Significance
The design of internal combustion engines relies on a good understanding of the kinetic mechanism of the autoignition of hydrocarbons. •OH and •HO2 radicals are known to be the key species governing all stages of the development of ignition. A direct measurement of these radicals under low-temperature oxidation conditions has been achieved by coupling the fluorescence assay by gas expansion technique, an experimental technique designed for the quantification of these radicals in the free atmosphere, to a jet-stirred reactor, an experimental device designed for the study of low-temperature combustion chemistry.
Keywords: HOx radicals, autoignition
Abstract
•OH and •HO2 radicals are known to be the key species in the development of ignition. A direct measurement of these radicals under low-temperature oxidation conditions (T = 550–1,000 K) has been achieved by coupling a technique named fluorescence assay by gas expansion, an experimental technique designed for the quantification of these radicals in the free atmosphere, to a jet-stirred reactor, an experimental device designed for the study of low-temperature combustion chemistry. Calibration allows conversion of relative fluorescence signals to absolute mole fractions. Such radical mole fraction profiles will serve as a benchmark for testing chemical models developed to improve the understanding of combustion processes.
In the context of a needed significant reduction of the emission of greenhouse gases, understanding the autoignition chemistry of hydrocarbons and biofuels is of critical importance to allow the development of new combustion strategies for clean and efficient internal combustion engines (1). Advanced combustion concepts that rely on compression self-ignition (1, 2), as well as the improvement of safety in oxidation processes (3), demand an improved understanding of the reaction kinetics governing the detailed mechanisms of organic compounds, particularly in the low-temperature oxidation regime. The chemistry of oxidation and autoignition is governed by species, with unpaired electrons, called free radicals, which are present in trace amounts. Among free radicals important in oxidation are H atoms, alkyl radicals, and hydroxyl (•OH) or hydroperoxy (•HO2) radicals, the last two being the most important. It is then of critical importance for a better understanding of this chemistry to develop methods to measure radicals under all possible reaction conditions governing autoignition, including low-temperature oxidation (below 1,000 K).
Because •OH radicals are present in relatively high concentrations at high temperatures, they have been measured for a long time in flames by molecular beam mass spectrometry (4) and by laser-based methods such as laser-induced fluorescence (LIF) or absorption spectroscopy (5, 6). The detection of •HO2 radicals has recently been achieved in a flame using a photofragmentation laser-induced fluorescence technique (7) as well as during the oxidation of dimethylether in a laminar flow reactor using midinfrared Faraday rotation spectroscopy (8). A method to measure the ratio between •HO2 and •RO2 radicals based on electron spin resonance spectroscopy was proposed by Carlier and Sochet (9) but has never been applied to real hydrocarbons and low-temperature oxidation.
H atoms (10) or •OH radicals (11) were also followed under high-temperature (T > 1,200 K) shock tube conditions. Finally, planar LIF using a laser sheet has been used in optical access engines to image OH radical concentrations to map the hot combustion zones (12). This technique does not directly give access to a real quantitative measurement of the radical concentration due to differences in quenching and line broadening caused by local gradients of fuel/air equivalence ratio and temperature (13).
In this work, we present the coupling of an optical technique named Fluorescence Assay by Gas Expansion (FAGE), initially designed for the measurement of absolute concentrations of •OH and •HO2 radicals in the atmosphere (14), to a combustion device called a jet-stirred reactor (JSR), with the goal of quantifying both radicals under reaction conditions similar to those found before development of autoignition (2). A JSR allows studying gas-phase reactions at temperatures up to 1,000 K without external radical production. Such measurements will be particularly valuable for improving knowledge of the chemistry and detailed kinetic models in the temperature zone (650–900 K), in which reaction mechanisms are the most complex and the kinetic data are the least accurate (15).
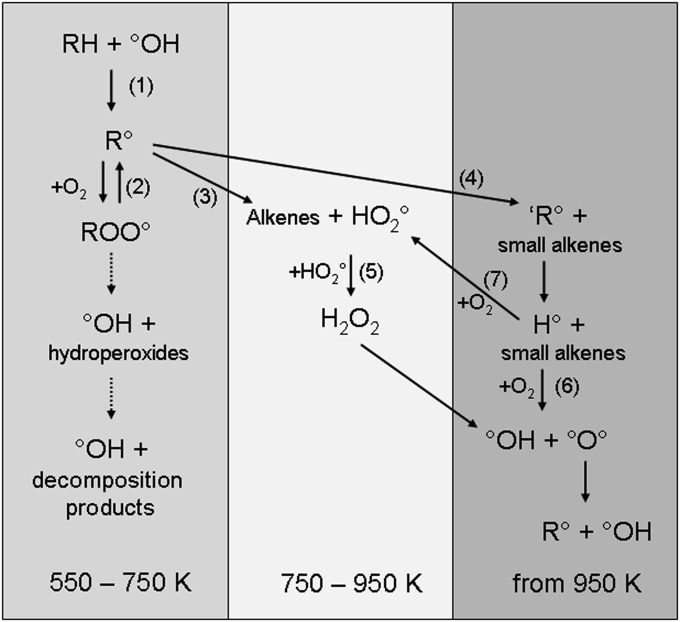
The schematic reaction mechanism currently accepted for the low-temperature combustion of many typical hydrocarbon fuel molecules is shown in Fig. 1, in which the key reactions are numbered to facilitate the following discussion. The starting point of the chain reaction leading to ignition is the reaction of a hydroxyl radical (•OH), the main chain carrier, with the fuel molecule, named hereafter RH. Abstraction of an H atom by the OH radical (reaction 1) leads to formation of a radical R•. At low temperatures (below around 750 K), R• rapidly yields a peroxy radical (ROO•) after a barrierless reaction with an oxygen molecule (reaction 2). This ROO• radical leads then to the formation of hydroperoxides and an •OH radical, mainly by a complex mechanism involving two isomerizations and addition of a second oxygen molecule, not described here. The obtained hydroperoxides can easily decompose giving two radicals, including a second •OH radical. This degenerate chain-branching reaction explains the high reactivity of hydrocarbon/oxygen mixtures at low temperatures.
Fig. 1.
Simplified scheme of the mechanism of oxidation of hydrocarbons (dotted arrows represent a succession of several elementary steps).
An increase in temperature enhances the reversibility of reaction 2, thus hindering the formation of peroxy radicals and consequently of •OH radicals. At moderate temperatures (between around 750 and 950 K), the radical R• reacts with oxygen, yielding an unsaturated hydrocarbon (named alkenes in Fig. 1) and an •HO2 radical (reaction 3), or, if temperature is high enough, decomposes into a small alkene and another radical ‘R• (reaction 4). •HO2 radicals are much less reactive than •OH radicals and mainly react in a termination step leading to hydrogen peroxide (H2O2) (reaction 5). This reduced importance of degenerate chain-branching reactions in favor of chain-terminating reactions leads to a slowdown of the reactivity and explains the occurrence of a feature specific to mixtures of organic compounds with oxygen (16): the commonly called “negative temperature coefficient” (NTC) zone; i.e., a zone (usually around 650 K under atmospheric pressure) where the reactivity decreases with increasing temperature.
With further increased temperature, the decomposition of H2O2 leading to two •OH radicals becomes faster and is the dominant degenerate chain-branching reaction, again promoting high reactivity. Above around 950 K, small hydrocarbon radicals such as obtained by reaction 4 can in turn decompose, leading to alkenes and •H atoms. The reaction of an •H atom with oxygen at high temperatures leads to an •OH radical and to an •O• atom (reaction 6), which in turn regenerates an •OH radical by H abstraction from the fuel. Reaction 6 is a true branching step, ensuring the full development of ignition and complete combustion. However, the pressure-dependent reaction 7 can compete with reaction 6, depending on temperature and pressure, and leads to the formation of the less reactive •HO2 radicals.
This scheme shows clearly the critical importance of •OH and •HO2 radicals in all stages linked to the development of ignition. The purpose of this study is therefore to present an experimental device allowing the quantification of both radicals under conditions observed during the reaction period before the autoignition of hydrocarbons. n-Butane has been studied as it is the smallest hydrocarbon structure that exhibits an oxidation behavior representative to species commonly present in gasoline and diesel fuels.
The goal of this work was to reproduce the chemistry leading to this phenomenon rather than to observe unsteady phenomena directly such as autoignition. For this purpose, a heated JSR has been used, a device that has already been used many times for studying the chemistry of organic compounds before ignition (15). Note that this reactor has been recently used to follow the formation of alkylhydroperoxides (17) and H2O2 (18) through its coupling with a reflectron time-of-flight mass spectrometer and cw-Cavity Ring Down Spectroscopy (CRDS) cell, respectively. CRDS is known to be a very sensitive technique to measure •HO2 radicals, but surprisingly these radicals could not be detected in these experiments, even though concentrations much above the detection limit of this technique (19, 20) were expected (18).
Results and Discussion
FAGE is a highly sensitive method for quantifying •OH and •HO2 radicals, originally designed for the quantification of these radicals in the free atmosphere. Mole fractions of •OH and •HO2 radicals found in the atmosphere are typically 0.2 and 8 ppt, respectively (21). Today eight research groups in the world have operational FAGE systems (22–28), but all of them are used in research solely linked to atmospheric chemistry. To our knowledge, no other work has been published where the advantages of radical detection by FAGE technique are exploited in the field of combustion research.
FAGE is based on LIF after expansion of the gas mixture (in general, the ambient atmosphere) through a small orifice (few 100 µm to 1 mm) to pressures of around 0.5 torr. •HO2 radicals, which do not fluoresce, are converted to •OH radicals by their fast reaction with NO. Indirect detection of •HO2 after conversion to •OH radicals through addition of NO to the reaction system is a common technique (29), leading possibly to complications due to the reaction of NO with other intermediates. In the case of FAGE, however, NO is added only after the expansion of the gas mixture into the FAGE cell; i.e., when the reaction system is already at low temperature and pressure. Recent experiments have shown that, even under these conditions, •RO2 radicals might also be converted to •OH radicals in a two-step reaction sequence with the conversion efficiency depending strongly on the NO concentration (30). In the present experiments, the NO concentration was kept very low (SI Text) and no interference has been detected.
The FAGE system of Lille has been described in detail several times (31–33), so only its coupling with the JSR will be described here. The spherical quartz JSR (diameter around 5.5 cm), in which a gas mixture is continuously flowing, was operated at atmospheric pressure and was electrically heated to a constant temperature for each data point of a temperature profile (see Figs. 3 and 4). This type of reactor, which can be heated up to 1,200 K, is well adapted for mechanistic studies (see SI Text for more details): the gas phase within the reactor is well stirred, meaning that concentrations and temperatures are close to homogenous.
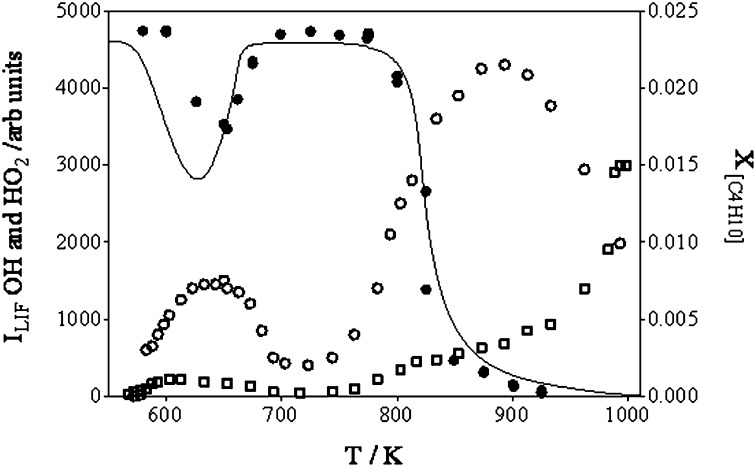
Fig. 3.
•OH (□) and •HO2 (○) radical fluorescence intensity signals (in arbitrary units; Left y axis) as a function of temperature, as well as experimental (●) and predicted (black line; see SI Text for details) n-butane mole fraction profiles (18) (Right y axis).
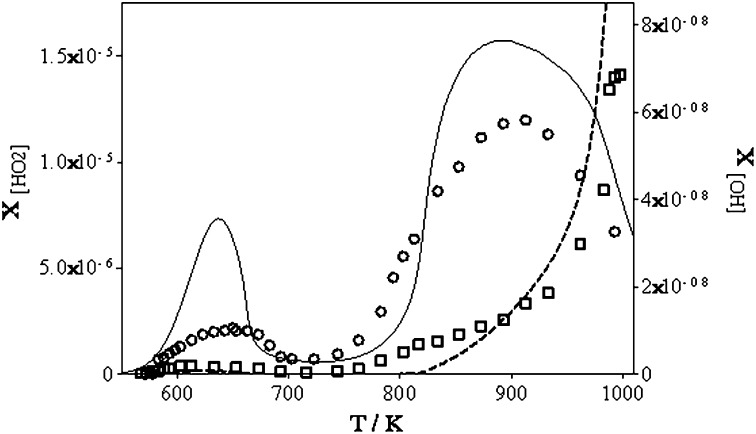
Fig. 4.
Estimated •OH (□) and •HO2 (○) radical mole fractions obtained from calibrated LIF signals compared with predictions using a detailed kinetic model (18).
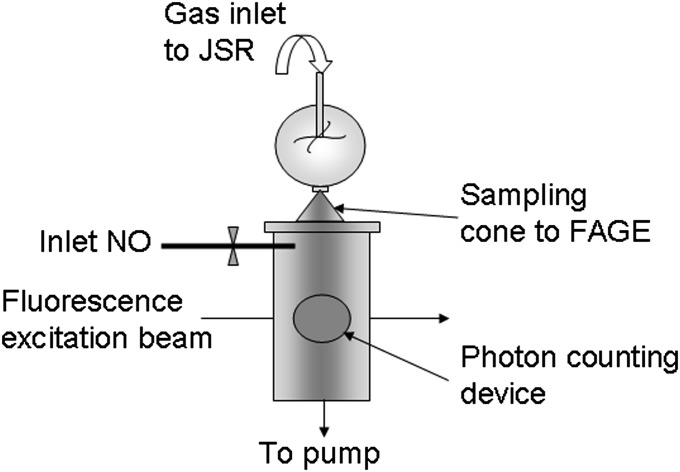
As schematically shown in Fig. 2, the JSR was adapted for the coupling with the FAGE system by transforming the gas exhaust from the usual tube into an orifice with a diameter of 7 mm. Only a small fringe of roughly 2 mm at the rim of the opening was kept to maintain the heating coils. The inlet of the FAGE was made from a stainless-steel cone with an orifice of 400 µm: this cone was placed in the center of the exhaust hole of the JSR. The exhaust of the JSR was not connected leak tight to the FAGE inlet to avoid perturbing the chemistry within the JSR; the air intake into the FAGE is higher than the output of the JSR. For calibration purposes, it has been estimated from flow rate measurements that the gas mixture of the JSR is diluted by a factor of 2.6–5.2 during the intake into the FAGE (SI Text). This contamination with ambient laboratory air has no impact on the chemistry, because ambient indoor air contains only extremely low radical concentrations. A loss of •OH radicals during the short transition period from the JSR to the FAGE inlet due to reaction with trace gases possibly present in the laboratory air can safely be neglected compared with the concentration of n-butane and its oxidation products. The JSR is surrounded by a Plexiglas cylinder to avoid air drafts: for a few experiments, this cylinder has been flooded with N2 (Alpha 3.0), and no differences have been observed in the LIF signal.
Fig. 2.
Simplified scheme of the experimental device (not to scale). The upper part (JSR) is at atmospheric pressure and heated at high temperatures, and the lower part (FAGE) is at low pressure (<1 torr) and ambient temperature.
Fig. 3 shows the •OH and •HO2 profiles obtained during the oxidation of a mixture of 2.3% n-butane/13% O2 in helium at a residence time of 6 s within the JSR. Each data point of such a profile is the result of an individual experiment: the electrical heating of the JSR is set to obtain the desired temperature, the gas flows are adapted to keep the residence time constant (the change in gas density with temperature needs to be taken into account), and once the chemical system has stabilized to the new conditions, the steady-state concentrations of the different species are measured at the exit of the JSR. Fig. 3 also presents the n-butane mole fraction profile measured by gas chromatography under the same conditions (18). The reaction starts when the JSR temperature reaches around 600 K, visible through a sharp increase in •OH and •HO2 LIF signal following the evolution of the n-butane consumption. The LIF signal intensity reaches a maximum for both radicals roughly at the same JSR temperature at which the n-butane concentration reaches a minimum. When further increasing the temperature of the JSR, the NTC zone is clearly visible above 650 K, the LIF intensity decreases to a minimum for both radicals, but nonzero LIF signal is still present near 700 K, a temperature for which no conversion of n-butane is observed. Further increase of the JSR temperature leads to a second increase in reactivity with an initially steeper rise in the •HO2 signal compared with that of the •OH signal. Above 900 K, the •HO2 signal decreases again, whereas the •OH signal sharply rises.
Because FAGE is based on LIF, it is only a relative method, and a calibration is needed for obtaining absolute concentrations. Different calibration techniques have been developed for the quantification of radicals in the atmosphere (34). One of these methods, based on the photolysis of H2O, has been set up for the FAGE system in Lille. However, it cannot be applied straightforward for the calibration of the measurements presented in this work because typical radical concentrations in atmospheric chemistry and in combustion systems differ by many orders of magnitude, especially for HO2 radicals (parts per trillion in the atmosphere, parts per million in combustion systems). Thus, the FAGE detection system, if directly used in the “atmospheric” configuration, would saturate when analyzing combustion exhaust gases and the sensitivity of the instrument has been attenuated (SI Text).
However, the calibration system is designed for generating typical atmospheric concentrations, and therefore the signals would be very weak if the “atmospheric” calibration system would be used in the attenuated “combustion” configuration. An attempt has nevertheless been made to convert relative LIF signals into absolute concentrations and then into mole fractions for both, •OH and •HO2 radicals. The profiles are shown in Fig. 4, together with model predictions.
For this purpose, several corrections have been applied to the calibration factors such as obtained by the atmospheric calibration system (for details, see SI Text). Note that the mole fractions shown for both radicals in Fig. 4 are probably only lower limits, because (i) the dilution factor (SI Text) has been calculated by considering that the JSR reactor flow is completely sampled into the FAGE, which is probably not true, and (ii) loss of radicals can be expected between the exit of the JSR and the expansion into the FAGE. •OH radicals are much more reactive than •HO2 radicals and therefore their concentration will probably decrease faster. Therefore, the agreement between the model and the measurement is excellent for the maximal mole fractions of •HO2 radicals at both temperatures with the model predicting somewhat higher •HO2 concentrations. As for the concentration of •OH radicals, the model underpredicts the concentration at nearly all temperatures; only at temperatures above 900 K is the measured OH concentration lower than the predicted.
Summary
We have presented here direct measurements of •OH and •HO2 radical profiles under combustion conditions combining the advantages of a radical detection by FAGE to a chemical reactor well adapted for studying low-temperature combustion phenomena, a JSR. Although the absolute concentrations are somewhat uncertain due to calibration and sampling issues, the relative temperature profiles are well defined. Such measurements will certainly lead to a big step forward in refining current models of combustion chemistry, as these radical profiles will be a powerful benchmark for testing models that have so far been developed mostly on the basis of concentration profiles for stable species only.
Supplementary Material
Acknowledgments
PhysicoChimie des Processus de Combustion et de l’Atmosphère participates in the Institut de Recherche en Environnement Industriel financed by the Région Nord Pas-de-Calais, the Ministère de l’Enseignement Supérieur et de la Recherche, the Centre National de la Recherche Scientifique, and the European Regional Development Fund. This project was supported by the French Agence Nationale de la Recherche under Contract ANR-11-LabEx-0005-01 “Chemical and Physical Properties of the Atmosphere,” by the European Commission through the “Clean ICE” Advanced Research Grant of the European Research Council, and by European Cooperation in Science and Technology Action CM0901.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314968110/-/DCSupplemental.
References
- 1.Lu X, Han D, Huang Z. Fuel design and management for the control of advanced compression-ignition combustion modes. Prog Energ Combust. 2011;37(6):741–783. [Google Scholar]
- 2.Westbrook CK, Mizobuchi Y, Poinsot TJ, Smith PJ, Warnatz J. Computational combustion. Proc Combust Inst. 2005;30(1):125–157. [Google Scholar]
- 3.Pasman HJ, et al. Playing with fire—safety and reaction efficiency research on gas phase hydrocarbon oxidation processes: Project SAFEKINEX. Process Saf Environ. 2005;83(B4):317–323. [Google Scholar]
- 4.McEnally CS, Pfefferle LD, Atakan B, Kohse-Höinghaus K. Studies of aromatic hydrocarbon formation mechanisms in flames: Progress towards closing the fuel gap. Prog Energ Combust. 2006;32(3):247–294. [Google Scholar]
- 5.Cheskis S, Goldman A. Laser diagnostics of trace species in low-pressure flat flame. Prog Energ Combust. 2009;35(4):365–382. [Google Scholar]
- 6.Mercier X, Therssen E, Pauwels JF, Desgroux P. Cavity ring-down measurements of OH radical in atmospheric premixed and diffusion flames. A comparison with laser-induced fluorescence and direct laser absorption. Chem Phys Lett. 1999;299(1):75–83. [Google Scholar]
- 7.Johansson O, et al. Photofragmentation laser-induced fluorescence imaging in premixed flames. Combust Flame. 2011;158(10):1908–1919. [Google Scholar]
- 8.Brumfield B, Sun W, Ju Y, Wysocki G. Direct in situ quantification of HO2 from a flow reactor. J Phys Chem Lett. 2013;4(6):872–876. doi: 10.1021/jz400143c. [DOI] [PubMed] [Google Scholar]
- 9.Carlier M, Sochet L-R. Quantitative measurements of hydroperoxy and alkylperoxy radicals in gas-phase oxidation reactions from overlapping electron paramagnetic resonance spectra. J Chem Soc Faraday Trans. 1983;79(4):815–821. [Google Scholar]
- 10.Fernandes R, Fittschen C, Hippler H. Kinetic investigations of the unimolecular decomposition of dimethylether behind shock waves. React Kinet Catal Lett. 2009;96(2):279–289. [Google Scholar]
- 11.Hong Z, Cook RD, Davidson DF, Hanson RK. A shock tube study of OH + H2O2 —> H2O + HO2 and H2O2 + M —> 2OH + M using laser absorption of H2O and OH. J Phys Chem A. 2010;114(18):5718–5727. doi: 10.1021/jp100204z. [DOI] [PubMed] [Google Scholar]
- 12.Dec JE. Advanced compression-ignition engines—understanding the in-cylinder processes. Proc Combust Inst. 2009;32(2):2727–2742. [Google Scholar]
- 13.Singh S, Musculus MPB, Reitz RD. Mixing and flame structures inferred from OH-PLIF for conventional and low-temperature diesel engine combustion. Combust Flame. 2009;156(10):1898–1908. [Google Scholar]
- 14.Heard DE, Pilling MJ. Measurement of OH and HO2 in the troposphere. Chem Rev. 2003;103(12):5163–5198. doi: 10.1021/cr020522s. [DOI] [PubMed] [Google Scholar]
- 15.Battin-Leclerc F, et al. Towards cleaner combustion engines through groundbreaking detailed chemical kinetic models. Chem Soc Rev. 2011;40(9):4762–4782. doi: 10.1039/c0cs00207k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westbrook CK. Chemical kinetics of hydrocarbon ignition in practical combustion systems. Proc Combust Inst. 2000;28(2):1563–1577. [Google Scholar]
- 17.Battin-Leclerc F, et al. Experimental confirmation of the low-temperature oxidation scheme of alkanes. Angew Chem Int Ed Engl. 2010;49(18):3169–3172. doi: 10.1002/anie.200906850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahrini C, et al. Quantification of hydrogen peroxide during the low-temperature oxidation of alkanes. J Am Chem Soc. 2012;134(29):11944–11947. doi: 10.1021/ja305200h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahrini C, Parker A, Schoemaecker C, Fittschen C. Direct Detection of HO2 radicals in the vicinity of TiO2 photocatalytic surfaces using cw-CRDS. Appl Catal B. 2010;99(3-4):413–419. [Google Scholar]
- 20.Thiebaud J, Crunaire S, Fittschen C. Measurements of line strengths in the 2nu1 band of the HO2 radical using laser photolysis/continuous wave cavity ring-down spectroscopy (cw-CRDS) J Phys Chem A. 2007;111(30):6959–6966. doi: 10.1021/jp0703307. [DOI] [PubMed] [Google Scholar]
- 21.Dusanter S, Vimal D, Stevens PS, Volkamer R, Molina LT. Measurements of OH and HO2 concentrations during the MCMA-2006 field campaign. Part 1: Deployment of the Indiana University laser-induced fluorescence instrument. Atmos Chem Phys. 2009;9(5):1665–1685. [Google Scholar]
- 22.Brune WH, Stevens PS, Mather JH. Measuring OH and HO2 in the troposphere by laser-induced fluorescence at low pressure. J Atmos Sci. 1995;52(19):3328–3336. [Google Scholar]
- 23.Kanaya Y, Sadanaga Y, Hirokawa J, Kajii Y, Akimoto H. Development of a ground-based LIF instrument for measuring HOx radicals: Instrumentation and calibrations. J Atmos Chem. 2001;38(1):73–110. [Google Scholar]
- 24.Fuchs H, Holland F, Hofzumahaus A. Measurement of tropospheric RO2 and HO2 radicals by a laser-induced fluorescence instrument. Rev Sci Instrum. 2008;79(8):084104. doi: 10.1063/1.2968712. [DOI] [PubMed] [Google Scholar]
- 25.Kubistin D, et al. Hydroxyl radicals in the tropical troposphere over the Suriname rainforest: Comparison of measurements with the box model MECCA. Atmos Chem Phys. 2010;10(19):9705–9728. [Google Scholar]
- 26.Creasey DJ, Halford-Maw PA, Heard DE, Pilling MJ, Whitaker BJ. Implementation and initial deployment of a field instrument for measurement of OH and HO2 in the troposphere by laser-induced fluorescence. J Chem Soc Faraday Trans. 1997;93(16):2907–2913. [Google Scholar]
- 27.Yoshino A, et al. Measurement of total OH reactivity by laser-induced pump and probe technique–comprehensive observations in the urban atmosphere of Tokyo. Atmos Environ. 2006;40(40):7869–7881. [Google Scholar]
- 28.Dusanter S, et al. Measurements of OH and HO2 concentrations during the MCMA-2006 field campaign: Part 2: Model comparison and radical budget. Atmos Chem Phys. 2009;9(18):6655–6675. [Google Scholar]
- 29.Bohn B, Zetzsch C. Formation of HO2 from OH and C2H2 in the presence of O2. J Chem Soc Faraday Trans. 1998;94(9):1203–1210. [Google Scholar]
- 30.Fuchs H, et al. Detection of HO2 by laser-induced fluorescence: Calibration and interferences from RO2 radicals. AMT. 2011;4(6):1209–1225. [Google Scholar]
- 31.Amedro D, Miyazaki K, Parker A, Schoemaecker C, Fittschen C. Atmospheric and kinetic studies of OH and HO2 by the FAGE technique. J Environ Sci (China) 2012;24(1):78–86. doi: 10.1016/s1001-0742(11)60723-7. [DOI] [PubMed] [Google Scholar]
- 32.Amedro D, Parker AE, Schoemaecker C, Fittschen C. Direct observation of OH radicals after 565 nm multi-photon excitation of NO2 in the presence of H2O. Chem Phys Lett. 2011;513(1-3):12–16. [Google Scholar]
- 33.Parker A, Amedro D, Schoemaecker C, Fittschen C. OH reactivity measurements by FAGE. EEMJ. 2011;10(1):107–114. [Google Scholar]
- 34.Dusanter S, Vimal D, Stevens PS. Technical note: Measuring tropospheric OH and HO2 by laser-induced fluorescence at low pressure. A comparison of calibration techniques. Atmos Chem Phys. 2008;8(2):321–340. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.