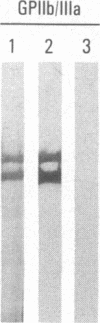
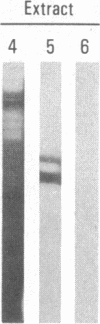
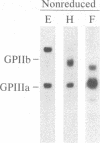
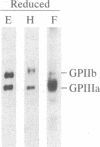
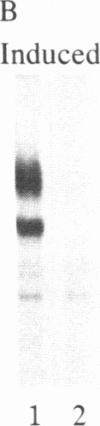
Abstract
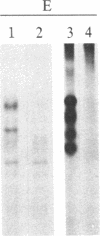
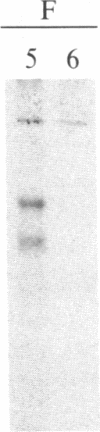
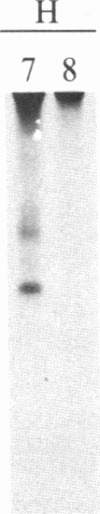
A polyclonal antiserum to platelet membrane glycoprotein GPIIb/IIIa was used to detect antigenically related molecules on a diverse panel of human cells. Umbilical vein endothelial cells, erythroleukemic HEL cells, and diploid fetal lung GM1380 fibroblasts expressed GPIIb/IIIa-related molecules, as judged by immunofluorescence and immunoprecipitation of surface-labeled proteins. The GPIIb and GPIIIa subunits were both present and were of similar molecular weight in these cell types. These molecules were synthetic products of the cells, as shown by immunoprecipitation of intrinsically labeled proteins. Promyeloid U937 cells could be induced by 4 beta-phorbol 12-myristate 13-acetate to synthesize and express GPIIb/IIIa-related molecules on their cell surface. The GPIIb/IIIa-related molecules were not precisely identical in the various cell types, based on slight differences in electrophoretic mobility and their failure to react with monoclonal antibodies specific for each subunit of platelet GPIIb/IIIa. These results suggest the existence of a widely distributed family of GPIIb/IIIa-related molecules. This family of "cytoadhesins" may share a common function in cellular adhesive reactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada K. M. Synthetic peptides competitively inhibit both direct binding to fibroblasts and functional biological assays for the purified cell-binding domain of fibronectin. J Biol Chem. 1985 Sep 5;260(19):10402–10405. [PubMed] [Google Scholar]
- Bai Y., Durbin H., Hogg N. Monoclonal antibodies specific for platelet glycoproteins react with human monocytes. Blood. 1984 Jul;64(1):139–146. [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G., Cines D. B. Identification of the fibrinogen receptor on human platelets by photoaffinity labeling. J Biol Chem. 1982 Jul 25;257(14):8049–8054. [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucaut J. C., Darribère T., Poole T. J., Aoyama H., Yamada K. M., Thiery J. P. Biologically active synthetic peptides as probes of embryonic development: a competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984 Nov;99(5):1822–1830. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray P. F., Rosa J. P., Lingappa V. R., Kan Y. W., McEver R. P., Shuman M. A. Biogenesis of the platelet receptor for fibrinogen: evidence for separate precursors for glycoproteins IIb and IIIa. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1480–1484. doi: 10.1073/pnas.83.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson K. J., McGregor J. L., McEver R. P., Jacques Y. V., Bainton D. F., Domzig W., Baggiolini M. Absence of platelet membrane glycoproteins IIb/IIIa from monocytes. J Exp Med. 1985 May 1;161(5):972–983. doi: 10.1084/jem.161.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Languino L. R., Polentarutti N., Balconi G., Ryckewaert J. J., Larrieu M. J., Donati M. B., Mantovani A., Marguerie G. Interaction between fibrinogen and cultured endothelial cells. Induction of migration and specific binding. J Clin Invest. 1985 Jan;75(1):11–18. doi: 10.1172/JCI111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Vergara-Dauden M., Balconi G., Pietra A., Cherel G., Donati M. B., Larrieu M. J., Marguerie G. Specific binding of human fibrinogen to cultured human fibroblasts. Evidence for the involvement of the E domain. Eur J Biochem. 1984 Mar 15;139(3):657–662. doi: 10.1111/j.1432-1033.1984.tb08054.x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion and the molecular processes of morphogenesis. Annu Rev Biochem. 1985;54:135–169. doi: 10.1146/annurev.bi.54.070185.001031. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Charo I. F., Phillips D. R. Human and bovine endothelial cells synthesize membrane proteins similar to human platelet glycoproteins IIb and IIIa. J Biol Chem. 1985 Sep 15;260(20):10893–10896. [PubMed] [Google Scholar]
- Gardner J. M., Hynes R. O. Interaction of fibronectin with its receptor on platelets. Cell. 1985 Sep;42(2):439–448. doi: 10.1016/0092-8674(85)90101-1. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Bennett J. S. The tetrapeptide analogue of the cell attachment site of fibronectin inhibits platelet aggregation and fibrinogen binding to activated platelets. J Biol Chem. 1985 Oct 5;260(22):11891–11894. [PubMed] [Google Scholar]
- Ginsberg M. H., Forsyth J., Lightsey A., Chediak J., Plow E. F. Reduced surface expression and binding of fibronectin by thrombin-stimulated thrombasthenic platelets. J Clin Invest. 1983 Mar;71(3):619–624. doi: 10.1172/JCI110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M., Pierschbacher M. D., Ruoslahti E., Marguerie G., Plow E. Inhibition of fibronectin binding to platelets by proteolytic fragments and synthetic peptides which support fibroblast adhesion. J Biol Chem. 1985 Apr 10;260(7):3931–3936. [PubMed] [Google Scholar]
- Gogstad G., Hetland O., Solum N. O., Prydz H. Monocytes and platelets share the glycoproteins IIb and IIIa that are absent from both cells in Glanzmann's thrombasthenia type I. Biochem J. 1983 Aug 15;214(2):331–337. doi: 10.1042/bj2140331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Gottlieb D. I. Monoclonal antibodies which alter the morphology of cultured chick myogenic cells. J Cell Biochem. 1982;18(2):221–229. doi: 10.1002/jcb.1982.240180209. [DOI] [PubMed] [Google Scholar]
- Haverstick D. M., Cowan J. F., Yamada K. M., Santoro S. A. Inhibition of platelet adhesion to fibronectin, fibrinogen, and von Willebrand factor substrates by a synthetic tetrapeptide derived from the cell-binding domain of fibronectin. Blood. 1985 Oct;66(4):946–952. [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- Knudsen K. A., Horwitz A. F., Buck C. A. A monoclonal antibody identifies a glycoprotein complex involved in cell-substratum adhesion. Exp Cell Res. 1985 Mar;157(1):218–226. doi: 10.1016/0014-4827(85)90164-8. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., Rao P. E., Damsky C. H., Buck C. A. Membrane glycoproteins involved in cell--substratum adhesion. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6071–6075. doi: 10.1073/pnas.78.10.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin E. G. Latent tissue plasminogen activator produced by human endothelial cells in culture: evidence for an enzyme-inhibitor complex. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6804–6808. doi: 10.1073/pnas.80.22.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Marguerie G. A., Thomas-Maison N., Ginsberg M. H., Plow E. F. The platelet-fibrinogen interaction. Evidence for proximity of the A alpha chain of fibrinogen to platelet membrane glycoproteins IIb/III. Eur J Biochem. 1984 Feb 15;139(1):5–11. doi: 10.1111/j.1432-1033.1984.tb07968.x. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Baenziger N. L., Majerus P. W. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980 Dec;66(6):1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Birchmeier W. New surface component of fibroblast's focal contacts identified by a monoclonal antibody. Cell. 1982 Dec;31(3 Pt 2):671–679. doi: 10.1016/0092-8674(82)90322-1. [DOI] [PubMed] [Google Scholar]
- Parise L. V., Phillips D. R. Reconstitution of the purified platelet fibrinogen receptor. Fibrinogen binding properties of the glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Sep 5;260(19):10698–10707. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977 Sep;60(3):535–545. doi: 10.1172/JCI108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M., Hayman E. G., Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1224–1227. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Birdwell C., Ginsberg M. H. Identification and quantitation of platelet-associated fibronectin antigen. J Clin Invest. 1979 Mar;63(3):540–543. doi: 10.1172/JCI109334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Bader R., de Marco L. Glanzmann thrombasthenia: deficient binding of von Willebrand factor to thrombin-stimulated platelets. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6038–6041. doi: 10.1073/pnas.79.19.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadle P. J., Ginsberg M. H., Plow E. F., Barondes S. H. Platelet-collagen adhesion: inhibition by a monoclonal antibody that binds glycoprotein IIb. J Cell Biol. 1984 Dec;99(6):2056–2060. doi: 10.1083/jcb.99.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz K. M., Zhen Y., August J. T. A human granulocyte-specific antigen characterized by use of monoclonal antibodies. Blood. 1983 Jan;61(1):19–26. [PubMed] [Google Scholar]
- Springer W. R., Cooper D. N., Barondes S. H. Discoidin I is implicated in cell-substratum attachment and ordered cell migration of Dictyostelium discoideum and resembles fibronectin. Cell. 1984 Dec;39(3 Pt 2):557–564. doi: 10.1016/0092-8674(84)90462-8. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tabilio A., Rosa J. P., Testa U., Kieffer N., Nurden A. T., Del Canizo M. C., Breton-Gorius J., Vainchenker W. Expression of platelet membrane glycoproteins and alpha-granule proteins by a human erythroleukemia cell line (HEL). EMBO J. 1984 Feb;3(2):453–459. doi: 10.1002/j.1460-2075.1984.tb01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan P., Shapiro S. S., Levine E., DeMarco L., Yalcin A. A monoclonal antibody to human platelet glycoprotein IIIa detects a related protein in cultured human endothelial cells. J Clin Invest. 1985 Mar;75(3):896–901. doi: 10.1172/JCI111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]