Significance
Antigen-presenting cells (APCs) function as the sentinels of the immune system by ingesting foreign pathogens, generating peptide fragments of these pathogens in endo-/lysosomes, where the peptides bind to MHC class II molecules, and expressing these peptide–MHC class II complexes (pMHC-II) on the APC surface. Antigen-specific CD4 T cells scan APC surfaces for appropriate pMHC-II, and, if not recognized by T cells, pMHC-II are degraded. We now show that internalizing pMHC-II are targeted for degradation by ubiquitination by the E3 ligase March-I in early endosomes. The “arrive at the surface, internalize from the surface, become ubiquitinated, then die” life cycle for pMHC-II ensures that APCs have ample opportunity to present a wide variety of foreign antigens to the immune system.
Abstract
As sentinels of the immune system, dendritic cells (DCs) continuously generate and turnover antigenic peptide–MHC class II complexes (pMHC-II). pMHC-II generation is a complex process that involves many well-characterized MHC-II biosynthetic intermediates; however, the mechanisms leading to MHC-II turnover/degradation are poorly understood. We now show that pMHC-II complexes undergoing clathrin-independent endocytosis from the DC surface are efficiently ubiquitinated by the E3 ubiquitin ligase March-I in early endosomes, whereas biosynthetically immature MHC-II–Invariant chain (Ii) complexes are not. The inability of MHC-II–Ii to serve as a March-I substrate is a consequence of Ii sorting motifs that divert the MHC-II–Ii complex away from March-I+ early endosomes. When these sorting motifs are mutated, or when clathrin-mediated endocytosis is inhibited, MHC-II–Ii complexes internalize by using a clathrin-independent endocytosis pathway and are now ubiquitinated as efficiently as pMHC-II complexes. These data show that the selective ubiquitination of internalizing surface pMHC-II in March-I+ early endosomes promotes degradation of “old” pMHC-II and spares forms of MHC-II that have not yet loaded antigenic peptides or have not yet reached the DC surface.
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that capture protein antigens by endocytosis and digest these internalized proteins into peptides in endo-/lysosomal compartments (1). These peptides are loaded onto MHC class II molecules (MHC-II) in antigen-processing compartments, and peptide-loaded MHC-II (pMHC-II) traffics to the DC plasma membrane. The interaction of specific pMHC-II on APCs with specific receptors on naïve CD4 T cells stimulates the activation and proliferation of CD4 T cells (1, 2). Each DC potentially expresses thousands of distinct pMHC-II complexes in which the MHC-II–bound peptides represent a sampling of the DC microenvironment.
Resting (i.e., immature) DCs generate and express pMHC-II complexes on their surface, and, at steady state, the rates of MHC-II synthesis and degradation are equal. Stimulation of APCs, either by Toll-like receptor (TLR) ligands or by exposure to other “danger” signals, ultimately reduces the rate of MHC-II synthesis and prolongs MHC-II t1/2 (3, 4), processes that allow the DC to preserve on their surface pMHC-II complexes generated at the time of DC activation. Given the importance of antigenic pMHC-II in the initiation of antigen-specific T cell responses, there is intense interest in elucidating the mechanisms regulating pMHC-II generation and turnover in professional APCs.
Newly synthesized MHC-II αβ-dimers begin their transport to the plasma membrane by binding to a chaperone protein termed the invariant chain (Ii) in the ER (5). Most MHC-II–Ii complexes then leave the ER, traverse the Golgi apparatus, and are transported to cell surface. When they are at the cell surface, MHC-II–Ii complexes are rapidly internalized by clathrin-mediated endocytosis, pass through early endosomes, and are ultimately sorted to late endosomal/prelysosomal compartments, where Ii proteolysis generates an MHC-II αβ-dimer. In these antigen presentation compartments, the class II-associated Ii-polypeptide is removed from the peptide-binding groove of MHC-II by the enzymatic activity of HLA-DM (6), thereby liberating the peptide-binding site to allow for high-affinity peptide binding to MHC-II before its movement to the plasma membrane.
pMHC-II expressed on the surface of APCs is not static, and these complexes are internalized and can recycle back to the cell surface or be targeted for degradation in lysosomes (7–9). We have reported that, unlike MHC-II-Ii complexes, pMHC-II complexes internalize by using a clathrin-independent endocytosis pathway that involves passage through Arf6+Rab35+ tubular endosomes (10). Thus, the fate of internalized pMHC-II strongly influences the overall stability of pMHC-II in DCs.
Ubiquitination of membrane proteins acts as a signal for clathrin-dependent and clathrin-independent endocytosis and targets ubiquitinated receptors to multivesicular bodies (MVBs), a process that ultimately leads to lysosomal degradation of ubiquitinated cargo molecules (11). Cell surface expression of pMHC-II is also regulated by ubiquitination of a cytosolic lysine residue (Lys225) present in the cytosolic domain of the MHC-II β-chain (12, 13). The E3 ligase March-I ubiquitinates MHC-II in B cells (14) and DCs (15) and dramatically affects MHC-II surface expression in these APCs. March-I is expressed on immature (resting) DCs, and activation of DCs with a variety of TLR-ligands leads to March-I downregulation (15, 16) and prevents MHC-II ubiquitination (12, 13, 15, 16). Curiously, it has been reported that Ii-associated MHC-II is not ubiquitinated in DCs (13, 15), suggesting that ubiquitination of MHC-II is tightly controlled in APCs.
Although a role for ubiquitination in regulating surface stability of pMHC-II has been revealed (15, 17), the mechanism behind the selectivity of March-I for different forms of MHC-II remains to be determined. In this study, we have asked in what intracellular compartment MHC-II is ubiquitinated and how MHC-II–Ii complexes are spared from ubiquitination by March-I. We found that the integrity of the dileucine sorting motif in Ii is required for the MHC-II–Ii complex to avoid ubiquitination by March-I. Although pMHC-II complexes are internalized and distributed in March-I+ endosomes, internalized MHC-II–Ii complexes are not. Furthermore, we found that inhibition of clathrin-dependent endocytosis directly promotes ubiquitination of MHC-II–Ii complexes. Our data thus show that MHC-II–Ii complexes avoid ubiquitination by March-I by using a rapid clathrin-dependent endocytosis pathway that limits the amount of time MHC-II–Ii complexes are present in March-I+ endosomes.
Results
Peptide-Loaded MHC-II Is Ubiquitinated Shortly After Endocytosis.
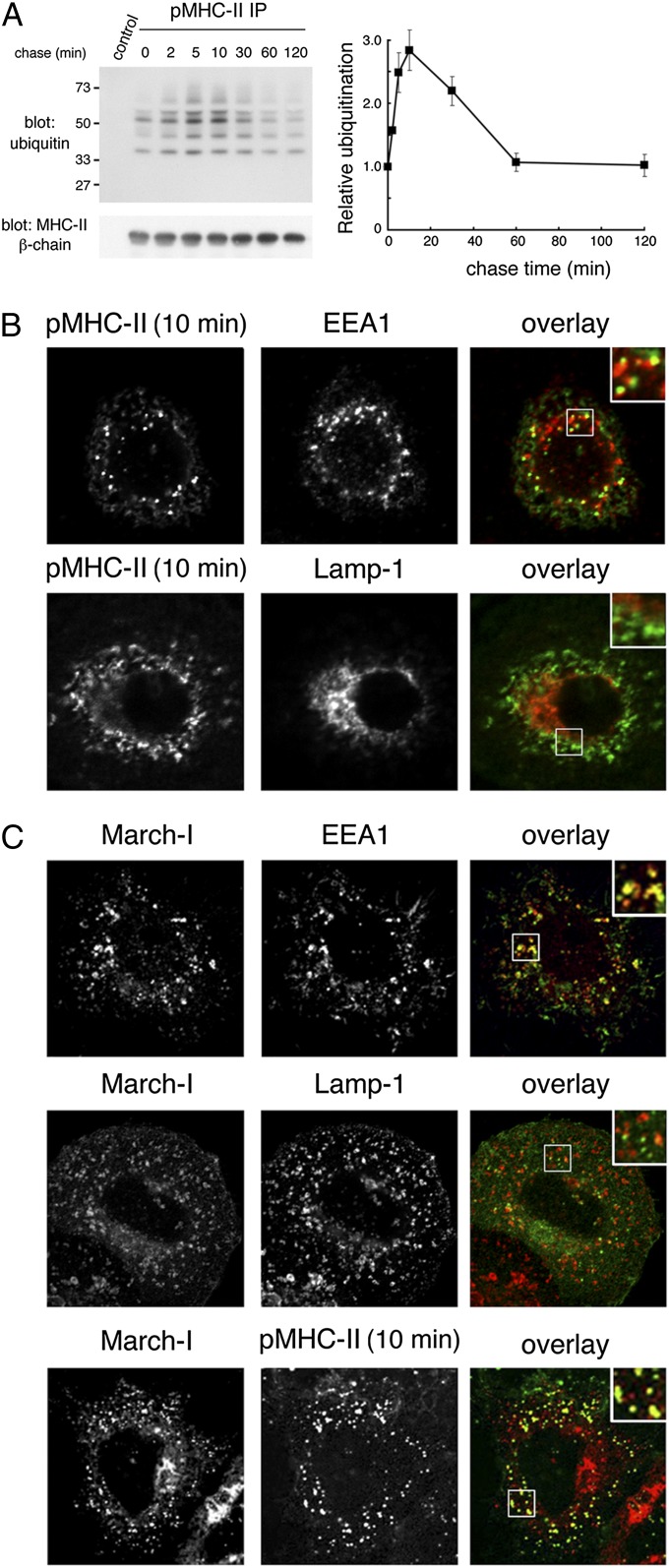
Cell-surface EGF receptors are ubiquitinated after EGF binding, thereby stimulating their eventual degradation in lysosomes (18). To investigate the time course of ubiquitination of cell-surface pMHC-II in DCs, cell-surface pMHC-II present on the surface of human immature DCs was labeled with pMHC-II–specific mAb and the cells were cultured at 37 °C for various times to study the kinetics of surface MHC-II ubiquitination. Ubiquitination of pMHC-II was observed even at time 0, demonstrating that ubiquitinated pMHC-II is present at the cell surface. After incubation at 37 °C, ubiquitination of pMHC-II increased and reached a maximum at 10 min of chase, after which ubiquitination of pMHC-II is gradually decreased by 60 min (Fig. 1A). This result demonstrates that pMHC-II ubiquitination increases rapidly after internalization. We therefore examined the localization of internalized pMHC-II by immunofluorescence microscopy. After 10 min of chase at 37 °C, pMHC-II colocalized with the early endosome marker protein EEA1 but not with the late endosomal marker Lamp-1 (Fig. 1B and Fig. S1A), demonstrating that plasma membrane pMHC-II was present in early endosomes 10 min after internalization.
Fig. 1.
Peptide-loaded MHC-II is ubiquitinated on the plasma membrane and in early endosomes. (A) Human DCs were incubated with anti–pMHC-II mAb L243 or IgG isotype control on ice, washed, and recultured at 37 °C for the indicated times. The cells were lysed in Triton X-100, and mAb L243-bound MHC-II was isolated using protein A–agarose beads. The samples were analyzed by immunoblotting by using anti-ubiquitin or anti–MHC-II β-chain antibodies. The amount of MHC-II ubiquitination and total amount of MHC-II β-chain present at each time point was quantitated by densitometry. MHC-II ubiquitination was normalized to the total amount of MHC-II present in the sample and was expressed relative to the amount of ubiquitination observed at time t 0. The data shown are the mean ± SD obtained from three independent experiments. (B) Human DCs were incubated with anti–pMHC-II mouse mAb L243 for 10 min at 37 °C, and residual cell surface antibodies were blocked by using unlabeled goat anti-mouse IgG serum on ice. The cells were fixed, permeabilized, and stained with Alexa Fluor-conjugated secondary antibody recognizing internalized pMHC-II (L243) and mAb recognizing early endosomes (EEA1) or late endosomes/lysosomes (Lamp-1). (C) HeLa-CIITA cells were transfected with a plasmid encoding V5 epitope-tagged March-I. The cells were incubated with anti–pMHC-II antibody (L243) on ice, washed, and recultured for 10 min at 37 °C. The cells were fixed, permeabilized, and stained with anti-V5 antibody, anti-EEA1 antibody, anti–Lamp-1 antibody, or a secondary antibody allowing detection of internalized pMHC-II mAb L243. (Insets, Right) Enlarged region of the cell.
We attempted to examine the distribution of endogenous March-I in human DCs, but we (and others) have been unable to detect endogenous March-I protein by using available March-I antibodies (14, 15, 19). For this reason, we transfected epitope-tagged March-I in HeLa cells expressing endogenous MHC-II driven by expression of the class II transactivator (CIITA). In HeLa-CIITA cells, March-I is present at low levels at the cell surface, and intracellular March-I colocalizes with EEA1 in early endosomes but not Lamp-1 in late endosomes/lysosomes (Fig. 1C and Fig. S1B). This finding is in excellent agreement with other studies localizing March-I in these cells (15). Most importantly, pMHC-II internalized for 10 min colocalizes very well with March-I (although there are numerous March-I+ vesicles that do not contain internalized pMHC-II). In agreement with our microscopy data in human DCs, internalized pMHC-II did not colocalize with the lysosomal marker GFP-CD63 until 30 min after endocytosis (Fig. S2), demonstrating that (at least some) internalized pMHC-II moved on to lysosomes after internalization. Taken together, these data show that March-I is localized primarily on the cell surface and early endosomes and colocalizes with internalized pMHC-II present in early endosomes.
MHC-II Bound to Ii Endocytosis/Sorting Mutants Is Effectively Ubiquitinated by March-I.
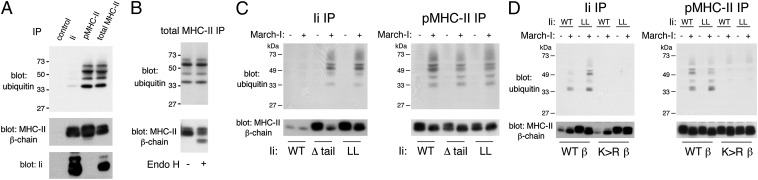
It has been reported that Ii-associated MHC-II (MHC-II-Ii) is not ubiquitinated in mouse bone marrow-derived DCs (15), human monocyte-derived DCs (15), or a human DC line (13). We confirmed that Ii-MHC is poorly ubiquitinated in human DCs compared with pMHC-II when normalized for the total amount of MHC-II present in each immunoprecipitate, but MHC-II–Ii ubiquitination is not completely absent (Fig. 2A). We asked whether the ER resident MHC-II is ubiquitinated by examining the susceptibility of the glycans on ubiquitinated MHC-II to digestion with endoglycosidase H (Endo H). Although Endo H revealed an ER-resident (i.e., Endo H-sensitive) pool of MHC-II β-chain, the MHC-II ubiquitination pattern was not affected by Endo H treatment (Fig. 2B). These results demonstrate that MHC-II becomes sensitive to ubiquitination after leaving the ER and that, even though pMHC-II is the preferred substrate for ubiquitination, post-ER Ii-associated MHC-II can be ubiquitinated to a small extent.
Fig. 2.
The dileucine sorting motif in Ii prevents ubiquitination of MHC-II–Ii complexes. Human DCs were lysed in Triton X-100, and aliquots of the lysate were subjected to immunoprecipitation by using an isotype control, anti-Ii antibody, anti–pMHC-II antibody, or anti–total MHC-II antibody. (A) The immunoprecipitates were analyzed by immunoblotting by using anti-ubiquitin, anti–MHC-II β-chain, or anti-Ii antibodies. (B) The anti–total MHC-II immunoprecipitate was mock-treated or treated with Endo H before SDS/PAGE and immunoblotting by using anti-ubiquitin or anti–MHC-II β-chain antibodies. (C) HEK-293T cells were transfected with plasmids encoding MHC-II and various forms of Ii (Ii WT, Δtail, or LL mutant) alone or together with March-I. The cells were lysed in Triton X-100, and aliquots of the lysate were subjected to immunoprecipitation by using anti-Ii antibody or anti–pMHC-II antibody. The immunoprecipitates were analyzed by immunoblotting as described earlier with anti-ubiquitin or anti–MHC-II β-chain antibodies. (D) HEK-293T cells were transfected with plasmids encoding WT Ii or the Ii LL mutant, MHC-II α-chain, and WT MHC-II β-chain or MHC-II β-chain K225R ubiquitination mutant alone or together with March-I. The cells were lysed and immunoprecipitates were analyzed by immunoblotting as described earlier.
As Lys225 in the cytosolic domain of the MHC-II β-chain is ubiquitinated by March-I, it is possible that the inability of March-I to effectively ubiquitinated MHC-II–Ii complexes is because the cytoplasmic tail of Ii sterically hinders access of March-I to the MHC-II β-chain Lys225 in the MHC-II–Ii complex. We therefore examined the role of the cytoplasmic tail of Ii in regulating MHC-II ubiquitination by expressing MHC-II with WT Ii or a form of Ii lacking most of its cytoplasmic domain (Ii Δtail). However, it is well known that the cytoplasmic tail of Ii contains two dileucine endocytosis/sorting motifs that regulate clathrin-mediated endocytosis of MHC-II–Ii complexes from the plasma membrane (20–23). Therefore, to examine the role of this dileucine motif in MHC-II ubiquitination, we also generated a dileucine motif mutant of Ii (Ii LL mutant) that was full-length but was mutated to eliminate the endocytosis/sorting function of Ii. Expression of the Ii Δtail mutant or the Ii LL mutant significantly impairs the ability of Ii-bound MHC-II to internalize and sort to antigen processing compartments (22, 24). When WT Ii, Ii Δtail, or Ii LL mutant were coexpressed in HEK-293T cells with or without March-I in the absence of MHC-II ubiquitination of free Ii was not observed, indicating that Ii itself is not ubiquitinated by March-I (Fig. S3). To examine the role of Ii in regulating MHC-II ubiquitination, MHC-II α- and β-chains and each form of Ii were expressed together with March-I in these cells. pMHC-II or Ii-associated MHC-II were isolated by immunoprecipitation, and our results demonstrate that just as in human DCs, MHC-II–Ii complexes are ubiquitinated poorly in HEK-293T cells (Fig. 2C). By contrast, when MHC-II was expressed with March-I together with Ii Δtail or the Ii LL mutant, we found robust ubiquitination of pMHC-II and Ii-associated MHC-II. In all conditions, the ubiquitination was on MHC-II β-chain Lys225, as an MHC-II β-chain Lys225Arg mutant was completely devoid of ubiquitination (Fig. 2D). As the cytoplasmic tails of the WT and dileucine mutant of Ii are identical length, these results demonstrate that the cytoplasmic tail of Ii does not simply block access of March-I to MHC-II in the MHC-II–Ii complex and shows that the integrity of the Ii dileucine endocytosis/sorting motif prevents ubiquitination of the MHC-II–Ii complex.
Ubiquitinated MHC-II–Ii Complexes Are Degraded Rapidly.
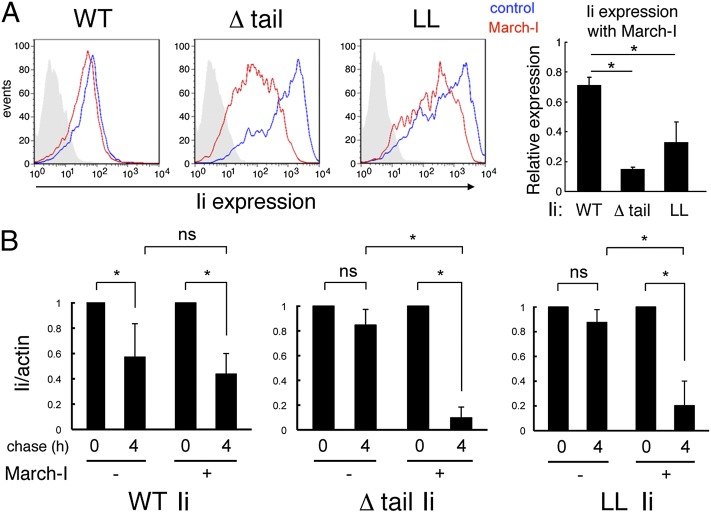
In agreement with studies examining the role of ubiquitination in regulating protein stability, ubiquitination of pMHC-II complexes leads to enhanced degradation kinetics (15, 17). We therefore set out to determine whether the forced ubiquitination of MHC-II–Ii complexes also promoted the degradation of these March-I substrates. In agreement with the observation that MHC-II–Ii complexes are poor ubiquitination substrates, we found that expression of March-I had little effect on expression of WT Ii on the surface of HEK-293T cells (Fig. 3A). By contrast, Ii expression was profoundly reduced if the cells expressed MHC-II and the Ii Δtail or the Ii LL mutant. It is important to note that the reduced expression of Ii on these cells is a consequence of MHC-II β-chain ubiquitination and not direct Ii ubiquitination. To determine if the reduced expression of Ii on transfected cells was a result of ubiquitin-dependent reduction in protein stability, cell surface proteins were biotinylated and their stability at 37 °C was determined. WT Ii had a t1/2 of ∼4 h in these cells, and this was not altered by expression of March-I (Fig. 3B). By contrast, surface Ii on cells expressing MHC-II and the Ii Δtail or the Ii LL mutant alone were very stable, and expression of March-I dramatically reduced surface Ii stability and promoted its degradation. These results extend our analysis of MHC-II–Ii ubiquitination in cells expressing MHC-II and various forms of Ii and show that ubiquitination of MHC-II-Ii mutants by March-I results in rapid degradation of these complexes.
Fig. 3.
Ubiquitinated MHC-II–Ii complexes are subject to rapid degradation. HEK-293T cells were transfected with plasmids encoding MHC-II α- and β-chain and various forms of Ii (Ii WT, Δtail, or LL mutant) alone or together with March-I. (A) The cell surface expression level of Ii in cells not expressing March-I (blue lines) or expressing March-I (red lines) was determined by FACS analysis by using anti-Ii antibody. The amount of Ii on the cell surface under each condition is expressed relative to the amount of Ii expressed on cells that were not transfected with March-I plasmid. The data shown are the mean ± SD of three independent experiments (*P < 0.05 vs. cells expressing WT Ii). (B) The cell surface proteins on each cell type were biotinylated on ice and the cells were kept on ice for 4 h (0 h chase) or returned to culture at 37 °C for 4 h (4 h chase). The cells were lysed, and biotinylated proteins were isolated by using streptavidin–agarose beads. The amount of biotinylated Ii remaining in the samples was analyzed by immunoblotting by using anti-Ii antibody. Each value was normalized to the amount of total actin present in the cell lysates (as a loading control). The amount of surface Ii present at each time point was expressed as a percentage of the total amount of surface Ii present on biotinylated cells at control time 0. The data shown are mean ± SD of three independent experiments (*P < 0.05). ns, not significant.
Internalized pMHC-II, but Not MHC-II–Ii Complexes, Is Present in March-I–Positive Early Endosomes.
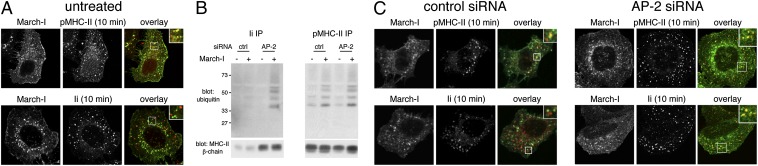
Efficient ubiquitination of MHC-II bound to the Ii LL mutant suggests that differences in intracellular localization regulate the differential ubiquitination of pMHC-II and MHC-II–Ii complexes. To test this hypothesis, we examined the intracellular distribution of pMHC-II and MHC-II–Ii complexes after endocytosis in March-I–expressing HeLa-CIITA cells. After 10 min of endocytosis (the time point at which maximum ubiquitination occurs), much of the internalized pMHC-II was distributed in March-I+ endosomes. By contrast, internalized MHC-II–Ii complexes were largely absent from March-I+ endosomes (Fig. 4A and Fig. S4A), demonstrating that internalized MHC-II–Ii complexes are poorly sorted to/retained in March-I+ early endosomes.
Fig. 4.
Disruption of clathrin-mediated endocytosis promotes endosomal localization and ubiquitination of MHC-II–Ii complexes. (A) HeLa-CIITA cells were transfected with a plasmid encoding V5 epitope-tagged March-I. The cells were incubated with anti–pMHC-II mouse mAb L243 or anti-Ii mouse mAb for 10 min at 37 °C, residual cell surface antibodies were blocked, and the distribution of internalized pMHC-II or Ii and March-I in these untreated cells was determined as described in the text. (Insets, Right) Enlarged region of the cell. (B and C) HeLa-CIITA cells were transfected with control siRNA or siRNA targeting the μ2 subunit of AP-2. After 2 d, the cells were supertransfected with a plasmid encoding V5 epitope-tagged March-I. (B) The cells were lysed in Triton X-100, and the aliquots of each lysate were subjected to immunoprecipitation by using anti-Ii or anti–pMHC-II mAb and analyzed by immunoblotting by using anti-ubiquitin, anti-MHC-II β-chain, or anti-Ii antibodies. (C) HeLa-CIITA cells transfected with control siRNA or siRNA targeting the μ2 subunit of AP-2 were stained for March-I and pMHC-II or Ii allowed to internalize for 10 min. (Insets, Right) Enlarged region of the cell.
Inhibition of Clathrin-Dependent Endocytosis Promotes Ubiquitination of MHC-II–Ii Complexes.
Endocytosis of surface-expressed MHC-II–Ii complexes is very rapid and clathrin-dependent (10, 25, 26). When clathrin-mediated endocytosis is inhibited by siRNA-mediated knockdown of clathrin heavy-chain or AP-2 μ-chain, endocytosis of MHC-II–Ii complexes is prevented, and these complexes accumulate on the cell surface (10, 25, 26). By contrast, pMHC-II internalization is clathrin-independent and relatively slow, and involves passage of pMHC-II through Arf6+Rab35+ endosomes (10). We therefore tested the hypothesis that inhibition of clathrin-mediated endocytosis would divert the endocytosis pathway of MHC-II–Ii complexes from clathrin-dependent to clathrin-independent and that this would, in turn, allow ubiquitination of MHC-II–Ii complexes containing a WT form of Ii. Introduction of AP-2 μ-chain siRNA in HeLa-CIITA cells dramatically reduced expression of AP-2 (Fig. S4B). As we observed in human DCs and HEK-293T cells, MHC-II–Ii complexes were very poor March-I substrates in transfected HeLa-CIITA cells (Fig. 4B). However, knockdown of AP-2 resulted in a dramatic increase in MHC-II ubiquitination in the Ii immunoprecipitate. Note also that AP-2 knockdown had no effect on ubiquitination of pMHC-II complexes, a finding that is in excellent agreement with our previous observation that pMHC-II endocytosis/sorting is clathrin-independent (10).
We also examined the intracellular location of the MHC-II–Ii complexes that internalized in HeLa-CIITA cells with defective clathrin-dependent endocytosis. Although treatment with control siRNA did not affect the distribution of MHC-II–Ii complexes and March-I from that observed in untreated cells, AP-2 knockdown altered the distribution of MHC-II–Ii complexes such that, in these clathrin-mediated endocytosis mutant cells, internalized MHC-II–Ii complexes colocalized beautifully with March-I (Fig. 4C and Fig. S4C). As expected, AP-2 knockdown had no effect on the overlap of March-I with MHC-II bound to the Ii LL mutant (Fig. S5). These results demonstrate that clathrin-dependent endocytosis sorts MHC-II–Ii complexes to March-I–negative endosomes whereas clathrin-independent endocytosis sorts pMHC-II to March-I+ endosomes. Taken together, these data show that the endocytosis/sorting signals present in Ii divert MHC-II–Ii complexes away from March-I+ early endosomes and that this is the mechanism by which MHC-II–Ii complexes avoid ubiquitination by March-I.
Discussion
It has been reported that pMHC-II is ubiquitinated in immature DC whereas Ii-associated forms of MHC-II are not (13, 15); however, the molecular mechanisms responsible for selective ubiquitination of pMHC-II and the immunological implications of differential MHC-II ubiquitination have not been investigated. We now report that pMHC-II is ubiquitinated at the plasma membrane and in early endosomes by March-I. Although MHC-II–Ii complexes also exist transiently on the cell surface, these complexes avoid ubiquitination because they are rapidly internalized by a clathrin-dependent endocytosis pathway that selectively regulates MHC-II-Ii, and not pMHC-II, internalization.
Ubiquitination of cell surface-tagged pMHC-II increases rapidly after endocytosis, with a peak of ubiquitination detected 10 min after endocytosis. At the time point of maximum ubiquitination, internalized pMHC-II was present primarily in early endosomes and not in late endosomes. We found that March-I is also localized mainly in early endosomes but can also be observed at low levels at the plasma membrane in HeLa-CIITA cells, a result that is in excellent agreement with results obtained by others (15). It is important to note that, although small amounts of ubiquitinated pMHC-II can be found on the surface of human DCs and HeLa-CIITA cells, this is not necessarily a result of March-I–mediated pMHC-II ubiquitination at the plasma membrane. For example, it is possible this pool of pMHC-II was ubiquitinated in early endosomes and arrived at the plasma membrane by recycling. Although MHC-II–Ii complexes are also present on the cell surface (27), we have found that they are very poor substrates for March-I. However, MHC-II–Ii complexes internalize very rapidly from the cell surface, whereas pMHC-II internalization is much more slow (22), and, after 10 min of internalization, the MHC-II–Ii complex does not colocalize with March-I in early endosomes. These results strongly suggest that March-I is able to ubiquitinated pMHC-II on the cell surface/early endosomes as a result of its prolonged residence time in these cellular compartments.
Our finding that pMHC-II complexes are ubiquitinated whereas MHC-II–Ii complexes are not led us to investigate the mechanism by which MHC-II–Ii complexes escape ubiquitination by March-I. One simple possibility is that cytoplasmic tail of Ii prevents access of March-I to the Lys225 ubiquitination site of MHC-II β-chain. We examined this possibility by using a cytoplasmic tail-deleted form of Ii and found that, indeed, deletion of the Ii cytoplasmic domain allows robust ubiquitination of the MHC-II–Ii complex. However, within this truncated domain resides the dileucine internalization motif, leaving open the possibility that it the integrity of the internalization motif, and not merely the physical blocking of access to Lys225, that regulates MHC-II–Ii ubiquitination. Indeed, we found that MHC-II bound to the dileucine motif mutant of Ii as well as the complete cytoplasmic tail-deletion mutant of Ii were ubiquitinated by March-I to similar extents. This result indicates that the dileucine sorting motif, and not the cytoplasmic tail itself, prevents the MHC-II–Ii complex from being recognized as a substrate for March-I.
The dileucine motifs in the Ii cytosolic domain control clathrin-mediated endocytosis of MHC-II–Ii complexes (25, 28). By contrast, pMHC-II complexes do not contain obvious endocytosis motifs, and these molecules internalize by using a clathrin-independent endocytosis pathway (10, 25, 26). Given the striking difference between pMHC-II and MHC-II–Ii as March-I substrates, we investigated the importance of an intact clathrin-mediated endocytosis pathway in MHC-II–Ii ubiquitination. In agreement with the hypothesis that that rapid clathrin-mediated endocytosis limits MHC-II–Ii ubiquitination, MHC-II–Ii complex ubiquitination was robust in cells lacking a clathrin-mediated endocytosis pathway, whereas ubiquitination of the pMHC-II complex was completely unaffected by clathrin adaptor AP-2 depletion. We believe this is the reason MHC-II–Ii complex ubiquitination has been so difficult to observe at steady state: MHC-II–Ii complexes simply do not reside in March-I–positive compartments long enough to serve as efficient ubiquitination substrates. When the surface/early endosome residence time of these complexes is prolonged, either by mutation of the dileucine internalization motif or by inhibiting clathrin-mediated endocytosis, ubiquitination of the MHC-II–Ii complex is readily detected.
We propose that ubiquitination affects the intracellular trafficking/fate of MHC-II in a manner that is analogous to that of the EGF receptor. EGF receptor is ubiquitinated at the cell surface and early endosomes by the E3 ligase c-Cbl (29). Ubiquitination regulates the sorting of EGF receptors from early endosomes to MVBs. The multiprotein endosomal sorting complexes required for transport (ESCRT) complex recognizes ubiquitinated cargo proteins and targets them for sorting to the intralumenal vesicles of MVB (11). Ultimately, MVB-associated EGF receptors are degraded following fusion of MVB with terminal lysosomes. It should be pointed out that there are also ESCRT-independent mechanisms of protein sorting into MVBs (30), and it has already been determined that MVBs are not a homogenous collection of intracellular organelles, with some MVBs playing a role in lysosomal degradation of target proteins and other MVBs playing a role in concentrating proteins for intralumenal vesicle release from MVB-derived exosomes (31). Previous studies showing that ubiquitination does not affect MHC-II release on exosomes (31, 32) is consistent with the hypothesis that ubiquitination of MHC-II controls sorting leading to lysosomal degradation and not incorporation into exosomes. March-I–mediated ubiquitination has been shown to alter the trafficking of clathrin-independent endocytic cargo (33), a finding that is also in excellent agreement with data showing that pMHC-II endocytosis is clathrin-independent (10, 25, 26).
This study has identified the peptide-loaded form of MHC-II as the primary form of MHC-II that serves as a ubiquitination substrate for March-I. pMHC-II ubiquitination occurs on the cell surface and rapidly after endocytosis in March-I+ early endosomes. The selective ubiquitination of internalizing surface pMHC-II leads to pMHC-II turnover in APCs, a process that is required to replace “old” surface pMHC-II with “new” surface pMHC-II complexes at steady state. It is the property of ubiquitination of surface pMHC-II that allows for pMHC-II diversity in APCs, a process that is likely important for effective immune responses to pathogens in the face of abundant self-pMHC-II on the APC surface.
Materials and Methods
Cell and Reagents.
HeLa cells expressing CIITA were a gift from Peter Cresswell (Yale University School of Medicine, New Haven, CT), and HEK-293T cells were maintained as described previously. Human DCs were generated from elutriated human monocytes obtained from the National Institutes of Health (NIH) Blood Bank as described previously (10).
The antibodies and plasmids used in this study are described in SI Materials and Methods. FugeneHD reagent was from Promega. Sulfo-NHS-biotin was from Thermo Scientific . Endo H was purchased from New England BioLabs. Protein A–agarose beads and streptavidin–agarose beads were purchased from Sigma-Aldrich. Lipofectamine 2000 reagent, unconjugated goat anti-mouse IgG, and Alexa Fluor-conjugated antibodies were obtained from Invitrogen.
Transfections and siRNA Treatment.
HeLa-CIITA cells and HEK-293T cells were transfected by using FugeneHD reagent and were assayed 24 h after transfection. Control or AP-2-μ2 siRNA (20 μM) were transfected into HeLa-CIITA cells by using Lipofectamine 2000, and the cells were assayed 72 h after transfection. The sequence of the control and AP-2-μ2 siRNA was described previously (10).
Immunoprecipitation and Immunoblotting.
Cells were lysed in lysis buffer (10 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mg/mL BSA, 50 mM PMSF, 0.1 mM Nα-Tosyl-L-lysine chloromethyl ketone hydrochloride, and 25 mM N-ethylmaleimide) and analyzed by immunoprecipitation by using protein A–agarose beads and immunoblot analysis by using protocols described previously (10).
Cell Surface Biotinylation.
Plasma membrane proteins of cells were surface biotinylated by incubation of ∼10 × 106 cells per milliliter by using the membrane-impermeable biotinylation reagent sulfo-NHS-biotin (1 mg/mL) in HBSS for 30 min according to the manufacturer’s protocol. After cell surface biotinylation, free biotin was quenched by washing cells twice with 50 mM glycine in HBSS, and the cells were washed in ice-cold HBSS and finally resuspended in complete medium. The cells were lysed in cell lysis buffer, and biotin-labeled proteins were isolated by using streptavidin–agarose beads. Biotinylated proteins were detected by SDS/PAGE and immunoblotting by using the indicated antibodies.
Ubiquitination of Cell Surface pMHC-II.
Cells were incubated with the anti–pMHC-II mAb L243 (5 μg/mL) for 30 min on ice. The cells were then washed twice with ice-cold medium and incubated for the indicated times at 37 °C. The cells were then harvested and lysed, and the anti–pMHC-II immune complexes in the lysate were isolated by using protein A–agarose beads and analyzed by immunoprecipitation/immunoblot analysis as described earlier.
Immunofluorescence Microscopy.
HeLa-CIITA cells were grown on glass coverslips overnight. The cells were incubated with the anti–pMHC-II mAb L243 or anti-Ii mAb LL1 for 30 min on ice to label cell-surface pMHC-II and Ii/MHC-II–Ii complexes, respectively. Each unbound antibody was removed by washing the cells with ice-cold medium, and cells were then incubated for indicated times at 37 °C. The remaining cell surface primary mouse mAb was blocked with incubating the cells with unconjugated goat anti-mouse IgG serum (100 μg/mL) on ice for 1 h. Control experiments confirmed that this protocol completely blocked surface expressed MHC-II and Ii/MHC-II–Ii complexes. The cells were then fixed in 4% PFA (in PBS solution) for 15 min at room temperature and permeabilized by using 0.02% saponin/3% (vol/vol) goat serum in PBS solution and stained with appropriately labeled secondary antibodies. All cells were imaged by using a Zeiss LSM 510 META laser scanning confocal microscope using a 63× oil-immersion objective lens. Quantitative analysis of colocalization was determined by using Pearson correlation coefficient between green and red pixels in each cell as determined by using ImageJ software (NIH) with JACoP plugin (34) using at least 10 cells in each analysis.
Supplementary Material
Acknowledgments
We thank Peter Cresswell, Satoshi Ishido, and Juan Bonifacino for the gift of reagents used in this study; Mike Kruhlak and the Experimental Immunology Branch microscopy facility for expert technical advice; and Richard Hodes for critical reading of this manuscript. This work was supported by the Japan Society for the Promotion of Science (K.F.) and the Intramural Research Program of the National Institutes of Health (P.A.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312994110/-/DCSupplemental.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 3.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388(6644):782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 4.Pierre P, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388(6644):787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 5.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch R, et al. Achieving stability through editing and chaperoning: Regulation of MHC class II peptide binding and expression. Immunol Rev. 2005;207:242–260. doi: 10.1111/j.0105-2896.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 7.Reid PA, Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990;346(6285):655–657. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- 8.Reid PA, Watts C. Constitutive endocytosis and recycling of major histocompatibility complex class II glycoproteins in human B-lymphoblastoid cells. Immunology. 1992;77(4):539–542. [PMC free article] [PubMed] [Google Scholar]
- 9.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375(6532):603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 10.Walseng E, Bakke O, Roche PA. Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J Biol Chem. 2008;283(21):14717–14727. doi: 10.1074/jbc.M801070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacGurn JA, Hsu PC, Emr SD. Ubiquitin and membrane protein turnover: From cradle to grave. Annu Rev Biochem. 2012;81:231–259. doi: 10.1146/annurev-biochem-060210-093619. [DOI] [PubMed] [Google Scholar]
- 12.Shin JS, et al. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444(7115):115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 13.van Niel G, et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25(6):885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Matsuki Y, et al. Novel regulation of MHC class II function in B cells. EMBO J. 2007;26(3):846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Gassart A, et al. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc Natl Acad Sci USA. 2008;105(9):3491–3496. doi: 10.1073/pnas.0708874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walseng E, et al. Dendritic cell activation prevents MHC class II ubiquitination and promotes MHC class II survival regardless of the activation stimulus. J Biol Chem. 2010;285(53):41749–41754. doi: 10.1074/jbc.M110.157586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walseng E, et al. Ubiquitination regulates MHC class II-peptide complex retention and degradation in dendritic cells. Proc Natl Acad Sci USA. 2010;107(47):20465–20470. doi: 10.1073/pnas.1010990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21(6):737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Thibodeau J, et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur J Immunol. 2008;38(5):1225–1230. doi: 10.1002/eji.200737902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63(4):707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 21.Lotteau V, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348(6302):600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 22.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci USA. 1993;90(18):8581–8585. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieters J, Bakke O, Dobberstein B. The MHC class II-associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J Cell Sci. 1993;106(pt 3):831–846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- 24.Bremnes B, Madsen T, Gedde-Dahl M, Bakke O. An LI and ML motif in the cytoplasmic tail of the MHC-associated invariant chain mediate rapid internalization. J Cell Sci. 1994;107(pt 7):2021–2032. doi: 10.1242/jcs.107.7.2021. [DOI] [PubMed] [Google Scholar]
- 25.McCormick PJ, Martina JA, Bonifacino JS. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc Natl Acad Sci USA. 2005;102(22):7910–7915. doi: 10.1073/pnas.0502206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dugast M, Toussaint H, Dousset C, Benaroch P. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J Biol Chem. 2005;280(20):19656–19664. doi: 10.1074/jbc.M501357200. [DOI] [PubMed] [Google Scholar]
- 27.Koch N, Moldenhauer G, Hofmann WJ, Möller P. Rapid intracellular pathway gives rise to cell surface expression of the MHC class II-associated invariant chain (CD74) J Immunol. 1991;147(8):2643–2651. [PubMed] [Google Scholar]
- 28.Hofmann MW, et al. The leucine-based sorting motifs in the cytoplasmic domain of the invariant chain are recognized by the clathrin adaptors AP1 and AP2 and their medium chains. J Biol Chem. 1999;274(51):36153–36158. doi: 10.1074/jbc.274.51.36153. [DOI] [PubMed] [Google Scholar]
- 29.Thien CB, Walker F, Langdon WY. RING finger mutations that abolish c-Cbl-directed polyubiquitination and downregulation of the EGF receptor are insufficient for cell transformation. Mol Cell. 2001;7(2):355–365. doi: 10.1016/s1097-2765(01)00183-6. [DOI] [PubMed] [Google Scholar]
- 30.Theos AC, et al. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10(3):343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buschow SI, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 32.Gauvreau ME, et al. Sorting of MHC class II molecules into exosomes through a ubiquitin-independent pathway. Traffic. 2009;10(10):1518–1527. doi: 10.1111/j.1600-0854.2009.00948.x. [DOI] [PubMed] [Google Scholar]
- 33.Eyster CA, et al. MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation. Mol Biol Cell. 2011;22(17):3218–3230. doi: 10.1091/mbc.E10-11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(pt 3):213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.