Abstract
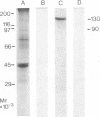
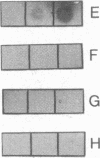
Monoclonal antibodies against some of the monomorphic determinants of major histocompatibility complex (MHC) class I molecules reduce insulin binding and precipitate 125I-labeled insulin receptor preparations. A monoclonal antibody with specificity for the insulin binding site on the cell membrane insulin receptor of human cells was used to precipitate insulin receptors from human cell lines and resulted in distinct bands of Mr approximately 130,000, 90,000, and 45,000. The Mr 45,000 molecules thus precipitated were subjected to NaDodSO4/PAGE, eluted from the gels, and found to react with monoclonal antibodies against monomorphic and a polymorphic MHC class I determinant known to be expressed on the cell line used as receptor source. Moreover, a murine thymoma line (RI) with MHC class I expression bound significant amounts of insulin, whereas a MHC class I-negative variant had low insulin binding capacity. Reduction in the density on human cells of the MHC class I heavy chain was obtained by capping with antibodies to beta 2-microglobulin or to the MHC class I heavy chain and resulted in decreased insulin binding, whereas down-regulation of insulin receptors induced increased density of MHC class I molecules. It is concluded that the MHC class I heavy chain and the tetrameric insulin receptor are structurally associated in the cell membrane and suggested that this association may occur by displacement of beta 2-microglobulin by the insulin receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen R. B., Killmann S. A., Olsson L. A monoclonal antibody (NAT-9 II:3F-6F) that identifies a differentiation antigen on human myeloid cells. Leuk Res. 1985;9(9):1161–1170. doi: 10.1016/0145-2126(85)90107-9. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. HLA structure and function: a contemporary view. Tissue Antigens. 1981 Jan;17(1):9–20. doi: 10.1111/j.1399-0039.1981.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Claas F. H., van Rood J. J. The interaction of drugs and endogeneous substances with HLA class-I antigens. Prog Allergy. 1985;36:135–150. [PubMed] [Google Scholar]
- Due C., Linnet K., Langeland Johansen N., Olsson L. Analysis of insulin receptors on heterogeneous eukaryotic cell populations with fluorochrome-conjugated insulin and fluorescence-activated cell sorter. Advantages and limitations to the 125I-labelled insulin methodology. Diabetologia. 1985 Oct;28(10):749–755. doi: 10.1007/BF00265023. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Peyron J. F., Samson M., Van Obberghen E., Brandenburg D., Brossette N. Molecular association between major histocompatibility complex class I antigens and insulin receptors in mouse liver membranes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8634–8637. doi: 10.1073/pnas.82.24.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Choi S., Sakamoto Y., Itakura K. Purification of insulin receptor with full binding activity. J Biol Chem. 1983 Apr 25;258(8):5045–5049. [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Iványi P. Some aspects of the H-2 system, the major histocompatibility system in the mouse. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):117–158. doi: 10.1098/rspb.1978.0060. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Kahn C. R., Hedo J. A., Van Obberghen E., Yamada K. M. Insulin-induced receptor loss in cultured human lymphocytes is due to accelerated receptor degradation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6917–6921. doi: 10.1073/pnas.78.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefford R. F., Calabi F., Fearnley I. M., Burrone O. R., Milstein C. Serum beta 2-microglobulin binds to a T-cell differentiation antigen and increases its expression. Nature. 1984 Apr 12;308(5960):641–642. doi: 10.1038/308641a0. [DOI] [PubMed] [Google Scholar]
- Kosmakos F. C., Roth J. Insulin-induced loss of the insulin receptor in IM-9 lymphocytes. A biological process mediated through the insulin receptor. J Biol Chem. 1980 Oct 25;255(20):9860–9869. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafuse W., Edidin M. Influence of the mouse major histocompatibility complex, H-2, on liver adenylate cyclase activity and on glucagon binding to liver cell membranes. Biochemistry. 1980 Jan 8;19(1):49–54. doi: 10.1021/bi00542a008. [DOI] [PubMed] [Google Scholar]
- Ohno S. The original function of MHC antigens as the general plasma membrane anchorage site of organogenesis-directing proteins. Immunol Rev. 1977 Jan;33:59–69. [PubMed] [Google Scholar]
- Olsson L., Forchhammer J. Induction of the metastatic phenotype in a mouse tumor model by 5-azacytidine, and characterization of an antigen associated with metastatic activity. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3389–3393. doi: 10.1073/pnas.81.11.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson L., Kronstrøm H., Cambon-De Mouzon A., Honsik C., Brodin T., Jakobsen B. Antibody producing human-human hybridomas. I. Technical aspects. J Immunol Methods. 1983 Jun 24;61(1):17–32. doi: 10.1016/0022-1759(83)90004-2. [DOI] [PubMed] [Google Scholar]
- Olsson L., Sorensen H. R., Behnke O. Intratumoral phenotypic diversity of cloned human lung tumor cell lines and consequences for analyses with monoclonal antibodies. Cancer. 1984 Nov 1;54(9):1757–1765. doi: 10.1002/1097-0142(19841101)54:9<1757::aid-cncr2820540902>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E., Beck-Nielsen H., Kromann H. Diabetes mellitus caused by insulin-receptor blockade and impaired sensitivity to insulin. N Engl J Med. 1981 Apr 30;304(18):1085–1088. doi: 10.1056/NEJM198104303041806. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Päbo S., Kämpe O., Severinsson L., Andersson M., Fernandez C., Peterson P. A. The association between class-I transplantation antigens and an adenovirus membrane protein. Prog Allergy. 1985;36:114–134. [PubMed] [Google Scholar]
- Rosa F., Berissi H., Weissenbach J., Maroteaux L., Fellous M., Revel M. The beta2-microglobulin mRNA in human Daudi cells has a mutated initiation codon but is still inducible by interferon. EMBO J. 1983;2(2):239–243. doi: 10.1002/j.1460-2075.1983.tb01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Festenstein H., Ward P. J., Sanderson A. R. Interspecies exchange of beta 2-microglobulin and associated MHC and differentiation antigens. Immunogenetics. 1981;13(6):483–491. doi: 10.1007/BF00343716. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Schlessinger J., Edidin M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J Cell Biol. 1984 Feb;98(2):725–731. doi: 10.1083/jcb.98.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen M., Olsson L. Possible roles of compound membrane receptors in the immune system. Ann Immunol (Paris) 1983 Jul-Aug;134D(1):85–92. doi: 10.1016/s0769-2625(83)80059-2. [DOI] [PubMed] [Google Scholar]
- Simonsen M., Skjødt K., Crone M., Sanderson A., Fujita-Yamaguchi Y., Due C., Rønne E., Linnet K., Olsson L. Compound receptors in the cell membrane: ruminations from the borderland of immunology and physiology. Prog Allergy. 1985;36:151–176. [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]