The function of sleep remains one of the mysteries of the ages, yet sleep is an evolutionarily preserved behavior manifest in diverse organisms including worms (1), flies (2), and all vertebrates thus far examined (3). Perhaps one of the most compelling arguments for a sleep function is its homeostatic control. In everyday language, this homeostasis usually refers to the tendency to sleep deeply after staying awake for a long period and, after sleeping over a long period, to stay awake. The mechanisms responsible for sleep homeostasis are likely to be intimately related to sleep function and therefore worthy of investigation. Morairty et al. (4) argue that a subset of interneurons expressing neuronal NOS (nNOS) and the substance P receptor [neurokinin-1 receptor (NK1)] is responsible for the link between sleep deprivation (SD)-induced “homeostatic sleep drive” and increased EEG slow wave activity (SWA). SWA is considered an index of the depth or efficacy of sleep as further discussed below. With increasing duration of SD, from 2 to 6 h, activity of these cortical nNOS neurons, assessed by Fos expression 2 h into the ensuing recovery sleep, increases in association with increasing SWA. In nNOS KO mice, the SWA homeostatic sleep response is attenuated, suggesting a role for nNOS in sleep homeostasis. The authors hypothesize that in WT mice, these cortical nNOS/NK1 neurons release NO in a Fos-marked, activity-dependent manner to elicit the SWA homeostatic sleep response. Somewhat surprisingly, in the nNOS KO mutants, the increased activity of nNOS neurons in response to SD (marked by Fos in NK1 neurons) remains unaffected. This implies that these neurons are not necessarily part of the homeostatic SWA generating circuit, in the sense that their increase in activity is not sufficient to generate the increased SWA. It remains to be tested whether their increase in activity in response to SD is necessary for the nNOS-dependent, homeostatic SWA response.
A construct, called the homeostatic sleep drive, is used in the study of Morairty et al. (4) to describe the underlying process(es) induced by SD. The authors index the homeostatic sleep drive by increased SWA, sleep consolidation (longer sleep episodes), and sleep duration, observed during recovery sleep. The relationships between these indices and the duration of SD are complex, and in some circumstances, the indices can be dissociated. Nevertheless, their examination and examination of the mechanisms involved would seem a reasonable approach to the function of sleep.
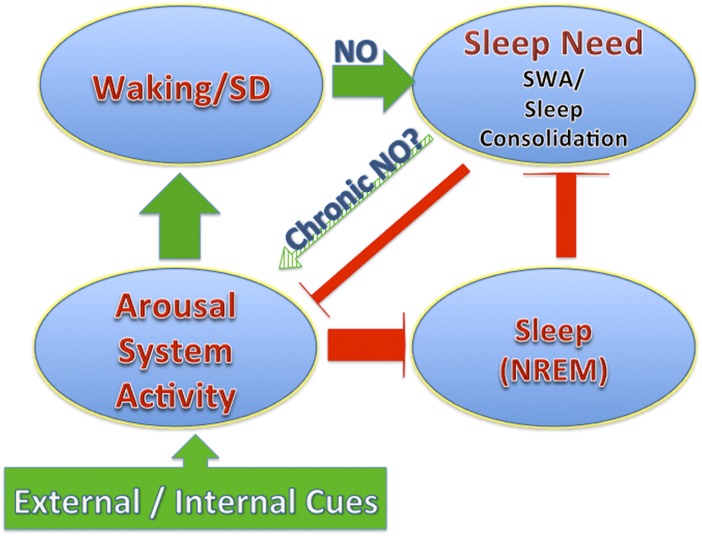
SWA refers to the low-frequency component (usually between 0.5 and 4.5 Hz) of the EEG field recording from the cortical surface and is often quantified by using a fast Fourier transform (FFT) to calculate power within the SWA bandwidth. SWA has long been considered a better indicator of the homeostatic sleep response than sleep duration because it is most rigorously and reproducibly correlated to SD duration (5, 6). Because of this correlation with previous waking, SWA power is considered an index for sleep need, a useful term in that it is independent of the tendency to fall asleep (as opposed to what may be implied by sleep drive), which is more likely to reflect a change in arousal (Fig. 1). For a particular genetic strain of mouse, the buildup of SWA as a function of prior waking duration and/or SD can be accurately described by a single exponential that is independent of circadian phase (7).
Fig. 1.
An illustration of the dissociable relationships of the arousal system and the sleep need or drive homeostatic system. The polarity of effects of the arousal system activation on waking is positive (green color) and on sleep is negative (red color). Waking promotes homeostatic sleep need, and sleep reduces homeostatic sleep need. In addition there is a mild but distinct effect of increased sleep need to reduce arousal system activity.
The SWA response to a given duration of SD is also relatively unaffected by different experiences during waking that have a large impact on multiple sleep latency tests (MSLTs) and sleep duration (Fig. 1). Effects on sleep duration and MSLTs are most closely associated with the level of arousal induced by differing SD techniques (8) or neuronal excitability as has, for example, been observed with mutations that reduce potassium channel activity in Drosophila (9).
We have all had the subjective experience of an increased tendency to fall asleep with prolonged waking (high sleep drive or sleep need). SD or prolonged synaptic release of glutamate is associated with the buildup of adenosine in basal forebrain cholinergic arousal centers (10) and brainstem cholinergic arousal centers (11). Increased adenosine tone is sufficient to decrease cholinergic neuron excitability (12, 13) and facilitate the transition to sleep (14). This is an effect on the arousal system (illustrated in Fig. 1). Although the actions of adenosine on arousal system neurons have been best characterized for the local circuit function of cholinergic arousal neurons, this kind of control likely extends to the monoaminergic nuclei that are also critical components of the arousal system. Furthermore, the adenosine-mediated facilitation to transition to sleep can be readily overcome by increased sensory activation of the arousal system activity as is routinely done experimentally to maintain SD even in the face of increased sleep drive.
Morairty et al. (4) observed a small but significant decrease in sleep duration under baseline conditions in nNOS KO mice and, somewhat paradoxically, a shorter latency to transition to sleep on the MSLT. It is likely that both phenomena indicate changes in the arousal system, but they are of opposite polarity. A decrease of arousal system activity may have been mediated by decreased NO-induced adenosine tone as has been reported in culture (15). What then mediates the reduced latency to sleep in nNOS KO mice during the MSLT? The study of Morairty et al. (4) suggests that nNOS does have a role in homeostatic sleep drive or need as indexed by attenuated SWA buildup following SD. Might this loss of drive be associated with a loss of sleep function needed for maintained arousal center activation with mild
The study of Morairty et al. suggests that nNOS does have a role in homeostatic sleep drive or need as indexed by attenuated SWA buildup following SD.
SD (mild SD is part of the MSLT protocol) or baseline conditions as was observed with the nNOS KO-induced decreased MSLT latencies? Reduced arousal system activity together with reduced homeostatic sleep drive is consistent with frequent transitions to sleep but with short sleep episode durations as was observed in the nNOS KO mice (Fig. 1).
The nNOS KO phenotype is most dramatic with respect to the attenuation of an SD-induced increase of SWA over baseline SWA. This phenotype is similar to that of the forebrain selective (i.e., does not affect GABAergic neurons, including the GSBSergic nNOS/NK1 neurons) conditional adenosine A1 receptor (AdoA1R) KO (16) with the notable difference that this conditional AdoA1R KO was selective for non-GABAergic neurons. Because the nNOS/NK1 neurons of the cortex are GABA neurons and the AdoA1R KO involves only non-GABA neurons of the cortex and subcortical structures, a necessary circuit for normal sleep homeostasis involving adenosine-activated AdoA1Rs on non-GABA neurons and NO released from nNOS/NK1, GABA neurons may be considered.
The dissociable effects on arousal and sleep homeostasis are observed in the study of Morairty et al., and with AdoA1R KO mice (16) as an attenuated increase of SWA in response to SD with small effect on sleep duration. A dissociation is also observed with increased sensory input to increase arousal system activity preventing sleep in the face of increased sleep need. These double dissociations are consistent with two related but distinct systems affecting sleep. nNOS and the nNOS/NK1 neurons appear to affect both systems to elicit different sleep-related phenomena, advancing our understanding of how sleep behavior is expressed.
Footnotes
The author declares no conflict of interest.
See companion article on page 20272.
References
- 1.Raizen DM, et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451(7178):569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 2.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287(5459):1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 3.Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr Biol. 2008;18(15):R670–R679. doi: 10.1016/j.cub.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morairty SR, et al. A role for cortical nNOS/NK1 neurons in coupling homeostatic sleep drive to EEG slow wave activity. Proc Natl Acad Sci USA. 2013;110:20272–20277. doi: 10.1073/pnas.1314762110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobler I, Borbély AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64(1):74–76. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 6.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 7.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21(8):2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki A, Sinton CM, Greene RW, Yanagisawa M. Behavioral and biochemical dissociation of arousal and homeostatic sleep need influenced by prior wakeful experience in mice. Proc Natl Acad Sci USA. 2013;110(25):10288–10293. doi: 10.1073/pnas.1308295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirelli C. The genetic and molecular regulation of sleep: From fruit flies to humans. Nat Rev Neurosci. 2009;10(8):549–560. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porkka-Heiskanen T, et al. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276(5316):1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brambilla D, Chapman D, Greene R. Adenosine mediation of presynaptic feedback inhibition of glutamate release. Neuron. 2005;46(2):275–283. doi: 10.1016/j.neuron.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Rainnie DG, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: Implications for EEG arousal. Science. 1994;263(5147):689–692. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arrigoni E, Rainnie DG, McCarley RW, Greene RW. Adenosine-mediated presynaptic modulation of glutamatergic transmission in the laterodorsal tegmentum. J Neurosci. 2001;21(3):1076–1085. doi: 10.1523/JNEUROSCI.21-03-01076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: A microdialysis study in the freely moving cat. Neuroscience. 1997;79(1):225–235. doi: 10.1016/s0306-4522(96)00640-9. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg PA, Li Y, Le M, Zhang Y. Nitric oxide-stimulated increase in extracellular adenosine accumulation in rat forebrain neurons in culture is associated with ATP hydrolysis and inhibition of adenosine kinase activity. J Neurosci. 2000;20(16):6294–6301. doi: 10.1523/JNEUROSCI.20-16-06294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29(5):1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]