Abstract
Fibered confocal fluorescence in vivo imaging with a fiber optic bundle uses the same principle as fluorescent confocal microscopy. It can excite fluorescent in situ elements through the optical fibers, and then record some of the emitted photons, via the same optical fibers. The light source is a laser that sends the exciting light through an element within the fiber bundle and as it scans over the sample, recreates an image pixel by pixel. As this scan is very fast, by combining it with dedicated image processing software, images in real time with a frequency of 12 frames/sec can be obtained.
We developed a technique to quantitatively characterize capillary morphology and function, using a confocal fluorescence videomicroscopy device. The first step in our experiment was to record 5 sec movies in the four quadrants of the tumor to visualize the capillary network. All movies were processed using software (ImageCell, Mauna Kea Technology, Paris France) that performs an automated segmentation of vessels around a chosen diameter (10 μm in our case). Thus, we could quantify the 'functional capillary density', which is the ratio between the total vessel area and the total area of the image. This parameter was a surrogate marker for microvascular density, usually measured using pathology tools.
The second step was to record movies of the tumor over 20 min to quantify leakage of the macromolecular contrast agent through the capillary wall into the interstitium. By measuring the ratio of signal intensity in the interstitium over that in the vessels, an 'index leakage' was obtained, acting as a surrogate marker for capillary permeability.
Keywords: Medicine, Issue 79, Cancer, Biological, Microcirculation, optical imaging devices (design and techniques), Confocal videomicroscopy, microcirculation, capillary leakage, FITC-Dextran, angiogenesis
Introduction
Angiogenesis is a complex process 1 that involves the formation of new blood vessels from pre-existing vessels. Pathological changes in tissue microcirculation, composed of arterioles, capillaries, and venules, are implicated in a large range of diseases such as cancer, inflammation, or diabetes. It is therefore essential to develop methods to quantitatively assess microvessel structure and function. Imaging enables the study of microvessels in a non- or micro-invasive manner, in real-time and in vivo, and repeated measures over time in the same animal 2.
Currently, dynamic contrast-enhanced (DCE) imaging 3 is commonly used to assess tissue microcirculation. Dynamic contrast-enhanced imaging is a technique which follows over time the biodistribution of a tracer injected intravenously. From this acquisition, quantitative parameters can be extracted reflecting tissue vascularization. DCE imaging has been most often used with CT, MRI or ultrasound. However, these imaging techniques do not allow direct viewing of the microvessels, since their resolution, other than with the use of specific experimental devices, most often remains macroscopic.
In this paper, we propose to study the tumor vasculature at the microscopic scale and in vivo using dynamic contrast-enhanced optical imaging, with fibered confocal videomicroscopy. We used a macromolecular contrast agent (FITC-dextran) which remains exclusively within vessels or leaks through the endothelial barrier into the interstitium, according to its molecular weight and the characteristics of the endothelium of the tissue studied 4. This allowed the study of both microvessel structure, by correctly delineating vessels, and capillary permeability, by leaking and accumulating in the interstitium.
Protocol
1. Preparation of the Contrast Agent
For FITC-dextran 70 kDa, the dose injected is 500 mg/kg (10 mg of FITC-dextran diluted in 0.1 ml of saline for a mouse weighing 20 g).
The agent should not be exposed too long to light. To avoid bleaching, it is recommended to cover the tube with aluminum foil.
2. Anesthesia
Mice were anesthetized by an intraperitoneal injection of a mixture of 1:4 of xylazine (Rompun 2%, Bayer, Puteaux, France) and Ketamine (Kétamine 500, Virbac, Carros, France), respectively 66 mg/kg and 264 mg/kg for a 20 g mouse.
3. Preparation of the Organ of Interest
We shaved the mice at the location of interest (for example, over a subcutaneous tumor). Animal hair is often auto-fluorescent when white. When black, it absorbs light.
The skin facing the organ to be imaged was incised. It is important to wait until the bleeding has stopped before injecting the contrast agent, otherwise it will leak in the blood and contaminate the image.
4. Acquisition
The contrast agent was injected through either the jugular vein or the caudal vein. There is no or little background signal in the organ observed in the absence of a fluorescent contrast agent.
The probe was placed in front of the organ to be imaged. In our study, this was the tumor.
The laser was turned on to illuminate the tumor and see the fluorescence in the capillaries.
The tumor was explored manually by moving the probe in a very slow movement while recording to visualize the capillary network. It is important to maintain a steady hand, and this technique requires a little experience. In our study, this first step allowed quantification of functional capillary density.
The second step was the dynamic acquisition over time. For this study, we used a 70 kDa FITC-dextran. There is no interstitial leakage in most normal organs but there is in tumors. To acquire images of the same location over time (as in our case), it is important to set up a system to maintain the probe on the area of interest. This was done by using a handmade support to hold the probe, and by placing a bit of ultrasound gel on the tip of the probe. Before recording, time was spent to stabilize the probe placed in contact with the tumor. Once the position was secured, there was only minimal motion due to the mouse's breathing. The laser was turned on to record 3 images every 30 sec for 20 min to detect the presence of capillary leakage. It was turned off between each recording to reduce contrast agent bleaching.
In our experiment, mice were sacrificed at the end of the procedure for histological analysis of tumors.
Representative Results
Using the data collected, we could quantitatively analyze different parameters reflecting microcirculation.
We studied in vivo the peripheral vascular network of a colon tumor implanted in balb-c mice using a fibered confocal fluorescence videomicroscopy system (Cellvizio, Maunakea Technology, Paris, France 2), after injection of a macromolecular fluorescent contrast agent Fluorescein IsoThioCyanate-dextran (FITC-dextran) with a molecular weight of 70 kDa (Sigma-Aldrich, Saint-Quentin Fallavier, France) and with excitation and emission wavelengths of 488 nm and 520 nm respectively (for compatibility with our imaging system).
The first step in our experiment was to record 5 sec movies in each of the four quadrants of the tumor to visualize the capillary network. This allowed representative sampling of the tumor vascularization. All movies were processed using a software (ImageCell, Mauna Kea Technology, Paris France) performing an automated segmentation of vessels in the images around a chosen diameter (10 μm in our case, which included vessels ranging from 5-20 μm in diameter). Thus, we could quantify the 'functional capillary density' (FCD), which is the ratio between the total vessel area and the total area of the image. This parameter was a surrogate marker of microvascular density, usually measured using pathology tools. Figure 1 shows an example of the type of images obtained and the result of vessel segmentation. In this example, FCD was measured as 36%.
Then, three images were recorded every 30 sec for 20 min to detect the presence of capillary leakage. Visual examination of the images was first performed, to evaluate the absence or presence of contrast agent leakage into the interstitium as well as its spatial distribution (homogeneous or heterogeneous).
We drew three regions of interest (ROI) in the capillaries and three ROI in the interstitium at time points 0, 5, 10 and 20 min. The signal intensities (SI) within the three different capillaries and contiguous interstitial areas were averaged at each time point. Index leakage (%) was calculated as follows = Σ [(Ip1/Ii1) + (Ip2/Ii2) + (Ip3/Ii3)] x 100/3, where Ip is perivascular (or interstitial) intensity and Ii is intravascular intensity 5-7. Figure 2 shows an example of contrast agent leakage in the interstitium. In this example, index leakage was measured as 1.47.
This dynamic contrast-enhanced optical imaging technique allows in vivo measurements of tumor microcirculation. It reflects the architecture of tumor vessels by quantifying capillary density, and their functionality by quantifying capillary permeability.
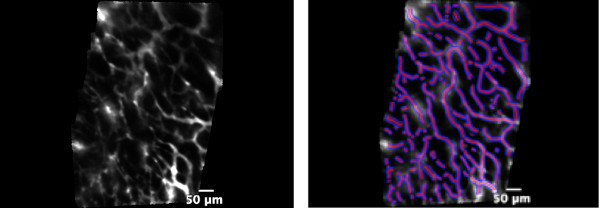 Figure 1. Left: picture of microvessels in the superficial layer of the tumor. Right: application of the vessel detection module to automatically segment vessels with diameters ranging from 5-20 μm (diameter of interest: 10 μm). The segmented vessels are highlighted in purple.
Figure 1. Left: picture of microvessels in the superficial layer of the tumor. Right: application of the vessel detection module to automatically segment vessels with diameters ranging from 5-20 μm (diameter of interest: 10 μm). The segmented vessels are highlighted in purple.
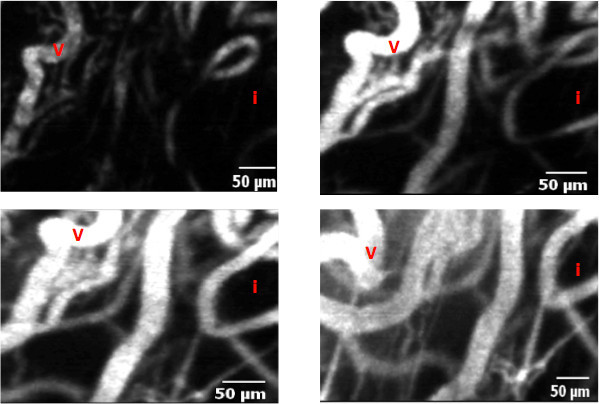 Figure 2. Leakage from the capillaries to the interstitium at different time points, respectively t0 (a), t5 (b), t10 (c) and t20 (d). Vessels (V) are seen as high signal linear structures. Before injection (t0), no signal is seen in the interstitium (I). Progressively, an enhancement can be seen in the interstitium due to a leak of the fluorescent contrast agent through the abnormal tumor endothelial barrier.
Figure 2. Leakage from the capillaries to the interstitium at different time points, respectively t0 (a), t5 (b), t10 (c) and t20 (d). Vessels (V) are seen as high signal linear structures. Before injection (t0), no signal is seen in the interstitium (I). Progressively, an enhancement can be seen in the interstitium due to a leak of the fluorescent contrast agent through the abnormal tumor endothelial barrier.
Discussion
The study of tumor microcirculation has become essential in understanding the pathophysiology of tumor growth, dissemination and response to therapy 1. Optical imaging is one of the techniques that can be used to observe the capillaries using a fluorescent contrast agent and to quantify morphological (Functional Capillary Density) and functional (index leakage) parameters.
The fluorescence microscopy imaging we used in this study has both advantages and limits. One advantage is being able to choose the size of the contrast agent used. Here, with a 70-kDa FITC-dextran, leakage through the endothelial barrier at the beginning of the experiment was minimal, which allowed us to observe the initial morphology (tortuosity, anarchic network, etc.) of the vessels with a good contrast between vessels and interstitium 8, and after a delay, a leakage of the contrast agent over 20 min of observation. The in-plane (xy) resolution was high (3.5 μm), which allowed us to visualize the vessels and the interstitium at a microscopic level, instead of a macroscopic level as with most other imaging techniques (MRI, CT, ultrasound, PET…). Finally, this is real-time imaging, which means that changes can be observed as they occur.
However, there are disadvantages to this technique. The apparatus is delicate to operate. Indeed, the probe is very small (1.8 mm) and slippery and it is difficult to stay in the same place on the tumor over long periods of time. The animal's breathing motions also compromise steadiness. To improve this, we used ultrasound gel to immobilize the probe and a handmade support to maintain the probe in position. Furthermore, we can explore only the superficial area of the tumor (from 100 μm to 170 μm), which means that the results obtained concern only the most superficial layers of the tumor.
The main limit, however, is the difficulty in reaching absolute quantification using optical imaging. Index leakage is a ratio, and therefore only a semi-quantitative parameter. First, there are artifacts due to partial voluming in the ROI. Indeed, though the in-plane resolution is high, the z-plane resolution is low (slice thickness of 70 μm), which means that it includes both vessels and interstitium. Therefore, when measuring signal intensity in a vessel with a 10-μm diameter, it is averaged with the surrounding interstitium included in the slice. Also, in optical imaging, there is a complex relation between signal intensity and contrast agent concentration. When a tissue is illuminated by photons, many events may occur simultaneously and influence the signal collected. There are natural chromophores in tissues that can absorb excitation or emission photons such as hemoglobin or collagen. There is also some diffusion which disperses photons in several directions. Finally, bleaching is probably one of the most important issues when using FITC, because it induces a loss of signal independent of concentration. Several research groups are working on quantifying the optical signal but this implies a complex modelization 9,10.
Finally, longitudinal studies are not easily performed. We had to incise the skin to reveal the tumor in order to acquire the images, and it may prove difficult to close up the incision, particularly when the organ observed is deep in the body cavity (e.g. liver or kidney).
Overall, we developed a dynamic contrast-enhanced fluorescence optical imaging technique to quantitatively characterize capillary anatomy and function, using a confocal fluorescence videomicroscopy device. This technique requires further validation, but may be useful for comparing tumor vascularization before and after therapy, or between tumor models.
Disclosures
We have nothing to disclose.
References
- Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3(7):643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- Laemmel E, Genet M, Le Goualher G, Perchant A, Le Gargasson JF, Vicaut E. Fibered confocal fluorescence microscopy (Cell-viZio) facilitates extended imaging in the field of microcirculation. A comparison with intravital microscopy. J Vasc Res. 2004;41(5):400–411. doi: 10.1159/000081209. [DOI] [PubMed] [Google Scholar]
- Charnley N, Donaldson S, Price P. Imaging angiogenesis. Methods Mol Biol. 2009;467:25–51. doi: 10.1007/978-1-59745-241-0_2. [DOI] [PubMed] [Google Scholar]
- Faye N, Fournier L, Balvay D, Taillieu F, Cuenod C, Siauve N, Clement O. Dynamic contrast enhanced optical imaging of capillary leakage. Technol Cancer Res Treat. 2011;10(1):49–57. doi: 10.7785/tcrt.2012.500179. [DOI] [PubMed] [Google Scholar]
- Kurose I, Kubes P, Wolf R, Anderson DC, Paulson J, Miyasaka M, Granger DN. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res. 1993;73(1):164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- Faye NFL, Balvay D, Thiam R, Orliaguet G, Clement O, Dewachter P. Macromolecular capillary leakage is involved in the onset of anaphylactic hypotension. Anesthesiology. 2012. [DOI] [PubMed]
- Faye N, Fournier L, Balvay D, Thiam R, Orliaguet G, Clement O, Dewachter P. Macromolecular Capillary Leakage Is Involved in the Onset of Anaphylactic Hypotension. Anesthesiology. 2012;117(5):1072–1079. doi: 10.1097/ALN.0b013e31826d3dc5. [DOI] [PubMed] [Google Scholar]
- Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5(6):423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- Ntziachristos V, Schellenberger EA, Ripoll J, Yessayan D, Graves E, Bogdanov A, Josephson L, Weissleder R. Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V-Cy5.5 conjugate. Proc Natl Acad Sci U S A. 2004;101(33):12294–12299. doi: 10.1073/pnas.0401137101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccia DJ, Bevilacqua F, Durkin AJ, Merritt S, Tromberg BJ, Gulsen G, Yu H, Wang J, Nalcioglu O. In vivo quantification of optical contrast agent dynamics in rat tumors by use of diffuse optical spectroscopy with magnetic resonance imaging coregistration. Appl Opt. 2003;42(16):2940–2950. doi: 10.1364/ao.42.002940. [DOI] [PubMed] [Google Scholar]


