Significance
An advantage of targeting reconsolidation to control reactions to learned threats is that the memory appears to be persistently altered, not inhibited. When these memories are diminished through extinction, the amygdala’s representation remains largely intact and the prefrontal cortex inhibits its expression, thus allowing the learned responses to recover. Targeting reconsolidation, therefore, should eliminate the necessity of prefrontal inhibition. We tested this hypothesis by contrasting standard extinction with extinction occurring during reconsolidation. We observed that behavioral interference of reconsolidation appears to bypass the prefrontal circuitry of extinction, inducing a more persistent loss of learned responses. Application of this strategy, which targets underlying learned threat processes, to fear and anxiety disorders may provide a more effective approach to treatment.
Keywords: fear, Pavlovian conditioning, defense, learning
Abstract
Controlling learned defensive responses through extinction does not alter the threat memory itself, but rather regulates its expression via inhibitory influence of the prefrontal cortex (PFC) over amygdala. Individual differences in amygdala–PFC circuitry function have been linked to trait anxiety and posttraumatic stress disorder. This finding suggests that exposure-based techniques may actually be least effective in those who suffer from anxiety disorders. A theoretical advantage of techniques influencing reconsolidation of threat memories is that the threat representation is altered, potentially diminishing reliance on this PFC circuitry, resulting in a more persistent reduction of defensive reactions. We hypothesized that timing extinction to coincide with threat memory reconsolidation would prevent the return of defensive reactions and diminish PFC involvement. Two conditioned stimuli (CS) were paired with shock and the third was not. A day later, one stimulus (reminded CS+) but not the other (nonreminded CS+) was presented 10 min before extinction to reactivate the threat memory, followed by extinction training for all CSs. The recovery of the threat memory was tested 24 h later. Extinction of the nonreminded CS+ (i.e., standard extinction) engaged the PFC, as previously shown, but extinction of the reminded CS+ (i.e., extinction during reconsolidation) did not. Moreover, only the nonreminded CS+ memory recovered on day 3. These results suggest that extinction during reconsolidation prevents the return of defensive reactions and diminishes PFC involvement. Reducing the necessity of the PFC–amygdala circuitry to control defensive reactions may help overcome a primary obstacle in the long-term efficacy of current treatments for anxiety disorders.
Efforts to control maladaptive defensive reactions through extinction or exposure therapy are sometimes short-lived because these techniques do not significantly alter the threat memory itself, but rather regulate its expression via the prefrontal cortex’s (PFC) inhibition of the amygdala (1, 2). Individual variation in the integrity of this amygdala–prefrontal circuitry has been linked to trait anxiety and posttraumatic stress disorder, suggesting that exposure-based techniques may be least effective in those who suffer from anxiety disorders (3–9).
Recently, it has been shown in mice (10, 11), rats (12), and humans (13–16) that precisely timing behavioral extinction to coincide with memory reconsolidation can persistently inhibit the return of defensive reactions (but see refs. 17–19 for a discussion of boundary conditions). Reconsolidation is the state to which memories enter upon retrieval, which makes them prone to interference (20–22). Behavioral interference of reconsolidation using extinction has been linked to alterations in glutamate receptor function in the amygdala, which plays a critical role in memory plasticity (10, 12). These findings are consistent with the suggestion that, in contrast to standard extinction training, extinction during reconsolidation may lead to long-lasting changes in the original threat memory (13, 16, 23).
To date, the impact of extinction occurring during threat memory reconsolidation on PFC involvement is unknown in humans and other species. Animal studies of standard extinction training (i.e., repeated presentations of a conditioned stimulus without the aversive outcome) show that extinction learning and recall are mediated via the infralimbic (IL) region of the medial PFC and its connections with the amygdala; IL projections activate inhibitory cells within the amygdala that block the generation of the defense response (24, 25). Functional MRI (fMRI) studies of extinction in humans typically show a decrease in blood-oxygenation level-dependent (BOLD) signal in the ventral medial PFC (vmPFC; the human homolog of IL) in acquisition and early extinction, and a gradual increase in BOLD activity with the progression of extinction training (26, 27). If extinction occurs during reconsolidation, how might the vmPFC’s role change? One possibility is that processes occurring during reconsolidation alter the extinction circuitry, diminishing vmPFC involvement. To test this hypothesis, we used fMRI to examine the vmPFC during behavioral interference of reconsolidation in humans.
BOLD responses were assessed during a 3-d protocol previously shown to interfere with reconsolidation (16). On day 1, two conditioned stimuli (CS+) were paired with a mild wrist shock (US, unconditioned stimulus); the third was not (CS−). On day 2, one CS+ (reminded CS+) but not the other (nonreminded CS+) was presented 10 min before extinction to reactivate the threat memory, followed by extinction training for all CSs. In this protocol, the reminded CS+ is presumably undergoing extinction during reconsolidation and the nonreminded CS+ is undergoing standard extinction training. On day 3, the threat memory was reinstated using four unsignaled shocks, followed by a test of recovery of the threat memory and another extinction session.
We found that timing extinction training to coincide with threat memory reconsolidation prevents the return of defensive reactions, and indeed significantly diminishes PFC involvement. During extinction, only the nonreminded CS+ engaged the vmPFC, but not the reminded CS+ or the CS−. The vmPFC, moreover, showed enhanced functional connectivity with the amygdala only during extinction of the nonreminded, but not the reminded CS+. This altered connectivity during extinction of the reminded CS+ may play a role in enabling extinction learning training to more persistently modify the original threat-memory trace within the amygdala, thus preventing the return of defensive reactions on subsequent recovery tests. Reducing the necessity of the prefrontal–amygdala circuitry to control learned defensive reactions may help overcome a primary obstacle in the long-term efficacy of current treatments for anxiety disorders.
Results
Behavioral Interference of Reconsolidation.
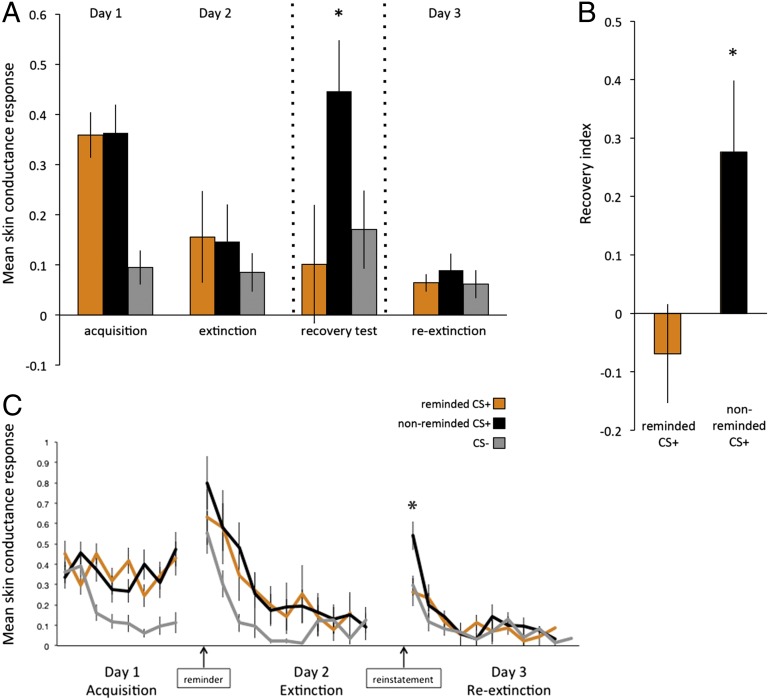
Consistent with behavioral interference of reconsolidation (16), skin conductance responses (SCR) showed no recovery for the reminded CS+ (Fig. 1). Specifically, on the first reextinction trial on day 3 we observed greater SCR for the nonreminded CS+ relative to the reminded CS+ (P < 0.01), and only the nonreminded CS+ significantly differed from the CS− (P < 0.05). A threat-memory recovery index was calculated as the first reextinction trial (first trial on day 3) minus the last extinction trial (last trial on day 2) for each of the CS+s minus the CS−. Importantly, the degree of acquisition and extinction learning for each CS+ before the recovery test was equivalent (this was the inclusion criteria; see Methods below). There was greater recovery for the nonreminded CS+ than the reminded CS+ (P < 0.01) in early reextinction, and only the nonreminded CS+ showed significant recovery (P < 0.05; all comparisons two-tailed t tests).
Fig. 1.
(A) Mean SCR (n = 19) by stimulus during late acquisition, extinction, and reextinction (second half); recovery test (first reextinction trial minus last extinction trial) shows threat memory reinstatement only to the nonreminded CS+ (between dashed lines). (B) Recovery index: recovery in SCR (first trial day 3 minus last trial day 2) for CS+ minus CS−. (C) Trial-by-trial mean SCR for each stimulus during acquisition, extinction, and reextinction. *P < 0.05 (two-tailed t-test).
Neuroimaging of Extinction During Reconsolidation.
Acquisition of threat memory.
Our primary regions of interests (ROI) were the amygdala and the vmPFC. On day 1, BOLD responses in both ROIs were consistent with previous studies of threat conditioning and did not differ for the reminded and nonreminded CS+s. Specifically, the amygdala ROI [defined based on day 1 US > baseline contrast, false-discovery rate (FDR) < 0.05] showed greater BOLD activation to both the reminded and nonreminded CS+ relative to CS− during late acquisition (Ps < 0.05), but not relative to each other (P > 0.5, not significant). The same contrast yielded activation in the vmPFC, which was characterized by decreased BOLD response to both the reminded and nonreminded CS+ relative to the CS− (P < 0.05), but not relative to each other (P > 0.9, not significant; all comparisons two-tailed t tests).
Extinction of threat memory.
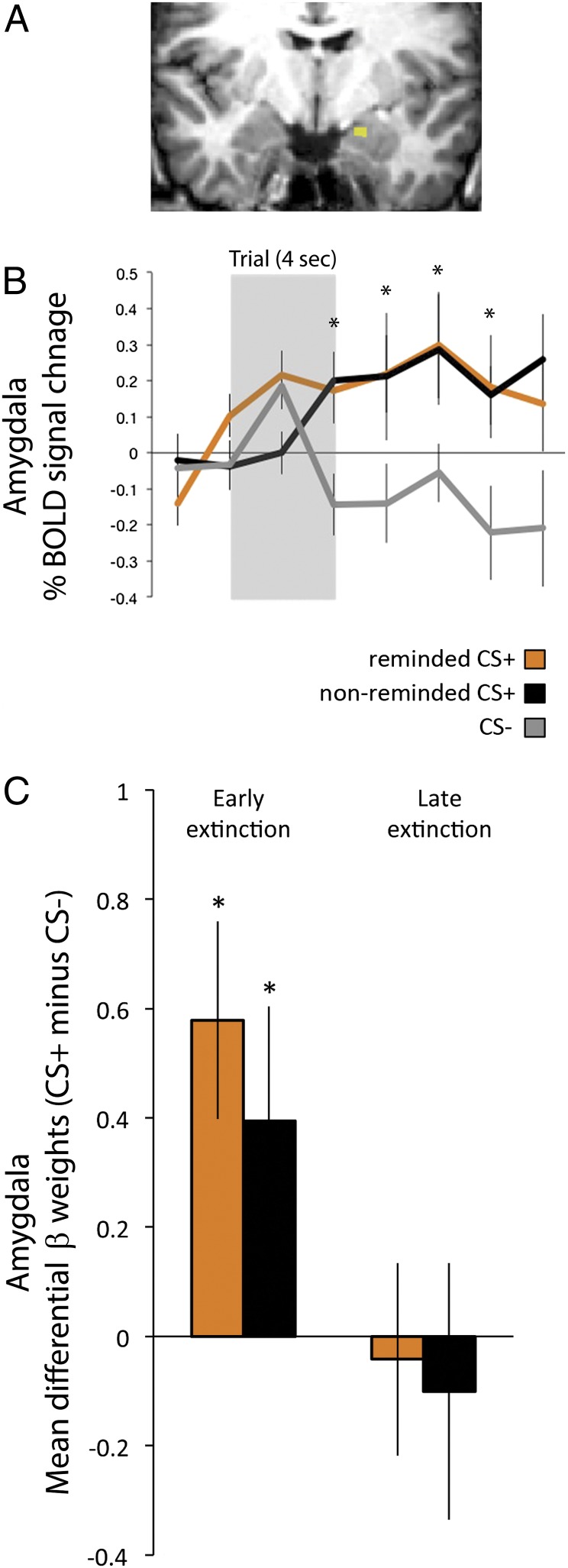
The primary BOLD analysis of interest was day 2 extinction training. The amygdala ROI showed increased BOLD signal on day 2 to both reminded and nonreminded CS+ in early extinction relative to CS−. As extinction progressed, this differential activation diminished, mirroring the diminished conditioned defensive responses during extinction training (Fig. 2). These results were supported by a two-way ANOVA on these difference scores with main factors time (early, late) and CS type (reminded, nonreminded, relative to CS−) yielding a significant main effect of time [F(1,36) = 6.34, P < 0.05] and no interaction (F < 1, not significant). Follow-up t tests showed that there was a significant differential BOLD increase for both reminded and nonreminded CS+ during early but not late extinction (P < 0.05).
Fig. 2.
(A) Amygdala ROI was defined based on the contrast US > fixation on day 1 (FDR < 0.05; x = −17, y = −12, z = −4). (B) On day 2, the BOLD time course of this region showed greater percent signal change to both reminded and nonreminded CS+ relative to CS− during early extinction. (C) As extinction progressed, the amygdala mean differential β weights (CS+ minus CS−) decreased for the reminded and nonreminded CS+. *P < 0.05 (one-tailed t-test); error bars, SEM.
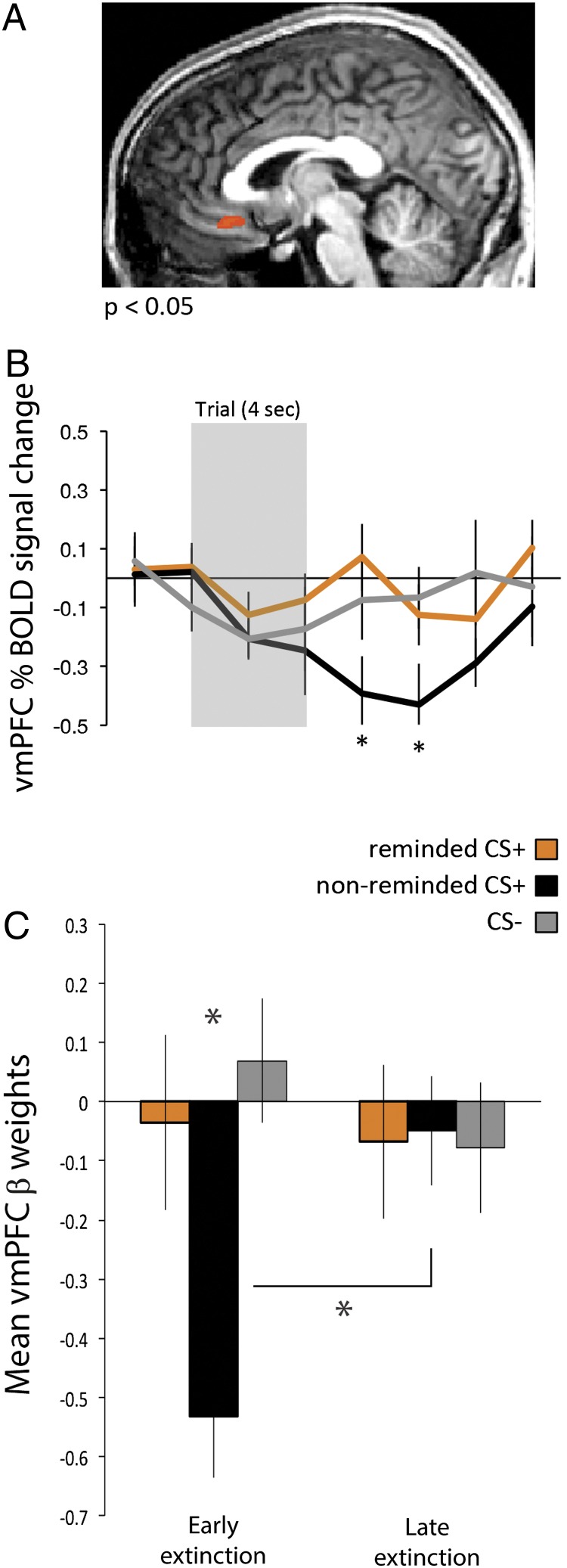
To test our primary hypothesis, we conducted a direct, whole-brain contrast of the two CS+s during early extinction. This process resulted in a single ROI in the vmPFC (Fig. 3A) (P < 0.05, corrected for multiple comparisons using cluster-level threshold estimator). An examination of the BOLD pattern in this region revealed that the nonreminded CS+ showed a decrease in BOLD signal relative to the CS− in early extinction, consistent with previous fMRI studies of extinction (26, 27). In contrast, the BOLD response to the reminded CS+ was similar to the CS−.
Fig. 3.
(A) Direct contrast of reminded vs. nonreminded CS+ during early extinction revealed only a vmPFC region (P < 0.05, corrected; BA 24). (B) Contrasting nonreminded CS+ vs. CS− in early extinction revealed an overlapping vmPFC region (P < 0.05, corrected). The BOLD time course of the nonreminded CS+ differed from the reminded CS+ during early extinction. The reminded CS+ did not differ from baseline (or CS−). (C) In early extinction, vmPFC mean BOLD responses (from the nonreminded CS+ vs. CS− in early extinction contrast) to the nonreminded CS+ were significantly lower than to the reminded CS+, which did not differ from CS−. As extinction progressed (from first to second half of extinction), the vmPFC mean BOLD responses increased only to the nonreminded CS+. Responses to the reminded CS+ and CS− remained at baseline level and did not change over time. *P < 0.05 (two-tailed t test); n.s., nonsignificant; error bars, SEM.
To assess if a similar vmPFC region emerged from a standard extinction contrast, and to select an ROI for further exploration independent of the critical reminded CS+ condition, we conducted a second direct contrast of the nonreminded CS+ vs. CS− during early extinction. This test revealed an overlapping vmPFC ROI (P < 0.05, corrected) with the same pattern of BOLD response (Fig. 3B). To explore how these vmPFC BOLD responses changed during extinction training, we compared early to late extinction for all three CSs (Fig. 3C). Consistent with previous studies (26, 27), as extinction progressed, vmPFC BOLD activation to the nonreminded CS+ increased. In contrast, BOLD responses did not change during extinction training to the reminded CS+ or CS−. A two-way ANOVA yielded a significant time × CS type interaction in the vmPFC [F(1,54) = 5.75, P < 0.01]. Follow-up t tests showed that the vmPFC BOLD to the nonreminded CS+ were significantly lower from the BOLD responses to the reminded CS+, which did not differ from CS−. As extinction progressed, the vmPFC BOLD responses increased from early to late extinction only for the nonreminded CS+ (P < 0.01), but not for the reminded CS+ or CS−.
Previous research has shown that BOLD responses during extinction are linked to later expression of the threat memory (26, 27). Consistent with this finding, we found that the increase in BOLD response to the nonreminded CS+ during extinction showed a marginally significant correlation with the recovery index (r = −0.39, P < 0.06, one-tailed), such that the greater the BOLD increase from early to late extinction on day 2, the less recovery of the conditioned response on day 3. No such correlation was observed for the reminded CS+ (r = −0.03, P = 0.45, one-tailed).
Condition-specific functional connectivity between vmPFC and amygdala during extinction.
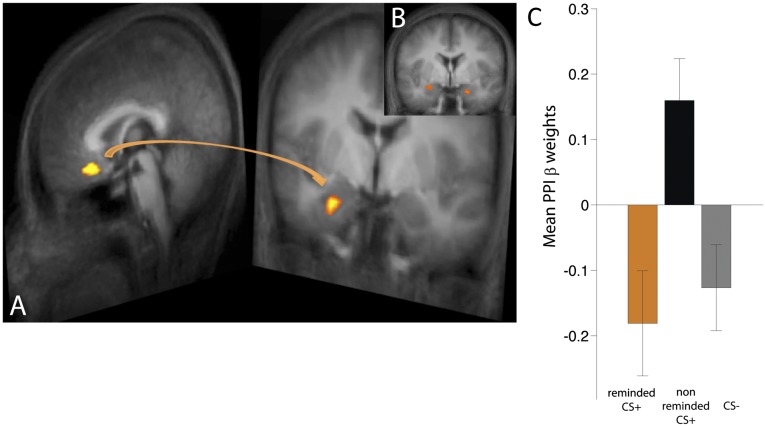
We further examined the pattern of connectivity during early extinction using the vmPFC ROI (from the reminded vs. nonreminded CS+ contrast) as seed (FDR < 0.05). We found robust coupling between vmPFC and amygdala (Fig. 4A). Follow-up psychophysiological interaction analysis (Fig. 4 B and C) revealed that the correlation between these regions was significantly stronger during the nonreminded CS+ versus the reminded CS+ and CS− (P < 0.05, corrected).
Fig. 4.
(A) Functional connectivity during early extinction using the vmPFC ROI as seed revealed robust coupling with amygdala (FDR < 0.05). (B) Psychophysiological interaction (PPI) analysis revealed stronger vmPFC–amygdala coupling during the nonreminded CS+ versus the reminded CS+ and the CS− (P < 0.05, corrected). (C) Mean β weights from the PPI.
Recovery of threat memory.
On day 3, the amygdala ROI showed evidence of a BOLD signal increase to the nonreminded CS+ versus the reminded CS+ during threat memory recovery (P < 0.05, one-tailed t test), consistent with Agren et al. (13), who tested the effects of extinction during reconsolidation on the amygdala BOLD signal during memory retrieval on day 3. No significant BOLD activation in the vmPFC was observed at this stage.
Discussion
This study reveals the neural processes that mediate extinction when it occurs during reconsolidation of the threat memory. Standard extinction is well known to depend on the integrity of the vmPFC (1, 2), and BOLD responses in this region tend to increase with extinction learning (26–28). Here we show that conducting extinction during reconsolidation may bypass this circuitry as it does not appear to engage the vmPFC. Our findings further show that the amygdala and the vmPFC were differentially involved when extinction of conditioned threat occurred during memory reconsolidation. The amygdala BOLD response mirrored the conditioned defensive SCR for all CSs. That is, there was increased activation in response to both the reminded and nonreminded CS+, but not CS−, early in extinction, and this activation diminished as extinction progressed. Thus, the pattern of amygdala response did not differ when extinction occurred during reconsolidation, consistent with the intact short-term threat memory observed with SCR (21). The pattern of amygdala’s functional connectivity with the vmPFC, however, did differ. These two regions showed enhanced functional connectivity during the extinction of the nonreminded CS+ (i.e., standard extinction) relative to the reminded CS+ (i.e., during reconsolidation) or the CS−.
In rodents, behavioral interference of reconsolidation alters molecular processes that underlie threat conditioning-induced plasticity in the amygdala, suggesting different processing there during this protocol (10, 12). Here, our finding of stronger coupling between the vmPFC and amygdala during the nonreminded CS+ versus the reminded CS+ and CS− suggests that although there are similar amygdala BOLD response patterns for both CS+s during early extinction, they may reflect different mechanisms. Specifically, the coupling between the amygdala and vmPFC may help establish an extinction memory for the nonreminded CS+, whereas the reminded CS+ may undergo an updating process whereby it is persistently altered. In contrast to the amygdala, there was no evidence of vmPFC involvement when extinction occurred during reconsolidation, consistent with the hypothesis that targeting reconsolidation may reduce the necessity for PFC inhibition.
Interestingly, limited PFC involvement has also been linked to a more persistent reduction of conditioned defensive reactions in two other circumstances: early in development when the PFC is not fully formed and following vmPFC damage. Kim and Richardson (29) have shown that the processes that mediate extinction at different stages of development are qualitatively different. In postweanling-aged rats (24-d-old) extinction was “adult-like” in the sense that the extinguished threat memory recovered following renewal, reinstatement, and spontaneous recovery, the three major assays indicating that the extinguished memory was never erased, only inhibited (30). Preweanling-aged rats (17-d-old), however, did not display these phenomena. Extinction in 24-d-old rats, moreover, involved the core brain regions of the extinction circuitry in adults (i.e., the amygdala and the vmPFC), whereas in 17-d-old rats extinction engaged the amygdala but not the vmPFC (31, 32).
These findings indicate that diminishing PFC involvement changes the nature of extinction or exposure so that it is more effective at preventing future defensive responses. Consistent with this idea, Koenigs et al. (33) studied a unique group of Vietnam War veterans who were not only exposed to emotionally traumatic events but also suffered brain injury. The authors found that veterans with damage either to the vmPFC (comprising the ventral portion of the medial PFC, below the level of the genu of the corpus callosum, and medial portion of the orbital surface, as well as the subjacent white matter) or a temporal lobe area that included the amygdala had substantially less occurrence of posttraumatic stress disorder. These results suggest the intriguing hypothesis that the consequences of vmPFC damage are not in releasing amygdala from inhibition (34), as one might expect, but rather in inducing a fundamental change in the memory processes occurring at the level of the amygdala itself.
Agren et al. (13) have recently examined that role of the amygdala during the retrieval, or lack thereof, of a memory that previously underwent extinction during reconsolidation. Consistent with previous studies, a conditioned threat memory that underwent standard extinction recovered a few days later, and this recovery was accompanied by amygdala BOLD activation. In contrast, a threat memory that underwent extinction during reconsolidation did not recover and did not engage the amygdala, a finding replicated in the present study. Agren et al., however, only examined the outcome of extinction training during reconsolidation. The present study examines the neural mechanisms underlying the process of altering reconsolidation through precisely timed extinction training. At this stage, there is significant amygdala activation, consistent with studies in rodents showing active amygdala involvement in memory modification with this technique (10). In contrast to standard extinction, there is no significant evidence for vmPFC involvement (but see also ref. 35). In addition, there is a relative disconnect between the amygdala and vmPFC. This altered connectivity may play a role in enabling extinction learning to more permanently modify the original threat memory trace, thus preventing the return of defensive reactions on subsequent recovery tests.
In conclusion, one hallmark of brain pathology in anxiety disorders is dysfunctional vmPFC–amygdala interactions (3–9). Here we found that extinction timed to coincide with threat memory reconsolidation diminishes the involvement of the vmPFC. By doing so, this threat prevention technique appears to circumvent a circuit that has been suggested to play a critical role in the development and expression of anxiety disorders.
Methods
Participants.
Final analysis included 19 healthy participants (10 females, ages 18–34). The study was approved by the New York University Committee on Activities Involving Human Subjects. All participants gave informed consent and were paid for their participation. We verified that none of the participants were taking any medication for psychiatric or neurological reasons. To explore the pattern of BOLD responses during reconsolidation interference, participants needed to meet three standard inclusion criteria for studies examining the alteration of conditioned threat in humans and other species (e.g., refs. 18, 36–38). First, the participants were required to show measurable SCR on all 3 d, because this was the primary dependent measure. Second, participants were required to show evidence of threat acquisition, because it is impossible to examine the mechanisms of learned threat alteration if it is not first acquired. Because our study used an unusual two-CS+ design to optimize our BOLD analyses, we also added an additional criterion of equivalent threat acquisition to both CS+s, because we cannot measure relative differential learned threat alteration to the two CS+s if the level of original threat acquisition differs. Third, given that our primary effect of interest was threat memory recovery after extinction training, we excluded participants who did not show equal and complete extinction to both CS+s. Specifically, participants who showed measurable SCR levels (> 0.02 μS, n = 72) were excluded after day 1 if they failed to show equivalent conditioned threat acquisition to the reminded and nonreminded CS+s (difference > 0.1 μs, n = 43). Participants who failed to show equivalent extinction to the reminded and nonreminded CS+s were excluded after day 2 (difference > 0.1 μS, n = 5). An additional five participants displayed the opposite behavioral effect (greater mean SCR of threat memory recovery to the nonreminded vs. reminded CS+). Given our goal of examining the neural systems of reconsolidation interference, we did not include these participants in our final BOLD analysis because they did not show the behavior of interest, although an exploratory analysis including these participants did not change the SCR or BOLD results. When these participants were included there remained significant threat memory recovery [first trial day 3 minus last trial day 2 for CS+ minus CS−) only to the nonreminded CS+ (P < 0.05), but not to the reminded CS+, P = 0.7; two-tailed t test].
Threat-Conditioning Paradigm and Physiological Assessment.
The paradigm consisted of three consecutive stages conducted 24-h apart: day 1, acquisition; day 2, reactivation and extinction; and day 3, reinstatement and reextinction, in a within-subject design. Day 1 consisted of a simple discrimination, partial reinforcement (38%) paradigm. Two colored squares (CS+; 4 s) coterminated with a mild electric shock to the wrist (200 ms). A third (CS−) was never paired with the shock. Acquisition included eight nonreinforced presentations of the two CS+s and the CS−, intermixed with an additional five presentations of each CS+ with the shock, presented in a pseudorandom order with a 12-s intertrial interval (ITI). On day 2, participants were reminded of one of the CS+s (two 4-s presentations and a 5-s ITI). The other CS+ and the CS− were not reminded. Participants then watched a video (BBC Planet Earth) for 10 min before extinction training for all CSs. Extinction included 10 trials of the reminded CS+, 11 of the nonreminded CS+, and 11 of CS− without the US. The day 3 recovery test began with four unsignaled presentations of the US (25-s ITI) to reinstate the threat memory. After a 10-min movie interval, participants underwent reextinction including 10 reminded CS+, 10 nonreminded CS+, and 11 CS− in a pseudorandom order with the first trial always a CS− to capture the orienting response. The trial order and color designation for CSs was counterbalanced across subjects.
Mild shocks were delivered through a stimulating bar electrode attached with a Velcro strap to the right wrist. A Grass Medical Instruments stimulator charged by a stabilized current was used, with cable leads that were magnetically shielded and grounded through an RF filter. The participants were asked to set the level of the shock themselves, using a work-up procedure before scanning. In this procedure, a participant was first given a very mild shock (20 V, 200 ms, 50 pulses per second), which was gradually increased to a level the participant indicated as “uncomfortable, but not painful” (with a maximum level of 60 V). Skin conductance was assessed with shielded Ag-AgCl electrodes, filled with standard NaCl electrolyte gel, and attached to the middle phalanges of the second and third fingers of the left hand. The electrode cables were grounded through an RF filter panel. The skin conductance signal was amplified and recorded with a BIOPAC Systems skin conductance module connected to a Macintosh computer. Data were continuously recorded at a rate of 200 samples per second. An off-line analysis of the analog skin conductance waveforms was conducted with AcqKnowledge software (BIOPAC Systems).
The level of skin conductance response was assessed for each trial as the base-to-peak amplitude difference in skin conductance of the largest deflection (in microsiemens, µS) in the 0.5- to 4.5-s latency window following stimulus onset. The minimal response criterion was 0.02 µS. Responses below this criterion were encoded as zero. The raw skin conductance scores were scaled according to each participant’s mean US response.
Neuroimaging Acquisition and Analysis.
A 3T Siemens Allegra head-only scanner and Siemens standard head coil (Siemens) were used for data acquisition. Anatomical images were acquired using a T1-weighted protocol (256 × 256 matrix, 176 1-mm sagittal slices). Functional images were acquired using a single-shot gradient echo EPI sequence (TR = 2,000 ms, TE = 25 ms, FOV = 192 cm, flip angle = 75°, bandwidth = 4,340 Hz/px, echo spacing = 0.29 ms). Thirty-nine contiguous oblique-axial slices (3 × 3 × 3-mm voxels) parallel to the AC–PC line were obtained. Analysis of the imaging data was conducted using BrainVoyager QX software package (Brain Innovation). Functional imaging data-preprocessing included motion correction, slice-scan time correction (using sinc interpolation), spatial smoothing using a 3D Gaussian filter (4-mm full-width half-maximum), and voxel-wise linear detrending and high-pass filtering of frequencies above three cycles per time-course.
A random-effects general linear model analysis was conducted on the fMRI signal during the task with separate predictors for each stimulus type (reminded CS+, nonreminded CS+, and CS−), at six stages: acquisition, extinction, and reextinction, each divided into early (first half) and late (second half) phases. We used separate predictors for the reminded and nonreminded CS+ trials terminating with the shock during acquisition. This process resulted in eight boxcar predictors for the acquisition stage, six for the extinction stage, and six for the reextinction stage. Each predictor corresponded to the length of each trial (4 s) and convolved with a standard canonical hemodynamic response function. Structural and functional data of each participant were transformed to standard Talairach stereotaxic space (39). For each region of interest, we compared the mean BOLD responses to the reminded CS+, nonreminded CS+, and CS− at each phase.
To examine condition-specific functional connectivity, each participant’s BOLD signal time-course of the vmPFC (ROI defined by the reminded vs. nonreminded CS+ contrast during early extinction on day 2; P < 0.05; corrected for multiple comparisons based on cluster-level threshold estimation) was extracted and averaged across voxels. A general linear model was constructed for each participant including the vmPFC time-course, which served as a seed region, the regressors for the different conditions, and separate regressors calculated as the seed region time-course multiplied by each condition’s regressor (reminded CS+, nonreminded CS+, and CS−). We then contrasted these weighted regressors (P < 0.05, corrected) to examine which regions showed higher functional connectivity with the vmPFC in the nonreminded vs. reminded CS+ condition.
Acknowledgments
We thank Candace Raio for assistance with an earlier version of this study and Avi Mendelsohn for assistance with the analysis. This study was supported by Grants R0 1MH080756, R0 1MH062104, R21MH081088, and James S. McDonnell Foundation grants (to E.A.P.); Grants 1R21MH086805 and 1R01MH091147 (to M.H.M.); and funds provided by New York University’s Center for Brain Imaging.
Footnotes
The authors declare no conflict of interest.
References
- 1.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: Historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60(4):329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Etkin A. Functional neuroanatomy of anxiety: A neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- 4.Graham BM, Milad MR. The study of fear extinction: Implications for anxiety disorders. Am J Psychiatry. 2011;168(12):1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes KC, Shin LM. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother. 2011;11(2):275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Front Behav Neurosci. 2011;5:44. doi: 10.3389/fnbeh.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim MJ, et al. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci. 2013;16(2):146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330(6007):1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao-Ruiz P, et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci. 2011;14(10):1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- 12.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agren T, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012a;337(6101):1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- 14.Agren T, Furmark T, Eriksson E, Fredrikson M. Human fear reconsolidation and allelic differences in serotonergic and dopaminergic genes. Transcult Psychiatry. 2012b;2:e76. doi: 10.1038/tp.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyarzún JP, et al. Updating fearful memories with extinction training during reconsolidation: A human study using auditory aversive stimuli. PLoS ONE. 2012;7(6):e38849. doi: 10.1371/journal.pone.0038849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golkar A, Bellander M, Olsson A, Ohman A. Are fear memories erasable? Reconsolidation of learned fear with fear-relevant and fear-irrelevant stimuli. Front Behav Neurosci. 2012;6:80. doi: 10.3389/fnbeh.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kindt M, Soeter M. Reconsolidation in a human fear conditioning study: A test of extinction as updating mechanism. Biol Psychol. 2013;92(1):43–50. doi: 10.1016/j.biopsycho.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Auber A, Tedesco V, Jones CE, Monfils MH, Chiamulera C. Post-retrieval extinction as reconsolidation interference: Methodological issues or boundary conditions? Psychopharmacology (Berl) 2013;226(4):631–647. doi: 10.1007/s00213-013-3004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudai Y. The restless engram: Consolidations never end. Annu Rev Neurosci. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- 21.Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 22.Sara SJ. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn Mem. 2000;7(2):73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 23.Schiller D, Phelps EA. Does reconsolidation occur in humans? Front Behav Neurosci. 2011;5:24. doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol. 2012;22(4):717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: More than just extinction. Curr Opin Neurobiol. 2010;20(2):231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milad MR, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14(6):268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: Theoretical and clinical implications. Biol Psychiatry. 2010;67(4):297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Hamlin AS, Richardson R. Fear extinction across development: The involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29(35):10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: Unlearning as a potential mechanism for extinction early in development. J Neurosci. 2008;28(6):1282–1290. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenigs M, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11(2):232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koenigs M, Grafman J. Posttraumatic stress disorder: The role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15(5):540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue YX, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336(6078):241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42(4):456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 37.Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex. 2009;19(2):474–482. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13(6):728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: An Approach to Medical Cerebral Imaging. New York, NY: Thieme Medical; 1998. [Google Scholar]