Significance
Several distinct congenital disorders can lead to tissue-iron overload with anemia. Tissue-iron accumulation is the major cause of mortality in these patients. Intestinal hypoxia-inducible factor-2α (HIF2α) and its downstream target gene divalent metal transporter-1 (DMT1) are essential for iron absorption during times of increased iron demand. However, the role of the intestinal HIF2α/DMT1 signaling axis in iron overload disorders has not been assessed. We demonstrate that HIF2α and DMT1 in the small intestine are highly activated early in mouse models of anemic iron overload and that disruption of their expression can prevent and improve tissue-iron accumulation in these disorders. These results demonstrate that HIF2α and DMT1 are ideal therapeutic targets in iron-overload disorders.
Keywords: thalassemia, HIF, Epas1, Slc11a2
Abstract
Several distinct congenital disorders can lead to tissue-iron overload with anemia. Repeated blood transfusions are one of the major causes of iron overload in several of these disorders, including β-thalassemia major, which is characterized by a defective β-globin gene. In this state, hyperabsorption of iron is also observed and can significantly contribute to iron overload. In β-thalassemia intermedia, which does not require blood transfusion for survival, hyperabsorption of iron is the leading cause of iron overload. The mechanism of increased iron absorption in β-thalassemia is unclear. We definitively demonstrate, using genetic mouse models, that intestinal hypoxia-inducible factor-2α (HIF2α) and divalent metal transporter-1 (DMT1) are activated early in the pathogenesis of β-thalassemia and are essential for excess iron accumulation in mouse models of β-thalassemia. Moreover, thalassemic mice with established iron overload had significant improvement in tissue-iron levels and anemia following disruption of intestinal HIF2α. In addition to repeated blood transfusions and increased iron absorption, chronic hemolysis is the major cause of tissue-iron accumulation in anemic iron-overload disorders caused by hemolytic anemia. Mechanistic studies in a hemolytic anemia mouse model demonstrated that loss of intestinal HIF2α/DMT1 signaling led to decreased tissue-iron accumulation in the liver without worsening the anemia. These data demonstrate that dysregulation of intestinal hypoxia and HIF2α signaling is critical for progressive iron overload in β-thalassemia and may be a novel therapeutic target in several anemic iron-overload disorders.
Secondary hemochromatosis is a group of distinct diseases that lead to iron accumulation, and several diseases within this group also have concomitant anemia (www.irondisorders.org). These disorders are very problematic to treat because phlebotomy, which is a very effective treatment for iron overload, is not an option due to the anemia. In many cases, diseases of iron overload with anemia are treated with a combination of erythropoietic stimulators, blood transfusions, and/or iron chelators (1). Increased iron loading in secondary hemochromatosis, particularly in the liver, is associated with increased morbidity and mortality (2). Because the main cause of death in β-thalassemia is iron overload (3), a major focus is to investigate the mechanisms involved in iron accumulation. Three major mechanisms have been attributed to the tissue-iron accumulation associated with several different anemic iron-overload diseases: (i) repeated blood transfusions, (ii) increased iron absorption, and (iii) chronic hemolysis. In conditions of severe anemia, such as β-thalassemia major, which is due to loss of functional β-globin protein, these patients have profound anemia as well as complications due to the production of abnormal red cells, requiring regular blood transfusions for survival. The transfused blood contains a significant amount of iron, which can lead to iron overload. Interestingly, patients with β-thalassemia due to partial loss of the β-globin gene product do not require blood transfusions for survival but exhibit iron overload (4, 5). This condition has been defined as β-thalassemia intermedia or nontransfusion-dependent thalassemia, and the mechanisms that contribute to iron overload are unclear (6). In addition, in diseases such as hemolytic and sideroblastic anemia the iron overload is due to chronic hemolysis. Through a mechanism similar to transfusions, increased hemolysis releases large of amounts of iron from red blood cells, which accumulates in tissues.
The liver is a central sensor and regulator of iron homeostasis, which is controlled through the expression of the hepatic hormone hepcidin. Hepcidin is a small peptide produced in the liver and secreted into the bloodstream that controls systemic iron homeostasis (7, 8). Hepcidin acts by binding to ferroportin (FPN1, also known as SLC40A1) (9–12), the only known mammalian iron exporter, which leads to its internalization and degradation (13). FPN1 is primarily expressed on enterocytes and macrophages of the reticuloendothelial system; thus, hepcidin acts to limit both duodenal iron absorption and release of iron from stores (14, 15). Hepcidin expression is decreased in several disorders of secondary hemochromatosis (16, 17). Multiple mechanisms control hepcidin expression in β-thalassemia (18), including the erythropoietic-induced repressors of hepcidin, growth differentiation factor-15 (GDF15) and twisted gastrulation (TWSG1) (19, 20). GDF15 and TWSG1 are secreted by erythropoietic precursors under conditions of erythropoietic stress and inhibit hepcidin expression. The prevailing thought is that the decrease in liver hepcidin is a critical factor in the hyperabsorption of iron. Increasing hepcidin levels inhibits iron overload in mouse models of β-thalassemia (21–23). Moreover, modulation of hepcidin levels can mobilize iron, suggesting that changes in hepcidin could be beneficial in several disorders that lead to iron overload with anemia that are not caused by hyperabsorption of iron. However, treatment with hepcidin does not completely correct serum and tissue-iron levels in iron-overload disorders with anemia (21–23).
The intestine is also a critical sensor and regulator of systemic iron homeostasis. Intestinal oxygen sensing is essential for adaptive increases in iron absorption. Intestinal hypoxia-inducible factor 2 alpha (HIF2α) is a critical regulator of iron absorption in the settings of iron deficiency, erythropoiesis, and hepcidin deficiency (24–29). HIF2α is a member of the family of hypoxia-inducible transcription factors, which control the cellular and systemic response to oxygen deficiency (30). The apical ferric reductase, duodenal cytochrome B [DcytB (31–33)] and the apical iron transporter, divalent metal transporter 1 (DMT1, also known as SLC11A2) (31, 34, 35), and the basolateral iron exporter FPN1 are direct HIF2α target genes (25, 26, 29). Despite the critical role of these apical transporters in iron absorption, no studies have examined the expression of these genes in β-thalassemia in mechanistic detail. Moreover, very little has been done to examine the integral role of local intestinal iron sensing and regulation in other anemic iron-overload disorders.
In this study, an intestinal hypoxia–HIF2α–DMT1 signaling axis was shown to be critical in the iron overload associated with β-thalassemia and hemolytic anemia. In addition, blocking intestinal HIF2α in mice with established iron overload led to a decrease in tissue iron while improving the anemia, providing a unique therapeutic target for patients with anemic iron-overload disorders.
Results
Hepcidin/FPN1 Signaling in the Intestine Is Not Required for Tissue-Iron Accumulation in Young β-Thalassemic Mice.
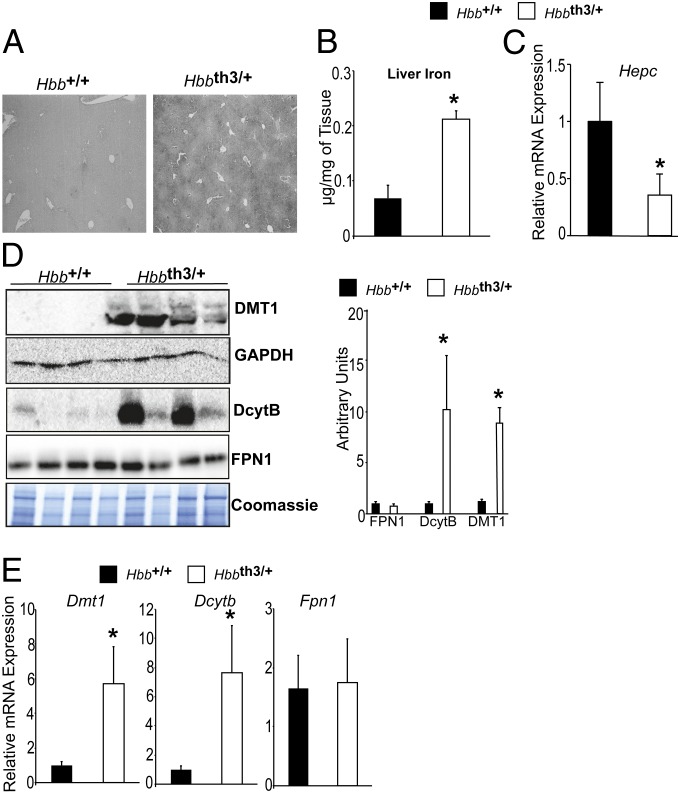
A mouse model of β-thalassemia intermedia (Hbbth3/+) was developed that recapitulates the human phenotype (36–38). These mice have a heterozygous deletion in the β-minor and β-major chain of hemoglobin. Hbbth3/+ mice develop severe anemia as well as progressive iron overload in the absence of blood transfusions, demonstrating that increased iron absorption plays a critical role in the disease pathogenesis. The mechanism of increased iron absorption is unclear and has not been targeted therapeutically in humans (39). One-month-old Hbbth3/+ mice have a significant increase in liver iron compared with wild-type littermate Hbb+/+ mice as determined by iron staining (Fig. 1A) and tissue nonheme iron quantitation (Fig. 1B). Consistent with previous reports, 3-wk-old Hbbth3/+ mice had lower liver hepcidin expression compared with littermate controls Hbb+/+ (Fig. 1C) (16). No change in duodenal FPN1 protein expression was observed (Fig. 1D). Robust and significant increases in DMT1 and DcytB were observed in 3-wk-old Hbbth3/+ mice (Fig. 1D). Gene expression analysis demonstrated a significant increase in DMT1 and DcytB but no change in FPN1 in Hbbth3/+ mice compared with littermate controls (Fig. 1E). These data demonstrate that mechanisms in addition to the hepcidin/FPN1 axis are critical for iron overload in β-thalassemia.
Fig. 1.
Apical iron absorption genes are increased early in a mouse model of β-thalassemia. (A) Enhanced Perls’ Prussian blue staining, (B) liver iron quantitation, and (C) qPCR analysis measuring liver hepcidin (Hepc) expression in 3-wk-old Hbbth3/+ and Hbb+/+ mice. Expression was normalized to β-actin. (D) Western blot analysis for DMT1, FPN1, and DcytB in 3-wk-old Hbbth3/+ and Hbb+/+ mice. DMT1 loading was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) whereas FPN1 and DcytB were normalized to coomassie staining, and the blots were quantitated and presented as fold changes compared with Hbb+/+ mice (Right). (E) qPCR analysis measuring intestinal Dmt1, Fpn1, and Dcytb gene expression. Expression was normalized to β-actin. Four to eight mice were assessed per each group, and each bar graph represents the mean value ± SD. *P < 0.01.
β-Thalassemic Mice Exhibit Systemic and Duodenal Hypoxia.
Intestinal DMT1 and DcytB were increased early in the pathogenesis of tissue-iron accumulation in a mouse model of β-thalassemia. These genes are direct hypoxia and HIF2α target genes (26). To determine whether mice with β-thalassemia are hypoxic, the hypoxia reporter mouse, ODD-luc, was crossed with the Hbbth3/+ mice (40). In vivo luminescence imaging of mice from this cross demonstrated that 3-wk-old thalassemic mice are systemically hypoxic (Fig. 2A). The increase in luminescence is evident in the abdomen and peripheral areas, such as the paws and tails. To obtain better resolution, tissues were excised and luciferase activity was measured. A significant increase in luciferase activity was observed in the liver, small intestine, and kidney (Fig. 2B). The increase in intestinal luminescence correlated to an increase in intestinal HIF2α and DMT1 expression, but not FPN1 (Fig. 2C). The increase in small-intestinal hypoxia and HIF2α in the β-thalassemic model compared with wild-type littermates (Hbb+/+) suggests that the increased iron absorption in β-thalassemia could be due to intestinal hypoxia.
Fig. 2.
Mice with β-thalassemia have systemic and duodenal hypoxia. ODD-luc mice were crossed with Hbbth3/+ mice and examined at 3 wk of age. (A) In vivo analysis of hypoxia in Hbbth3/+ mice compared with littermate controls. (B) Tissue luciferase assays from duodenum (Duo), colon (Col), Liver (Liv), kidney (Kid), heart (Ht), spleen (Spl), pancreas (Pan), white adipose tissue (WAT), brown adipose tissue (BAT), and lung (Lu). Luciferase values [relative luciferase units (RLU)] were normalized to total protein. (C) Western blot for duodenal HIF2α, DMT1, and FPN1 expression. HIF2α expression was normalized to lamin B1, DMT1 expression was normalized to GAPDH, and FPN1 expression was normalized to coomassie staining, and the blots were quantitated and presented as fold changes compared with Hbb+/+ mice (Right). Three to five mice were assessed per each group, and each bar graph represents the mean value ± SD. *P < 0.01.
Increased Iron Absorption and Tissue-Iron Overload in Mouse Models of β-Thalassemia Are Dependent on HIF2α.
Bone marrow transplantation (BMT) of Hbbth3/+ bone marrow into irradiated wild-type syngeneic mice leads to stable ineffective erythropoiesis and iron overload, hallmarks of β-thalassemia (36). This method provides a powerful and rapid means to assess genes critical in the disease progression of β-thalassemia. Intestinal HIF2α is a central transcription factor regulating iron absorption (25–27, 29); mice with the Hif2α gene floxed (Hif2αF/F) were crossed to mice expressing Cre under control of the villin promoter to specifically ablate HIF2α in the intestinal epithelium (Hif2αΔIE). To investigate the role of intestinal HIF2α in thalassemic iron absorption, Hif2αΔIE mice or littermate controls (Hif2αF/F) were lethally irradiated and transplanted with Hbbth3/+ or Hbb+/+ bone marrow (Fig. 3A). One month following BMT, expression of duodenal iron transporter genes was analyzed. The expression of the apical iron transporters Dmt1 and Dcytb was significantly increased in Hif2αF/F mice that were subjected to BMT from Hbbth3/+ donors (Fig. 3B). To understand the mechanistic basis for the increase in Dmt1 and Dcytb, bone marrow from Hbbth3/+ mice was transplanted into Hif2αΔIE mice. The increase in Dmt1 and Dcytb was completely abolished in the Hif2αΔIE mice (Fig. 3B). To understand whether the increase in apical iron absorption genes and intestinal HIF2α signaling is important in tissue-iron accumulation, liver iron was quantitated. As previously shown, Hif2αΔIE mice on an iron-replete diet for 1 mo do not have any significant difference in liver iron compared with Hif2αF/F mice (Fig. 3C). Irradiated Hif2αΔIE mice transplanted with wild-type bone marrow (Hbb+/+) demonstrate a significant drop in liver iron compared with irradiated littermate controls transplanted with wild-type bone marrow (Fig. 3C). This decrease may be due to the importance of intestinal HIF2α in iron absorption following erythropoiesis (27). Hif2αF/F mice transplanted with Hbbth3/+ bone marrow demonstrated a significant increase in liver iron 1 mo following BMT, but the increase in liver iron was entirely ameliorated in the Hif2αΔIE mice (Fig. 3C).
Fig. 3.
Disruption of intestinal HIF2α limits tissue-iron accumulation in a BMT-induced model of β-thalassemia. (A) Hif2αF/F and Hif2αΔIE mice were transplanted with Hbb+/+ or Hbbth3/+ bone marrow and analyzed 1 mo later for (B) intestinal Dmt1 and Dcytb mRNA expression and (C) liver iron content. mRNA expression was normalized to β-actin, and tissue-iron content was calculated per mg wet weight. Six to eight mice were assessed per each group, and each bar graph represents the mean value ± SD. *P < 0.01.
To confirm the role of the intestinal HIF2α response in thalassemic iron overload without the confounding effects of radiation, the Hif2αΔIE and Hif2αF/F mice were crossed to the Hbbth3/+ mice. The increase in Dmt1 and Dcytb gene expression and protein expression that was observed in the BMT model of β-thalassemia was recapitulated in the 2-mo-old Hif2αF/F/Hbbth3/+ mice, and this increase was abolished in Hbbth3/+/Hif2αΔIE mice (Fig. 4 A and B). Additionally, no change in FPN1 was observed in 2-mo-old thalassemic mice (Fig. 4B). When assessed for iron content by diaminobenzidine (DAB)-enhanced Perls’ Prussian blue staining, Hif2αF/F/Hbbth3/+ mice exhibited significantly higher accumulation of liver iron and spleen iron than wild-type littermates at 2 mo of age (Fig. 4C). The staining in the liver was mainly localized to hepatocytes and is consistent with what is observed in β-thalassemic patients (41). Disruption of intestinal HIF2α in the β-thalassemia model resulted in normalization of liver iron similar to the levels observed in wild-type mice (Fig. 4C). Liver-iron quantitation confirmed that the increase in iron accumulation observed in the Hif2αF/F/Hbbth3/+ mice is attenuated in the Hif2αΔIE/Hbbth3/+ mice (Fig. 4D). No change in serum iron, but a significant decrease in hepcidin expression, was noted at this time point, suggesting that an increase in tissue iron can relieve systemic increases in iron, or that tissue iron is a more sensitive measure of iron overload (Figs. S1 and S2). Moreover, the decrease in hepcidin expression was similar in the Hif2αΔIE/Hbbth3/+ mice compared with Hif2αF/F/Hbbth3/+ mice, suggesting that the decrease in iron accumulation is independent of hepcidin signaling (Fig. S2). Hematologic parameters were also assessed and demonstrated a slight but not statistically significant improvement compared with Hif2αF/F/Hbbth3/+ mice (Fig. 4E and Fig. S3). Together, the data suggest that HIF-2α is critical in the pathogenesis of iron accumulation in β-thalassemia.
Fig. 4.
β-thalassemic mice crossed to Hif2αΔIE mice demonstrate decreased tissue-iron accumulation. (A) qPCR analysis measuring intestinal Dmt1 and Dcytb gene expression in 2-mo-old Hif2αF/F/Hbb+/+, Hif2αΔIE/Hbb+/+, Hif2αF/F/Hbbth3/+, and Hif2αΔIE/Hbbth3/+ mice. Expression was normalized to β-actin. (B) Western blot analysis measuring intestinal DMT1, DcytB, and FPN1 protein expression in 2-mo-old Hif2αF/F/Hbb+/+, Hif2αΔIE/Hbb+/+, Hif2αF/F/Hbbth3/+, and Hif2αΔIE/Hbbth3/+ mice. DMT1 loading was normalized to GAPDH whereas FPN1 and DcytB were normalized to coomassie staining The blots were then quantitated and presented as fold changes compared with Hbb+/+/Hif2αF/F mice (values below the blots). (C) Enhanced Perls’ Prussian blue staining, (D) iron quantitation from the livers, and (E) RBC values in 2-mo-old Hif2αF/F/Hbb+/+, Hif2αΔIE/Hbb+/+, Hif2αF/F/Hbbth3/+, and Hif2αΔIE/Hbbth3/+ mice. Six to eight mice were assessed per each group, and each bar graph represents the mean value ± SD. *P < 0.01. For Western quantitation, the mean values ± SD are represented. *P < 0.01 compared with Hif2αF/F/Hbb+/+ mice.
Temporal Deletion of Intestinal HIF2α Improves Iron Accumulation in Thalassemic Mice with Established Iron Overload.
Blocking intestinal HIF2α restricts iron absorption leading to a decrease in tissue-iron accumulation. However, it is not clear whether existing iron that is accumulated in the liver can be cleared or mobilized more efficiently through inhibition of intestinal HIF2α and blocking of iron absorption. To answer this question, Hif2αF/F mice were crossed with villin-Cre-ERT2 transgenic mice to generate a temporal and conditional disruption of Hif2α (Hif2αF/F;VilERcre). The Cre is activated in intestinal epithelial cells only following tamoxifen administration, which leads to disruption of HIF2α. To confirm the inducibility, the Hif2αF/F;VilERcre mice were crossed to yellow fluorescent protein reporter (YFP) mice, in which Cre activation would be delineated by expression of YFP. Three 1.5-mg doses of tamoxifen led to efficient recombination in the small intestine and a robust decrease in intestinal HIF2α expression (Fig. S4). To investigate the role of intestinal HIF2α in mice with established iron overload due to thalassemia, Hif2αF/F;VilERcre or littermate controls (Hif2αF/F) were lethally irradiated and transplanted with Hbbth3/+ or Hbb+/+ bone marrow. Two months following BMT, mice were treated with vehicle or tamoxifen and killed at 4 mo following BMT (Fig. 5A). Transplant of Hbb+/+ bone marrow into Hif2αF/F and Hif2αΔIE mice led to no significant difference in the expression of Dmt1, Dcytb, and Fpn1 4 mo following BMT (Fig. 5B). Transplant of Hbbth3/+ bone marrow into Hif2αF/F and Hif2αF/F;VilERcre led to a significant increase in intestinal Dmt1 and Dcytb expression 2 mo following BMT. Interestingly, intestinal Fpn1 expression was also increased, confirming a previous study demonstrating that FPN1 expression is induced in aged thalassemic mice (Fig. 5B) (42). Gene-expression analysis of the small intestine of 4-mo-old Hif2αF/F and Hif2αF/F;VilERcre mice transplanted with Hbbth3/+ bone marrow and treated with tamoxifen at 2 mo was performed. The data demonstrated that the increase in Dmt1, Dcytb, and Fpn1 was completely attenuated in tamoxifen-treated Hif2αF/F;VilERcre mice 4 mo after transplant (Fig. 5B). Protein expression analysis was consistent with the gene-expression analysis in BMT of Hif2αF/F and Hif2αF/F;VilERcre mice (Fig. 5C). Liver iron was assessed to determine whether HIF2α disruption improved iron accumulation. Transplant of Hbb+/+ bone marrow into Hif2αF/F and Hif2αΔIE mice led to a significant decrease of liver iron in the Hif2αΔIE mice 4 mo following BMT. These data suggest that as the mice age, intestinal HIF2α may play a more important role in basal iron absorption (Fig. 5D). Transplant of Hbbth3/+ bone marrow into Hif2αF/F and Hif2αF/F;VilERcre mice led to a significant increase in liver iron 2 mo following BMT. Transplant of Hbbth3/+ bone marrow into Hif2αF/F and Hif2αF/F;VilERcre mice treated with tamoxifen at 2 mo with liver iron measured at 4 mo demonstrated that the Hif2αF/F mice have a further increase in liver iron compared with when they are assessed at 2 mo. The Hif2αF/F;VilERcre mice treated with tamoxifen have a significant attenuation of liver iron (Fig. 5D). Transplant of Hbbth3/+ bone marrow into Hif2αF/F mice also demonstrated a significant increase in serum iron, which was completely attenuated in similarly treated Hif2αF/F;VilERcre mice at 4 mo (Fig. S5). Assessment of liver hepcidin expression 4 mo after transplant demonstrated a decrease in hepcidin expression in all groups compared with Hif2αF/F mice receiving Hbb+/+ bone marrow. Hepcidin expression was not significantly different in mice, regardless of improvement in tissue-iron accumulation. (Fig. S6). Furthermore, the disruption of HIF2α 4 mo following BMT of Hbbth3/+ marrow demonstrated a significant improvement in hematological parameters compared with similarly treated Hif2αF/F mice (Fig. 5E and Fig. S7). Together, the data demonstrate that intestinal HIF2α can be therapeutically targeted in anemic iron-overload disorders.
Fig. 5.
Disruption of HIF2α decreases tissue-iron accumulation in thalassemic mice with established iron overload. (A) Hif2αF/F, Hif2αΔIE, and Hif2αF/F;VilERcre mice were lethally irradiated and transplanted with Hbbth3/+ or Hbb+/+ bone marrow. Two months following BMT, mice were treated with vehicle or 1.5 mg of tamoxifen for 3 consecutive days and killed 2 mo later. (B) qPCR analysis measuring Dmt1, Dcytb, and Fpn1. Expression was normalized to β-actin. (C) Western blot analysis measuring intestinal DMT1, DcytB, and FPN1 protein expression. DMT1 loading was normalized to GAPDH whereas FPN1 and DcytB were normalized to Coomassie staining. The blots were quantitated and presented as fold changes compared with Hbb+/+ bone marrow transplanted into Hif2αF/F mice (values below the blots). (D) Iron quantitation from the liver and (E) RBC values. Six to eight mice were assessed per each group, and each bar graph represents the mean value ± SD. *P < 0.01. For Western quantitation, the mean values are represented. *P < 0.01 compared with Hbbth3/+ bone marrow transplanted into Hif2αF/F mice at 4 mo following BMT. TP < 0.01 compared with mice at 2 mo following BMT.
Loss of Intestinal HIF2α Decreases Iron Accumulation due to Hemolysis.
The observation that HIF2α inhibition could be beneficial in β-thalassemic mice with established iron overload suggests that HIF2α could be a useful target in several conditions that cause iron loading with anemia. In patients with hemolytic anemia, the tissue-iron accumulation is due to hemolysis. Phenylhydrazine (PhZ)-induced hemolysis is a well characterized model for hemolytic anemia that leads to an increase in liver iron that is poorly mobilized over time (43, 44). In this model, intestinal iron absorption is important for erythropoietic recovery but is not required for iron accumulation (27). This model is optimal to study the role of intestinal HIF2α in decreasing iron accumulation following hemolysis. Hif2αF/F mice were treated with PhZ, and liver iron was measured at 2 and 7 d after treatment. Hif2αF/F mice had a significant increase in liver iron 2 d after PhZ treatment, and levels remained high 1 wk following treatment (Fig. 6A). Similar to wild-type mice, PhZ treatment of Hif2αΔIE mice causes substantial liver iron loading 2 d following treatment. However, 7 d after PhZ treatment, liver-iron content decreased to normal levels in the Hif2αΔIE mice (Fig. 6A). Given that the initial iron loading at 2 d after PhZ was significantly lower in the Hif2αΔIE mice compared with Hif2αF/F mice, the Hif2αF/F;VilERcre mice were also assessed. This model will allow similar hemolysis-induced iron loading and more accurate assessment of how fast iron can be mobilized and/or used from the liver following HIF2α disruption. Hif2αF/F and Hif2αF/F;VilERcre mice were treated with vehicle or PhZ, and liver iron was measured at 2 d after treatment or the mice were treated with tamoxifen and assessed for liver iron at 7 d following PhZ treatment (Fig. 6B, Inset). There was no significant difference in the iron accumulation at 2 d following PhZ treatment in the Hif2αF/F and Hif2αF/F;VilERcre mice. Acute disruption of intestinal HIF2α led to a significant decrease in liver iron 7 d following PhZ administration whereas Hif2αF/F mice still had significant iron accumulation at 7 d following PhZ treatment. These data demonstrate that inhibition of intestinal HIF2α could be beneficial in anemic iron-overload disorders due to hemolysis.
Fig. 6.
Intestinal disruption of HIF2α decreases hemolysis-induced liver iron accumulation. (A) Hif2αF/F and Hif2αΔIE mice were treated with vehicle (Con) or phenylhydrazine (PhZ), and liver iron was quantitated 2 and 7 d following treatment. (B) Hif2αF/F and Hif2αF/F;VilERcre mice were treated with vehicle (Con) or phenylhydrazine (PhZ), and liver iron was quantitated or the mice were additionally treated with 1.5 mg of tamoxifen for 3 consecutive days, and liver iron was quantitated 7 d following PhZ treatment. Four to eight mice were assessed per each group, and each bar graph represents the mean value ± SD. NS, not significant; *P < 0.01; #P < 0.05.
Disruption of DMT1 Decreases Iron Accumulation in Mouse Models of β-Thalassemia and Hemolytic Anemia.
The data suggest that the apical proteins DMT1 and DcytB are critical downstream effectors of HIF2α in mouse models of anemic iron overload. The role of DMT1 in iron-overload disorders with anemia was assessed. Mouse models with a temporal disruption of DMT1 in intestinal epithelial cells were generated. Disruption of DMT1 in intestinal epithelial cells leads to a rapid and progressive anemia (11). Therefore, a cross of Dmt1F/+ mice to the villin-Cre-ERT2 mice was generated to disrupt one Dmt1 allele. This cross led to no significant difference in the basal Dmt1 expression in the small intestine 1 mo following tamoxifen treatment but dramatically decreased the low iron-induced expression of Dmt1 (Fig. S8A). There was no significant difference in serum iron on iron-replete or low-iron diet for 2 wk in tamoxifen-treated Dmt1F/+ mice compared with Dmt1F/+;VilERcre mice. A low-iron diet for 2 wk significantly decreased liver iron in Dmt1F/+;VilERcre compared with Dmt1F/+ mice following tamoxifen treatment (Fig. S8 B and C). These data demonstrate that disrupting a single allele of Dmt1 in the intestine is an ideal model to understand the significance of intestinal DMT1 induction in β-thalassemia without the confounding systemic anemia that is associated with complete intestinal disruption. To understand the role of DMT1 in iron accumulation in β-thalassemia, Dmt1F/+ and Dmt1F/+;VilERcre were treated with tamoxifen, and irradiated mice were transplanted with Hbbth3/+ or Hbb+/+ bone marrow (Fig. 7A). One month following BMT, DMT1 gene expression and protein expression were assessed. Deletion of one allele of Dmt1 did not affect basal DMT1 expression in Dmt1F/+ and Dmt1F/+;VilERcre mice transplanted with Hbb+/+ bone marrow (Fig. 7 B and C). A significant increase in intestinal Dmt1 expression was observed in Dmt1F/+ transplanted with Hbbth3/+ bone marrow, which was significantly decreased in the Dmt1F/+;VilERcre mice (Fig. 7 B and C). Consistent with these data, liver iron was not altered in the Dmt1F/+ and Dmt1F/+;VilERcre mice transplanted with Hbb+/+ bone marrow. No change in serum iron was observed (Fig. S9); however, an increase in liver iron accumulation was observed in Dmt1F/+ mice transplanted with Hbbth3/+ bone marrow, which was significantly attenuated in the Dmt1F/+;VilERcre mice (Fig. 7D). A decrease in liver hepcidin expression was observed in Dmt1F/+ and Dmt1F/+;VilERcre mice transplanted with Hbbth3/+ bone marrow. These data suggest that disruption of DMT1 attenuates iron accumulation via a hepcidin-independent mechanism, similar to that observed following intestinal HIF2α disruption in the intestine (Fig. S10). The role of Dmt1 was also assessed in iron accumulation following hemolysis. Dmt1F/+ and Dmt1F/+;VilERcre mice were treated with vehicle or PhZ, and liver iron was measured at 2 d after treatment or the mice were treated with tamoxifen and assessed for liver iron at 7 d following PhZ treatment (Fig. 7E, Inset). There was no significant difference in the iron accumulation at 2 d following PhZ treatment in the Dmt1F/+ and Dmt1F/+VilERcre mice. However, following tamoxifen treatment, hemolysis-induced iron accumulation in the liver of Dmt1F/+;VilERcre mice was significantly lower compared with tamoxifen-treated Dmt1F/+ mice at 7 d following PhZ treatment (Fig. 7E). These data suggest that DMT1 is an important downstream target gene of HIF2α that modulates iron accumulation in iron-overload disorders with anemia.
Fig. 7.
DMT1 is a critical downstream effector of HIF2α in anemic iron overload. (A) Dmt1F/+ and Dmt1F/+;VilERcre mice were treated with 1.5 mg of tamoxifen for 3 consecutive days and then were lethally irradiated and transplanted with Hbbth3/+ or Hbb+/+ bone marrow and analyzed 1 mo later. (B) qPCR analysis measuring Dmt1 with expression normalized to β-actin. (C) Western blot analysis measuring intestinal DMT1 with loading normalized to GAPDH. The blot was then quantitated and presented as fold change compared with Hbb+/+ bone marrow transplanted into Dmt1F/+ mice (values below the blots). (D) Iron quantitation from the liver of BMT mice. (E) Dmt1F/+ and Dmt1F/+;VilERcre mice were treated with vehicle (Con) or phenylhydrazine (PhZ), and liver iron was quantitated or the mice were additionally treated with 1.5 mg of tamoxifen for 3 consecutive days, and liver iron was quantitated 7 d following PhZ treatment. Six to eight mice were assessed per each group, and each bar graph represents the mean value ± SD. *P < 0.01, #P < 0.05. For Western quantitation, the mean values are represented. *P < 0.01 compared with Hbbth3/+ bone marrow transplanted into Dmt1F/+ mice.
Discussion
Previous work has demonstrated an integral role of intestinal HIF2α signaling in iron absorption. HIF2α is a critical regulator of the apical iron absorption genes DMT1 and DcytB and the only known mammalian basolateral exporter FPN1. During increased systemic requirements for iron, intestinal HIF2α is highly activated, leading to an adaptive increase in iron absorption. Several studies have cemented the role of intestinal HIF2α in physiological regulation of iron homeostasis. However, the role of HIF2α signaling in the pathogenesis of iron-overload disorders was unclear. Recently, it was demonstrated that intestinal HIF2α signaling was essential for liver iron overload following hepcidin deficiency (28). It is not clear the pathway by which HIF2α is activated during hepcidin deficiency. We hypothesized that intestinal HIF2α would be critical in iron accumulation in specific anemic iron-overload disorders such as β-thalassemia because it has been shown that anemia induces intestinal hypoxia (27) and that hyperabsorption of iron contributes significantly to the iron overload (4, 5). Consistent with this hypothesis, HIF2α was activated early in the pathogenesis of iron accumulation in mouse models of β-thalassemia, and disruption of intestinal HIF2α corrected the iron overload. Moreover, a significant repression of hepcidin is observed in β-thalassemia; however, this decrease still persists in mice with a disruption of intestinal HIF2α. These data suggest that HIF2α and hepcidin operate independently or that HIF2α is downstream of hepcidin signaling to control iron overload in β-thalassemia.
Recent studies based on HIF2α structural analysis have identified compounds that specifically inhibit HIF2α function and present the possibility of HIF2α-based therapeutics, although these compounds have not yet been demonstrated to have in vivo efficacy (45–47). To determine whether HIF2α would be a good therapeutic target to decrease tissue-iron accumulation, a mouse model with a temporal disruption of intestinal HIF2α was used. The data clearly demonstrated that β-thalassemic mice with established iron overload significantly improved with respect to tissue-iron accumulation upon disruption of intestinal HIF2α. Moreover, limiting intestinal iron absorption improved the anemia associated with β-thalassemia. These data are consistent with previous reports demonstrating that decreased tissue-iron accumulation in β-thalassemia correlates to improvement in complete blood count (CBC) parameters (21–23, 48). Currently the mechanism leading to a decrease in iron accumulation in β-thalassemic mice with established iron overload is not completely clear. Disruption of intestinal HIF2α may work through two mechanisms: (i) blocking iron absorption, therefore inhibiting further iron accumulation, (ii) or leading to more efficient iron mobilization and utilization from the liver. These data suggest that blocking HIF2α may be beneficial in other conditions that lead to iron-overload disorders with anemia. To test this possibility, a hemolytic anemia model was used. Iron accumulation in this model is directly due to increased hemolysis and independent of iron absorption. Disruption of HIF2α led to decreased iron accumulation following hemolysis, providing further rationale that intestinal HIF2α is a highly efficacious target for anemic iron-overload disorders.
Dmt1, Dcytb, and Fpn1 are all direct HIF2α target genes, but the increase in intestinal DMT1 and DcytB preceded changes in FPN1 in the small intestine of β-thalassemic mice. These results are consistent with the difference in kinetics observed in the regulation of these genes by HIF2α (25). No significant change in FPN1 protein expression was observed until 2 mo following induction of β-thalassemia, and a further increase was observed at 4 mo. The increase in Fpn1 gene expression was completely dependent on HIF2α. The increase in protein expression was only partially dependent on intestinal HIF2α signaling. These findings are similar to FPN1 induction by chronic low-iron diet treatment, which is thought to be due to the regulation of FPN1 degradation by hepcidin. Indeed, hepcidin levels are decreased in mouse models of β-thalassemia and in thalassemic patients (17). Restoring hepcidin levels can improve iron accumulation in β-thalassemic mice, demonstrating the importance of hepcidin in anemic iron-overload disorders (21–23). However, the rapid increase in HIF2α, DMT1, and DcytB indicates the importance of the apical iron transport proteins early in the pathogenesis of iron accumulation in mouse models of β-thalassemia. To test this possibility, a mouse model was generated in which a single allele of Dmt1 was disrupted specifically in the intestine. The mice on a normal diet were indistinguishable from their wild-type littermates. However, the induction of Dmt1 that is observed in the intestine following low-iron diet or in thalassemic mice is significantly attenuated in mice disrupted for a single allele of Dmt1 in the intestine. These mice had decreased iron accumulation in mouse models of β-thalassemia and hemolytic anemia. These results demonstrate the importance of DMT1 in iron-overload disorders and provides good rationale to target DMT1 in anemic iron-overload disorders. Similar to HIF2α, compounds have been identified that inhibit DMT1 function (49, 50), and thus it may be possible to specifically inhibit DMT1 as a novel treatment modality in iron-overload disorders.
In summary, the present study demonstrates that the intestine, liver, and kidney as observed in the hypoxia reporter mice are the major susceptible tissues to systemic changes in oxygen delivery. This sensitivity may be due to their critical role in erythropoiesis and systemic iron homeostasis. In the pathogenesis of anemic iron-overload diseases such as β-thalassemia, intestinal hypoxia leads to activation of HIF2α, which increases DMT1 expression, contributing to tissue-iron accumulation. Inhibition of HIF2α or DMT1 prevents tissue iron loading in β-thalassemia. Moreover, inhibition of HIF2α or DMT1 can decrease tissue-iron accumulation in mouse models of β-thalassemia and hemolytic anemia with established iron overload. This finding suggests that inhibition of this signaling pathway could be a novel and robust treatment strategy for several conditions that cause iron overload with anemia.
Materials and Methods
Animals and Treatments.
Hif2αF/F, Hif2αΔIE, Hbbth3/+, and ODD-luc animals were described previously (25, 36, 40). The Hif2αF/F;VilERcre and Dmt1F/+;VilERcre mice were generated by crossing the Hif2αF/F mice or Dmt1F/F (51) to the villin-CreERT2 mice (52). To activate the CreERT2, mice were treated with 1.5 mg of tamoxifen (Sigma) on 3 consecutive days. PhZ (Sigma) treatment was performed as previously described (27). For BMT, mice received 12 Gy of total body irradiation split into two doses of 6 Gy in an interval of 4 h, and BMT was performed the day after irradiation. In vivo and ex vivo luminescence imaging was performed as previously described in 3-wk-old mice (40). Tissue luciferase activity was quantitated by homogenizing the tissues in 1× passive lysis buffer, and 1 μL of extract was used for standard luciferase assay (Promega). CBC analysis was performed by the Unit for Laboratory Animal Medicine Pathology Core for Animal Research. The University Committee on the Use and Care of Animals at the University of Michigan approved all animal studies.
Western Blot.
Membrane protein isolation was performed for the detection of DcytB and FPN1 as described previously (25). Whole-cell extracts were isolated for the detection of DMT1, and nuclear extracts were isolated for the detection of HIF2α as previously described (24). Antibodies against HIF2α (Novus Biologicals), GAPDH (Santa Cruz Biotechnology), Dmt1, DcytB, and Fpn1 (Alpha Diagnostic International), and Lamin B1 (Abcam) were used.
Iron Staining.
Tissue-iron detection was performed in formalin fixed paraffin-embedded sections stained with Perls’ Prussian blue enhanced with DAB and H2O2 with nuclear fast red counterstain.
Real-Time Quantitative PCR.
RNA isolation, reverse transcription, real-time quantitative PCR (qPCR), and primers used for qPCR were described previously (27).
Tissue-Iron Quantitation.
Tissue nonheme iron was quantitated as previously described (53). Briefly, tissues were homogenized in a 1-M HCl and 10% (wt/vol) trichloroacetic acid solution and heated for 1 h at 95 °C. Following a high-speed spin, iron was quantitated using a ferrozine solution and compared with a standard.
Statistics.
Results are expressed as mean ± SD. P values were calculated by independent t test, 1-way ANOVA, and two-way ANOVA.
Supplementary Material
Acknowledgments
We thank Dr. Nancy Andrews (Duke University) for generously providing the Dmt1F/F mice. This work was supported by a Rackham predoctoral fellowship (to E.R.A.), National Institutes of Health Grants CA148828 and DK095201, and the University of Michigan Gastrointestinal Peptide Center (Y.M.S.).
Footnotes
Conflict of interest statement: S. Rivella is a consultant for Exigo Management Consultant, Isis, Bayer AG, BioMarin, Merganser Biotech, and Novartis Pharmaceuticals. He also holds an equity/ownership interest in Merganser Biotech, Inc. In addition, he is a coinventor for the patents US8058061 B2 C12N 20111115 and US7541179 B2 C12N 20090602. The consulting work and intellectual property of S. Rivella did not affect in any way the design, conduct, or reporting of this research.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314197110/-/DCSupplemental.
References
- 1.Gattermann N. The treatment of secondary hemochromatosis. Dtsch Arztebl Int. 2009;106(30):499–504, I. doi: 10.3238/arztebl.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musallam KM, et al. Elevated liver iron concentration is a marker of increased morbidity in patients with β thalassemia intermedia. Haematologica. 2011;96(11):1605–1612. doi: 10.3324/haematol.2011.047852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg MHFB, Higgs DR, Nagel RL. Disorders of Hemoglobin: Genetics, Pathophysiology and Clincal Management. Cambridge, UK: Cambridge Univ Press; 2001. [Google Scholar]
- 4.Heinrich HC, et al. Absorption of inorganic and food iron in children with heterozygous and homozygous beta-thalassemia. Z Kinderheilkd. 1973;115(1):1–22. doi: 10.1007/BF00438987. [DOI] [PubMed] [Google Scholar]
- 5.Pitcher CS, Williams HS, Parsonson A, Williams R. The measurement of iron absorption by the double isotope technique. Br J Haematol. 1965;11(6):633–641. doi: 10.1111/j.1365-2141.1965.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 6.Ginzburg Y, Rivella S. β-thalassemia: A model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood. 2011;118(16):4321–4330. doi: 10.1182/blood-2011-03-283614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolas G, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98(15):8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolas G, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99(7):4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275(26):19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 10.Donovan A, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403(6771):776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 11.Donovan A, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.McKie AT, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5(2):299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 14.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102(5):1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frazer DM, et al. Hepcidin expression inversely correlates with the expression of duodenal iron transporters and iron absorption in rats. Gastroenterology. 2002;123(3):835–844. doi: 10.1053/gast.2002.35353. [DOI] [PubMed] [Google Scholar]
- 16.Adamsky K, et al. Decreased hepcidin mRNA expression in thalassemic mice. Br J Haematol. 2004;124(1):123–124. doi: 10.1046/j.1365-2141.2003.04734.x. [DOI] [PubMed] [Google Scholar]
- 17.Darshan D, Frazer DM, Anderson GJ. Molecular basis of iron-loading disorders. Expert Rev Mol Med. 2010;12:e36. doi: 10.1017/S1462399410001687. [DOI] [PubMed] [Google Scholar]
- 18.Anderson ER, Shah YM. Iron homeostasis in the liver. Compr Physiol. 2013;3(1):315–330. doi: 10.1002/cphy.c120016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanno T, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13(9):1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 20.Tanno T, et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114(1):181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardenghi S, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. J Clin Invest. 2010;120(12):4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo S, et al. Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice. J Clin Invest. 2013;123(4):1531–1541. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt PJ, et al. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(-/-) mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood. 2013;121(7):1200–1208. doi: 10.1182/blood-2012-09-453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue X, et al. Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 2012;72(9):2285–2293. doi: 10.1158/0008-5472.CAN-11-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor M, et al. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140(7):2044–2055. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9(2):152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson ER, Xue X, Shah YM. Intestinal hypoxia-inducible factor-2alpha (HIF-2alpha) is critical for efficient erythropoiesis. J Biol Chem. 2011;286(22):19533–19540. doi: 10.1074/jbc.M111.238667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mastrogiannaki M, et al. Deletion of HIF-2α in the enterocytes decreases the severity of tissue iron loading in hepcidin knockout mice. Blood. 2012;119(2):587–590. doi: 10.1182/blood-2011-09-380337. [DOI] [PubMed] [Google Scholar]
- 29.Mastrogiannaki M, et al. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119(5):1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie B, Garrick MD. Iron Imports. II. Iron uptake at the apical membrane in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289(6):G981–G986. doi: 10.1152/ajpgi.00363.2005. [DOI] [PubMed] [Google Scholar]
- 32.Latunde-Dada GO, Simpson RJ, McKie AT. Duodenal cytochrome B expression stimulates iron uptake by human intestinal epithelial cells. J Nutr. 2008;138(6):991–995. doi: 10.1093/jn/138.6.991. [DOI] [PubMed] [Google Scholar]
- 33.McKie AT, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291(5509):1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- 34.Fleming MD, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16(4):383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 35.Gunshin H, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 36.Rivella S, May C, Chadburn A, Rivière I, Sadelain M. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transfer. Blood. 2003;101(8):2932–2939. doi: 10.1182/blood-2002-10-3305. [DOI] [PubMed] [Google Scholar]
- 37.Ciavatta DJ, Ryan TM, Farmer SC, Townes TM. Mouse model of human beta zero thalassemia: Targeted deletion of the mouse beta maj- and beta min-globin genes in embryonic stem cells. Proc Natl Acad Sci USA. 1995;92(20):9259–9263. doi: 10.1073/pnas.92.20.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang B, et al. A mouse model for beta 0-thalassemia. Proc Natl Acad Sci USA. 1995;92(25):11608–11612. doi: 10.1073/pnas.92.25.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rechavi G, Rivella S. Regulation of iron absorption in hemoglobinopathies. Curr Mol Med. 2008;8(7):646–662. doi: 10.2174/156652408786241401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson ER, et al. The hypoxia-inducible factor-C/EBPα axis controls ethanol-mediated hepcidin repression. Mol Cell Biol. 2012;32(19):4068–4077. doi: 10.1128/MCB.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Origa R, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92(5):583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 42.Gardenghi S, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109(11):5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, et al. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood. 2011;118(7):1912–1922. doi: 10.1182/blood-2011-01-330324. [DOI] [PubMed] [Google Scholar]
- 44.Latunde-Dada GO, McKie AT, Simpson RJ. Animal models with enhanced erythropoiesis and iron absorption. Biochim Biophys Acta. 2006;1762(4):414–423. doi: 10.1016/j.bbadis.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Scheuermann TH, et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol. 2013;9(4):271–276. doi: 10.1038/nchembio.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Key J, Scheuermann TH, Anderson PC, Daggett V, Gardner KH. Principles of ligand binding within a completely buried cavity in HIF2alpha PAS-B. J Am Chem Soc. 2009;131(48):17647–17654. doi: 10.1021/ja9073062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheuermann TH, et al. Artificial ligand binding within the HIF2alpha PAS-B domain of the HIF2 transcription factor. Proc Natl Acad Sci USA. 2009;106(2):450–455. doi: 10.1073/pnas.0808092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, et al. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16(2):177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 49.Buckett PD, Wessling-Resnick M. Small molecule inhibitors of divalent metal transporter-1. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G798–G804. doi: 10.1152/ajpgi.90342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, et al. Discovery of benzylisothioureas as potent divalent metal transporter 1 (DMT1) inhibitors. Bioorg Med Chem Lett. 2012;22(15):5108–5113. doi: 10.1016/j.bmcl.2012.05.129. [DOI] [PubMed] [Google Scholar]
- 51.Gunshin H, et al. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115(5):1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.el Marjou F, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39(3):186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 53.Rebouche CJ, Wilcox CL, Widness JA. Microanalysis of non-heme iron in animal tissues. J Biochem Biophys Methods. 2004;58(3):239–251. doi: 10.1016/j.jbbm.2003.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.