D. E.. The Toxicology of Fishes; Taylor & Francis Group, Boca Raton, FL, 2008. [Google Scholar]
- Miguel-Queralt S.; Hammond G. L. Sex hormone-binding globulin in fish gills is a portal for sex steroids breached by xenobiotics. Endocrinol. 2008, 149, 4269–4275. [DOI] [PubMed] [Google Scholar]
- Kosjek T.; Heath E. Occurrence, fate and determination of cytostatic pharmaceuticals in the environment. Trends Anal. Chem. 2011, 30, 1065–1087. [Google Scholar]
- Brodin T.; Fick J.; Jonsson M.; Klaminder J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 2013, 339, 814–815. [DOI] [PubMed] [Google Scholar]
- Moen M. A.; Owen S. F.; Hutchinson T. H.; Hetheridge M. J.; Constantine L. A.; Sumpter J. P.; Huggett D. B.. Pharmaceutical plasma concentrations in fish as a prediction tool for toxicity: Case studies with 17α-ethinyl estradiol and propranolol. SETAC North America, 29th Annual Meeting, 2007.
- Schulz M.; Iwersen-Bergmann S.; Andresen H.; Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit. Care 2012, 16, R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner D. A.; Hall M.; Davis G. G.; Brissie R. M.; Robinson C. A. Fatal multiple drug intoxication following acute sertraline use. J. Anal. Toxicol. 1998, 22, 545–548. [DOI] [PubMed] [Google Scholar]
- Valenti T. W.; Perez-Hurtado P.; Chambliss C. K.; Brooks B. W. Aquatic toxicity of sertraline to Pimephales promelas at environmentally relevant surface water pH. Environ. Toxicol. Chem. 2009, 28, 2685–2694. [DOI] [PubMed] [Google Scholar]
- Zeilinger J.; Steger-Hartmann T.; Maser E.; Goller S.; Vonk R.; Länge R. Effects of synthetic gestagens on fish reproduction. Environ. Toxicol. Chem. 2009, 28, 2663–2670. [DOI] [PubMed] [Google Scholar]
- Giltrow E.; Eccles P. D.; Winter M. J.; McCormack P. J.; Rand-Weaver M.; Hutchinson T. H.; Sumpter J. P. Chronic effects assessment and plasma concentrations of the β-blocker propranolol in fathead minnows (Pimephales promelas). Aquat. Toxicol. 2009, 95, 195–202. [DOI] [PubMed] [Google Scholar]
- Owen S. F.; Huggett D. B.; Hutchinson T. H.; Hetheridge M. J.; Kinter L. B.; Ericson J. F.; Sumpter J. P. Uptake of propranolol, a cardiovascular pharmaceutical, from water into fish plasma and its effects on growth and organ biometry. Aquat. Toxicol. 2009, 93, 217–224. [DOI] [PubMed] [Google Scholar]
- Länge R.; Hutchinson T. H.; Croudace C. P.; Siegmund F.; Schweinfurth H.; Hampe P.; Panter G. H.; Sumpter J. P. Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2001, 20, 1216–1227. [DOI] [PubMed] [Google Scholar]
- Kugathas S.; Sumpter J. P. Synthetic glucocorticoids in the environment: First results on their potential impacts on fish. Environ. Sci. Technol. 2011, 45, 2377–2383. [DOI] [PubMed] [Google Scholar]
- Runnalls T. J.; Beresford N.; Losty E.; Scott A. P.; Sumpter J. P. Several synthetic progestins with different potencies adversely affect reproduction in fish. Environ. Sci. Technol. 2013, 47, 2077–2084. [DOI] [PubMed] [Google Scholar]
- Williams T. D.; Caunter J. E.; Lillicrap A. D.; Hutchinson T. H.; Gillings E. G.; Duffell S. Evaluation of the reproductive effects of tamoxifen citrate in partial and full life-cycle studies using fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2007, 26, 695–707. [DOI] [PubMed] [Google Scholar]
- Sun L.; Zha J.; Spear P. A.; Wang Z. Tamoxifen effects on the early life stages and reproduction of the Japanese medaka (Oryzias latipes). Comp. Biochem. Physiol. Part C 2007, 145, 533–541. [Google Scholar]
- Ankley G. T.; Kahl M. D.; Jensen K. M.; Hornung M. W.; Korte J. J.; Makynen E. A.; Leino R. L. Evaluation of the aromatase inhibitor fadrozole in a short-term reproduction assay with the fathead minnow (Pimephales promelas). Toxicol. Sci. 2002, 67, 121–130. [DOI] [PubMed] [Google Scholar]
- Sun L.; Zha J.; Spear P. A.; Wang Z. Toxicity of the aromatase inhibitor letrozole to Japanese medaka (Oryzias latipes) eggs, larvae and breeding adults. Environ. Toxicol. Pharmacol. 2007, 24, 23–29. [DOI] [PubMed] [Google Scholar]
- Prindiville J. S.; Menningen J. A.; Zamora J. M.; Moon T. W.; Weber J.-M. The fibrate drug gemfibrozil disrupts lipoprotein metabolism in rainbow trout. Toxicol. Appl. Pharmacol. 2011, 251, 201–208. [DOI] [PubMed] [Google Scholar]
- Gaworecki K. M.; Klaine S. J. Behavioural and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat. Toxicol. 2008, 88, 207–213. [DOI] [PubMed] [Google Scholar]
- Holmberg A.; Fogel J.; Albertsson E.; Fick J.; Brown J. N.; Paxeus N.; Forlin L.; Johnsson J. J.; Larsson D. G. J. Does waterborne citalopram affect the aggressive and sexual behaviour of rainbow trout and guppy?. J. Hazard. Mater. 2011, 187, 596–599. [DOI] [PubMed] [Google Scholar]
- Lorenzi V.; Mahinto A. C.; Denslow N. D.; Schlenk D. Effects of exposure to the β-blocker propranolol on the reproductive behaviour and gene expression of the fathead minnow, Pimephales promelas. Aquat. Toxicol. 2012, 116–117, 8–15. [DOI] [PubMed] [Google Scholar]
- Hong H. N.; Kim H. N.; Park K. S.; Lee S.-K.; Gu M. B. Analysis of the effects diclofenac has on Japanese medaka (Oryzias latipes) using real-time PCR. Chemosphere 2007, 67, 2115–2121. [DOI] [PubMed] [Google Scholar]
- Cuklev F.; Kristiansson E.; Fick J.; Asker N.; Forlin L.; Larsson D. G. J. Diclofenac in fish: Blood plasma levels similar to human therapeutic levels affect global hepatic gene expression. Environ. Toxicol. Chem. 2011, 30, 2126–2134. [DOI] [PubMed] [Google Scholar]
- Wang R.-L.; Bencic D.; Biales A.; Flick R.; Lazorchak J.; Villeneuve D.; Ankley G. T. Discovery and validation of gene classifiers for endocrine-disrupting chemicals in zebrafish (Danio rerio). BMC Genomics 2012, 13, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent K.; Sumpter J. P. Progress and promises in toxicogenomics in aquatic toxicology: Is technical innovation driving scientific innovation?. Aquat. Toxicol. 2011, 105S, 25–39. [DOI] [PubMed] [Google Scholar]
- Huggett D. B.; Benson W. H.; Chipman K.; Cook J. C.; Gray L. E.; Kinter L. B.; Meyerhoff R. D.; Trudeau V. L.. Role of mammalian data in determining pharmaceutical responses in aquatic species. In Human Pharmaceuticals; Williams R. T., Ed.; SETAC Press: Pensacola, FL, 2005; pp 149–181. [Google Scholar]
- Stout E. P.; La Clair J. J.; Snell T. W.; Shearer T. L.; Kubanek J. Conservation of progesterone hormone function in invertebrate reproduction. Proc. Natl. Acad. Sci. 2010, 107, 11859–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann L.-H.; Sibly R. M.; Timmermans M. J. T. N.; Callaghan A. Outlining eicosanoid biosynthesis in the crustacean Daphnia. Front. Zool. 2008, 5, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schistosoma Japonicum Genome Sequencing and Functional Analysis Consortium. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature, 2009, 460, 345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain R. A.; Reitsma T. S.; Lissemore L. I.; Bestari K.; Sibley P. K.; Solomon K. R. Herbicidal effects of statin pharmaceuticals in Lemna gibba. Environ. Sci. Technol. 2006, 40, 5116–5123. [DOI] [PubMed] [Google Scholar]
- Macreadie I. G.; Johnson G.; Schlosser T.; Macreadie P. I. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol. Lett. 2006, 262, 9–13. [DOI] [PubMed] [Google Scholar]
- Zapata R.; Piulachta M.-D.; Belles X. Inhibitors of 3-hydroxyglutaryl-CoA reductase lower fecundity in the German cockroach: Correlation between the effects on fecundity in vivo with the inhibition of enzymatic activity in embryo cells. Pest Manage. Sci. 2003, 59, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Stanley J. K.; Ramirez A. J.; Mottaleb M.; Chambliss C. K.; Brooks B. W. Enantiospecific toxicity of the β-blocker propranolol to Dapnia magna and Pimephales promelas. Environ. Toxicol. Chem. 2006, 25, 1780–1786. [DOI] [PubMed] [Google Scholar]
- Ericson H.; Thorsen G.; Kumblad L. Physiological effects of diclofenac, ibuprofen and propranolol on Baltic Sea blue mussels. Aquat. Toxicol. 2010, 99, 223–231. [DOI] [PubMed] [Google Scholar]
- Heckmann L.-H.; Connon R.; Hutchinson T. H.; Maund S. J.; Sibly R. M.; Callaghan A. Expression of target and reference genes in Daphnia magna exposed to ibuprofen. BMC Genomics 2006, 7, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann L.-H.; Sibly R. M.; Connon R.; Hooper H. L.; Hutchinson T. H.; Maund S. J.; Hill C. J.; Bouetard A.; Callghan A. Systems biology meets stress ecology: Linking molecular and organismal stress responses in Daphnia magna. Genome Biol. 2008, 9, R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks J. L.; Gilbert M.; Virani M. Z.; Watson R. T.; Meteyer C. U.; Rideout B. A.; Shivaprasad H. L.; Ahmed S.; Chaudhry M. J. I.; Arshad M.; Mahmood S.; Ali; Khan A. A. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [DOI] [PubMed] [Google Scholar]
- Bacchi S.; Palumbo P.; Sponta A.; Coppolino M. F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Anti-Inflammatory Anti-Allergy Agents Med. Chem. 2012, 11, 52–64. [DOI] [PubMed] [Google Scholar]
- Jayasutha J.; Nisha M. R.; Bhargav dilip S.; Ramasamy C. Assessments of NSAIDS induced renal diseases. Intl. J. Pharm. Sci. Rev. Res. 2012, 17, 77–79. [Google Scholar]
- Physician’s Desk Reference 2012. http://PDR3D.com
- Swan G.; Naidoo V.; Cuthbert R.; Green R. E.; Pain D. J.; Swarup D.; Prakash V.; Taggart M.; Bekker L.; Das D.; Diekmann J.; Diekmann M.; Killian E.; Meharg A.; Patra R. C.; Saini M.; Wolter K. Removing the threat of diclofenac to critically endangered Asian vultures. PLOS Biol. 2006, 4, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo V.; Diekmann M.; Wolters K.; Swan G. E. Establishment of selected baseline blood chemistry and haematologic parameters in captive and wild-caught African white-backed vultures (Gyps africanus). J. Wildlife Dis. 2008, 44, 649–654. [DOI] [PubMed] [Google Scholar]
- Rattner B. A.; Whitehead M. A.; Gasper G.; Meteyer C. U.; Link W. A.; Taggart M. A.; Meharg A. A.; Pattee O. H.; Pain D. J. Apparent tolerance of turkey vulture (Cathartes aura) to the non-steroidal anti-inflammatory drug diclofenac. Environ. Toxicol. Chem. 2008, 27, 2341–2345. [DOI] [PubMed] [Google Scholar]
- Green R. E.; Taggart M. A.; Das D.; Pain D. J.; Kumar C. S.; Cunningham A. A.; Cuthbert R. Collapse of Asian vulture populations: Risk of mortality from residues of the veterinary drug diclofenac in carcasses of treated cattle. J. Appl. Ecol. 2006, 43, 949–956. [Google Scholar]
- Naidoo V.; Duncan N.; Bekker L.; Swan G. Validating the domestic fowl as a model to investigate the pathophysiology of diclofenac in Gyps vultures. Environ. Toxicol. Pharmacol. 2007, 24, 260–266. [DOI] [PubMed] [Google Scholar]
- Flippin J. L.; Huggett D.; Foran C. M. Changes in the timing of reproduction following chronic exposure to ibuprofen in Japanese medaka Oryzias latipes. Aquat. Toxicol. 2007, 81, 73–78. [DOI] [PubMed] [Google Scholar]
- Schwaiger J.; Ferling H.; Mallow U.; Wintermayr H.; Negele R. D. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 2004, 68, 141–150. [DOI] [PubMed] [Google Scholar]
- Schulz M. M.; Painter M. M.; Bartell S. E.; Logue A.; Furlong E. T.; Werner S. L.; Schoenfuss H. L. Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat. Toxicol. 2011, 104, 38–47. [DOI] [PubMed] [Google Scholar]
- Skolness S. Y.; Durhan E. J.; Jensen K. M.; Kahl M. D.; Makynen E. A.; Villeneuve D. L.; Ankley G. T. Effects of gemfibrozil on lipid metabolism, steroidogenesis, and reproduction in the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2012, 11, 2615–2624. [DOI] [PubMed] [Google Scholar]
- Bhandari K.; Venables B. Ibuprofen bioconcentration and prostaglandin E2 levels in the bluntnose minnow Pimephales notatus. Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol. 2011, 153, 251–257. [DOI] [PubMed] [Google Scholar]
- Gomez C. F.; Constantine L.; Moen M.; Vaz A.; Wang W.; Huggett D. B. Ibuprofen metabolism in the liver and gill of rainbow trout Oncorhynchus mykiss. Bull. Environ. Contam. Toxicol. 2011, 86, 247–251. [DOI] [PubMed] [Google Scholar]
- Uchea C.; Sarda S.; Schulz-Utermoehl T.; Owen S.; Chipman K. J. In vitro models of xenobiotic metabolism in trout for use in environmental bioaccumulation studies. Xenobiotica 2012, 43, 421–431. [DOI] [PubMed] [Google Scholar]
- De Felice B.; Copia L.; Guida M. Gene expression profiling in zebrafish embryos exposed to diclofenac, an environmental toxicant. Mol. Biol. Rep. 2011, 39, 2119–2128. [DOI] [PubMed] [Google Scholar]
- Saravanan M.; Devi K. U.; Malarvizhi A.; Ramesh M. Effects of ibuprofen on haematological, biochemical and enzymological parameters of blood in an Indian major carp Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2012, 34, 14–22. [DOI] [PubMed] [Google Scholar]
- Ma Q.; Lu A. Y. H. Pharmacogenetics, pharmacogenomics, and individualised medicine. Pharmacol. Rev. 2011, 63, 437–459. [DOI] [PubMed] [Google Scholar]
- Wyatt J. E.; Pettit W. L.; Harirforoosh S. Pharmacogenetics of non-steroidal anti-inflammatory drugs. Pharmacogenomics J. 2012, 12, 462–467. [DOI] [PubMed] [Google Scholar]
- Hammond J. I.; Jones D. K.; Stephens P. R.; Relyea R. A. Phylogeny meets ecotoxicology: Evolutionary patterns of sensitivity to a common insecticide. Evol. Appl. 2012, 5, 593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick J.; Lindberg R. H.; Tysklind M.; Larsson D. G. J. Predicted critical environmental concentrations for 500 pharmaceuticals. Regul. Toxicol. Pharmacol. 2010, 58, 516–523. [DOI] [PubMed] [Google Scholar]
- Roos V.; Gunnarsson L.; Fick J.; Larsson D. G. J.; Ruden C. Prioritising pharmaceuticals for environmental risk assessment: Towards adequate and feasible first-tier selection. Sci. Total Environ. 2012, 421–422, 102–110. [DOI] [PubMed] [Google Scholar]
- Winter M. J.; Lillicrap A. D.; Caunter J. E.; Schaffner C.; Alder A. C.; Ramil M.; Ternes T. A.; Giltrow E.; Sumpter J. P.; Hutchinson T. H. Defining the chronic impacts of atenolol on embryo-larval development and reproduction in fathead minnow (Pimephales promelas). Aquat. Toxicol. 2008, 86, 361–369. [DOI] [PubMed] [Google Scholar]
- Brown J. N.; Paxeus N.; Forlin L.; Larsson D. G. J. Variations in bioconcentration of human pharmaceuticals from sewage effluents into fish blood plasma. Environ. Toxicol. Pharmacol. 2007, 24, 267–274. [DOI] [PubMed] [Google Scholar]
- Lahti M.; Brozinski J.-M.; Segner H.; Kronberg L.; Oikari A. Bioavailability of pharmaceuticals in waters close to wastewater treatment plants: Use of fish bile for exposure assessment. Environ. Toxicol. Chem. 2012, 31, 1831–1837. [DOI] [PubMed] [Google Scholar]
- Cuklev F.; Fick J.; Cvijovic M.; Kristiansson E.; Forlin L.; Larsson D. G. J. Does ketoprofen or diclofenac pose the lowest risk to fish?. J. Hazard. Mater. 2012, 229–230, 100–106. [DOI] [PubMed] [Google Scholar]
- Meredith-Williams M.; Carter L. J.; Fussell R.; Raffaelli D.; Ashauer R.; Boxall A. B. A. Uptake and depuration of pharmaceuticals in aquatic invertebrates. Environ. Pollut. 2012, 165, 250–258. [DOI] [PubMed] [Google Scholar]
- LaLone C. A.; Villeneuve D. L.; Olmstead A. W.; Medlock E. K.; Kahl M. D.; Jensen K. M.; Durhan E. J.; Makynen E. A.; Blanksma C. A.; Cavallin J. E.; Thomas L. M.; Seidl S. M.; Skolness S. Y.; Wehmas L. C.; Johnson R. D.; Ankley G. T. Effects of a glucocorticoid receptor agonist, dexamethasone, on fathead minnow reproduction, growth and development. Environ. Toxicol. Chem. 2012, 31, 611–622. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons P. N.; Fernandez J. D.; Hoffman A. D.; Butterworth B.; Nicholas J. Branchial elimination of superhydrophobic organic compounds by rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2001, 55, 23–34. [DOI] [PubMed] [Google Scholar]
- Oie S. Drug distribution and binding. J. Clin. Pharmacol. 1986, 26, 583–586. [DOI] [PubMed] [Google Scholar]
- Ankley G. T.; Bennett R. S.; Erickson R. J.; Hoff D. J.; Hornung M. W.; Johnson R. D.; Mount D. R.; Nichols J. W.; Russom C. L.; Schmieder P. K.; Serrano J. A.; Tietge J. E.; Villeneuve D. L. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [DOI] [PubMed] [Google Scholar]
- Di Poi C.; Darmaillacq S.-M.; Dickel L.; Boulouard M.; Bellanger C. Effects of perinatal exposure to waterborne fluoxetine on memory processing in the cuttlefish Sepia officinalis. Aquat. Toxicol. 2013, 132–133, 84–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.