Significance
The epidermis of the skin is composed of keratinocytes that are organized in several layers. Basal cells divide and produce cells moving outwards the epidermis while undergoing the process of terminal differentiation, crucial for the barrier function of the skin. Calcium is an indispensible ion for differentiation, and calcium channels are of primary importance. Unexpectedly, we discovered that the Orai1 calcium channel is mainly expressed in the basal layer, functioning to negatively control differentiation. The Orai1 channel supplies calcium to sustain proliferation and, in particular, to drive migration of keratinocytes, both processes being the feature of basal keratinocytes.
Keywords: calcium signaling, cell migration, orai1 KO mice
Abstract
To achieve and maintain skin architecture and homeostasis, keratinocytes must intricately balance growth, differentiation, and polarized motility known to be governed by calcium. Orai1 is a pore subunit of a store-operated Ca2+ channel that is a major molecular counterpart for Ca2+ influx in nonexcitable cells. To elucidate the physiological significance of Orai1 in skin, we studied its functions in epidermis of mice, with targeted disruption of the orai1 gene, human skin sections, and primary keratinocytes. We demonstrate that Orai1 protein is mainly confined to the basal layer of epidermis where it plays a critical role to control keratinocyte proliferation and polarized motility. Orai1 loss of function alters keratinocyte differentiation both in vitro and in vivo. Exploring underlying mechanisms, we show that the activation of Orai1-mediated calcium entry leads to enhancing focal adhesion turnover via a PKCβ-Calpain-focal adhesion kinase pathway. Our findings provide insight into the functions of the Orai1 channel in the maintenance of skin homeostasis.
The involvement of calcium-dependent mechanisms in the induction and regulation of keratinocyte proliferation, migration, and differentiation is now well established (1–3). Keratinocytes are arranged in highly organized, specialized layers according to their functions and the programmed life cycle. Proliferating keratinocytes comprise the stratum basale. Basal-cell proliferation is appreciably higher and inversely correlated with the calcium gradient in the skin, reflecting the importance of calcium signaling in differentiation (3). As a result of proliferation, keratinocytes leave the stratum basale, moving toward the exterior with the onset of differentiation in the stratum spinosum. Differentiation is completed in the stratum granulosum, thereby constituting the enucleated stratum corneum, which plays the major role as a permeability barrier (1). Besides differentiation and proliferation, the balance of which determines the epidermis physiology, the polarized motility of keratinocytes follows the same vertical pathway, suggesting its crucial importance for skin homeostasis (4).
For years, calcium has been considered as a potent inducer of keratinocyte differentiation; for this reason, calcium channels have been suggested to be indispensable in its promotion. Of them, store-operated calcium channels (SOCs) are a major mechanism of Ca2+ entry in nonexcitable cells (5–7). A molecular candidate for SOC termed Orai1 has been identified and characterized (8–12). Numerous studies have demonstrated that Orai1 mediates calcium release-activated currents and SOC in a large variety of cells and is involved in a wide range of cell functions, including endothelial cell proliferation (13), lymphocyte proliferation (14), and mast cell activation (15), as well as skeletal muscle development and a contractile function (16). However, the role of Orai1 in skin physiology remains poorly understood. The phenotypic features of the homozygous orai1−/− mice have been recently shown as sporadic hair loss, resembling the cyclical alopecia, thinner epidermis with lower cell density, and narrower follicles (17), which indicates the important role of the Orai1 channel in skin homeostasis. Although the first findings on the role of Orai1 in differentiation and migration of isolated keratinocytes have very recently appeared (18, 19), they do not reflect the complex role of this channel in the overall processes of skin homeostasis. In the present study, using both human primary keratinocytes and the keratinocytes obtained from orai1−/− mice, we found a previously undescribed role of Orai1 in epidermal physiology. Indeed, in contrast to its expected prodifferentiative role, we show that Orai1 constitutively inhibits terminal keratinocyte differentiation and is indispensable for the physiological control of proliferation and migration of basal keratinocytes. We demonstrate that Orai1 protein is mainly confined to the basal layer of the epidermis where it plays a critical role in the control of keratinocyte proliferation and polarized motility by enhancing focal adhesion turnover via the EGFR-PKCβ-Calpain-focal adhesion kinase (FAK) pathway. Orai1 loss of function decreases keratinocyte proliferation and inhibits directional migration, thereby accelerating the expression of differentiation-regulating genes. Finally, Orai1 loss of function alters the skin homeostasis in an in vivo mice model, confirming our findings obtained on primary keratinocytes.
Results
Orai1 Protein Is Mostly Expressed in Stratum Basale and Diminishes During Differentiation.
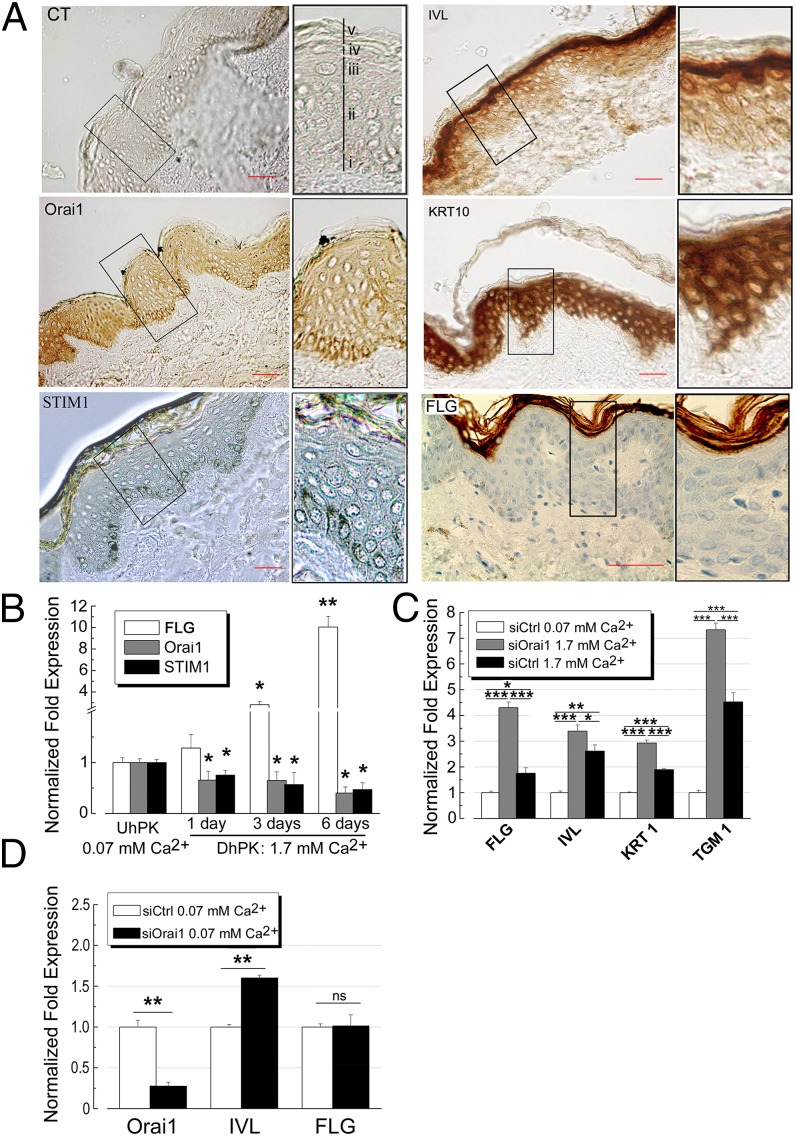
Firstly, we have studied the expression of Orai1 protein in human skin sections (Fig. 1). Immunohistochemical studies showed that the Orai1 protein is mostly expressed in the stratum basale of human epidermis, with a slight presence in upper layers of the skin (Fig. 1A). The same tendency was observed for the STIM1 protein, which was also predominantly expressed in basal layers. In contrast to these proteins, the staining of early differentiation markers like involucrin (IVL) and cytokeratin 10 (KRT10) showed patterns of expression with an increasing gradient toward upper layers such as the stratum spinosum, granulosum. The late differentiation marker fillagrin (FLG) is mainly expressed in the most superficial layer of the epidermis: the stratum corneum (Fig. 1A). The distribution of STIM1 was found to mirror that of Orai1 and is mostly confined to the basal layer of skin although, according to immunohistochemical data, the expression of both is still conserved in the upper layers of epidermis. Although several but various differentiation markers are shown in each experiment, the expression of all of the differentiation markers, such as early (e.g., IVL, KRT1 and -10), intermediate (e.g., TGM1), and late (e.g., Filaggrin, Loricrin), was studied.
Fig. 1.
Orai1 expression in human skin and its role in keratinocytes differentiation. (A) immunohistochemical studies for the expression of Orai1, STIM1, IVL, KRT10, and FLG in human skin sections. Top Left shows the respective control for our stainings using secondary antibody only. Insets (Right) show enlarged images of the boxed regions. Different layers (Inset, Top Left) in epidermis are: (i) stratum basale; (ii) stratum spinosum; (iii) stratum granulosum; (iv) stratum lucidum; (v) stratum corneum. (Scale bars: 50 µm.) (B) Real-time quantitative PCR from three independent experiments showing the expression levels of Orai1, STIM1, and FLG in hPK kept in 0.07 mM Ca2+ (UhPK), or hPK cells treated with 1.7 mM Ca2+ during 1, 3, or 6 d (DhPK). Data were normalized compared with HPRT gene expression. Data represented are mean ± SEM; *P < 0.05; **P < 0.01, n = 3. (C) The effects of Orai1 knockdown on the expression of FLG, IVL, KRT1, and TGM1 transcripts induced by calcium switch (1.7 mM Ca2+, 24 h). Data were normalized compared with HPRT gene expression. Data represented are mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; n = 3. (D) The expression of IVL and FLG in hPK kept in 0.07 mM Ca2+ induced by Orai1 knockdown for 24 h. Data were normalized compared with HPRT gene expression. Data represented are mean ± SEM; **P < 0.01; n = 3.
To confirm the immunohistochemical studies, we have studied the expression of Orai1, STIM1, and the late differentiation marker FLG in human primary keratinocytes (hPKs) using real-time quantitative PCR (Fig. 1B). Quantification of the mRNA transcripts from three independent experiments showing the expression levels of Orai1, STIM1, and FLG in hPK kept in 0.07 mM Ca2+ [undifferentiated hPK (UhPK)] or hPK cells treated with 1.7 mM Ca2+ during 1, 3, or 6 d [differentiated hPK (DhPK)] is indicated in Fig. 1B. The expression data for Orai1, STIM1, and FLG confirmed those of immunohistochemical studies indicating the down-regulation of these proteins during the differentiation process in contrast to the FLG, whose expression increases during differentiation.
To study the role of Orai1 in calcium-induced differentiation of hPK, we used a so-called Ca2+-switch protocol. The aim was to see whether the knockdown of Orai1 protein may influence the onset of keratinocyte differentiation. Thus, the cells are pretransfected with the Orai1-specific siRNA tested preliminarily as shown in Fig. S1A. After 24 h, 1.7 mM Ca2+ is added to UhPK to trigger differentiation for an additional 24 h. The control hPK cells kept in 0.07 mM Ca2+ and control hPK cells with the induced Ca2+ switch were transfected with siCtrl. The pretransfection of hPK cells with siOrai1 significantly induced the expression of differentiation markers such as cytokeratin 1 (KRT1), IVL, keratinocyte transglutaminase (TGM1), and even FLG, which was superior to the extent of Ca2+ switch itself (Fig. 1C). These data strongly indicate the important role of Orai1 in the process of keratinocyte differentiation.
Because the previous experiment suggested the probable negative role of Orai1 in differentiation, we decided to see whether the knockdown of Orai1 protein in the low Ca2+ solution (undifferentiated, UhPK) is able to trigger differentiation per se (Fig. 1D). The pretransfection of UhPK cells with siOrai1 in 0.07 mM Ca2+ for 24 h has slightly but significantly increased the expression of early differentiation marker IVL whereas the expression of late differentiation marker FLG remained unchanged, confirming the crucial role of calcium (superior to 0.07 mM, entering via other channels) in differentiation processes. These data suggest that a decrease in Ca2+ entry through the Orai1 channels contributes to the onset and/or progression of differentiation.
Orai1 Is the Main Component of the Store-Operated Current in Human Keratinocytes.
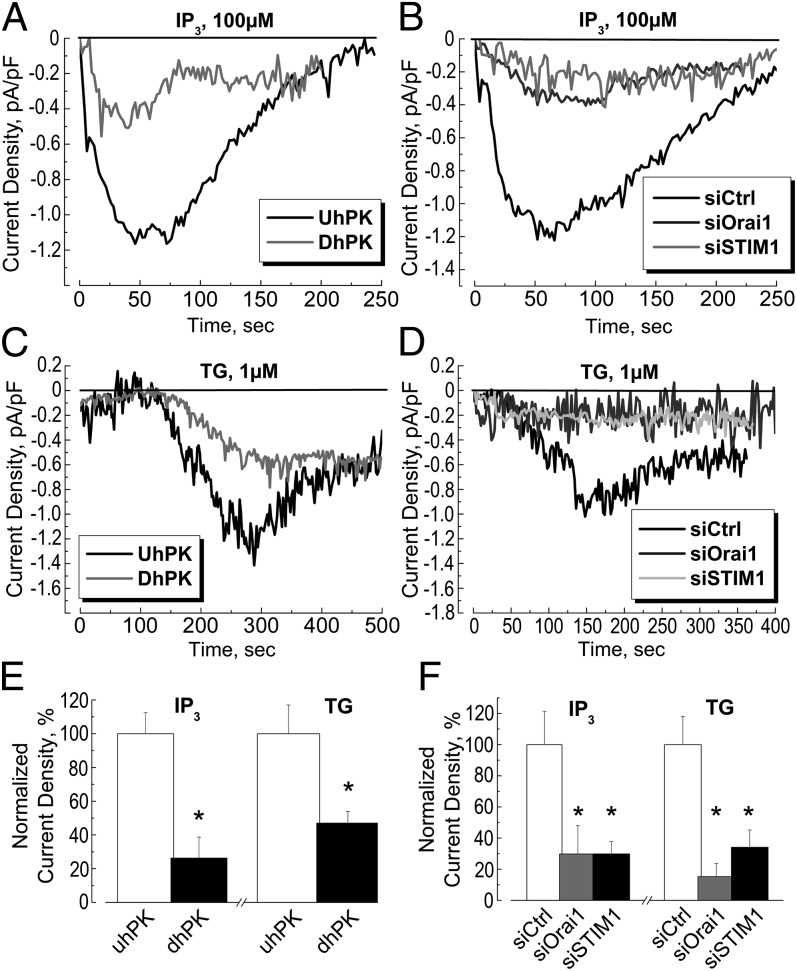
The entry of calcium through the plasma membrane into the cytoplasm [Ca2+]i in response to the decrease in calcium content of the endoplasmic reticulum [Ca2+]ER was studied (Fig. 2). Depletion of endoplasmic reticulum (ER) stores via activation of IP3 receptors or by inhibition of the ER SERCA pump with thapsigargin (TG) induced a significant store-operated calcium entry (SOCE) in undifferentiated hPK (UhPK) compared with differentiated (DhPK) cells (Fig. 2 A and C, respectively). The normalized quantification of this current is given in Fig. 2E. This entry, induced either by IP3 or TG, was successfully blocked by inhibition of the Orai1 channel and STIM1 protein using the transfection of UhPK with the respective siRNAs against these proteins (Fig. 2 B and D). The quantification histogram of this SOCE inhibition is shown in Fig. 2F. We can conclude that the Orai1 channel and STIM1 proteins are the main components of store-operated calcium current in human keratinocytes and that this SOCE is significantly down-regulated following differentiation of hPK.
Fig. 2.
Store-operated current in human primary keratinocytes. (A and C) Representative time courses of ISOC development (current density, measured at −100 mV holding potential) in UhPK cells (black traces, kept in 0.07 mM Ca2+) and DhPK cells (gray traces, kept in 1.7 mM Ca2+) in response to the dialysis of 100 µM IP3 (A) or 1 µM TG (C). (B and D) Representative time courses of ISOC development (current density, measured at −100 mV holding potential) in UhPK cells (kept in 0.07 mM Ca2+) pretreated with siRNA against Orai1 (gray traces), STIM1 (light gray traces), compared with siCtrl (black traces) in response to the dialysis of 100 µM IP3 (B) or 1 µM TG (D). In the case of TG-induced SOCE (C and D), the presence of 10 mM BAPTA in the pipette may partially account for the SOCE development. (E) Quantification of the maximum amplitude of IP3- and TG-induced ISOC densities in UhPK cells (white column, kept in 0.07 mM Ca2+) and DhPK cells (black column, kept in 1.7 mM Ca2+). Data shown are normalized. Mean ± SEM; *P < 0.05; n = 32 for each condition. (F) Quantification of the maximum amplitude of IP3- and TG-induced ISOC densities in UhPK cells (kept in 0.07 mM Ca2+) pretreated with siRNA against Orai1 (gray columns), STIM1 (black columns), compared with siCtrl (white columns). Data shown are mean ± SEM; *P < 0.05; n = 34 for each condition.
Orai1 Channel Is Involved in Proliferation and Migration of Undifferentiated hPK.
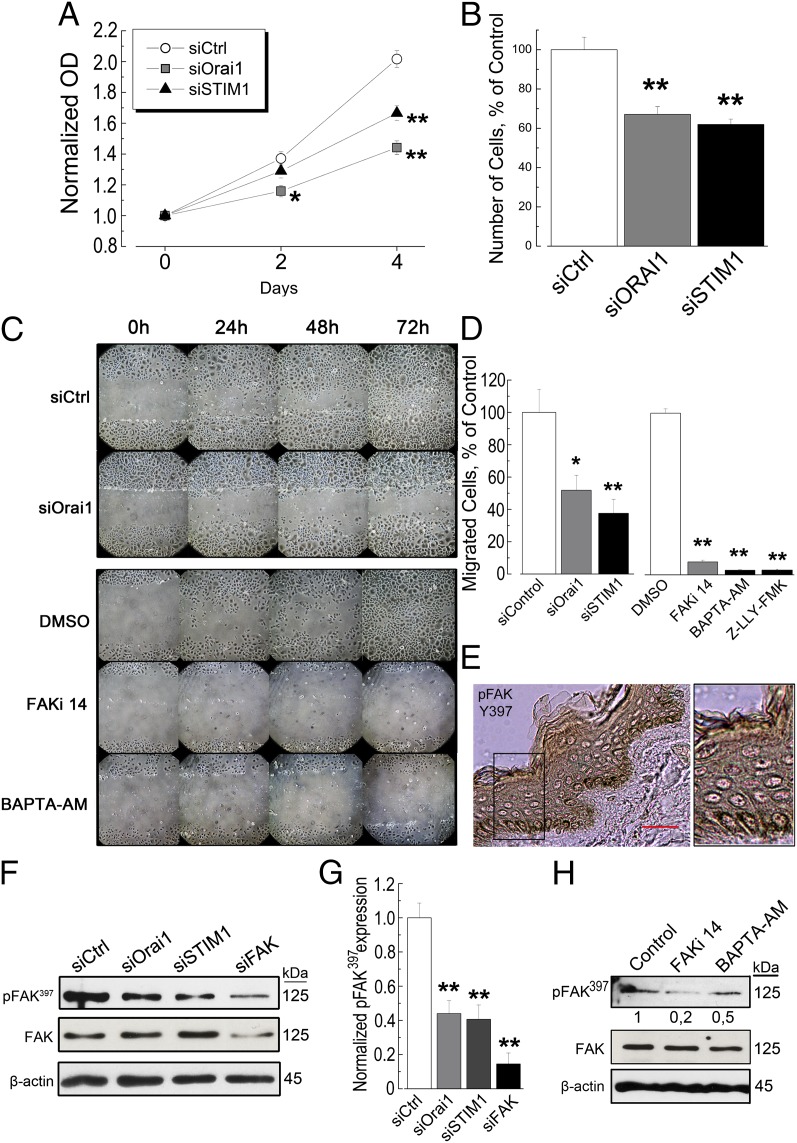
Having discovered the expression and the role of the Orai1 channel in SOCE of UhPK corresponding to undifferentiated keratinocytes, we have sought to study its role in cell proliferation and migration, the characteristic features of keratinocytes derived from stratum basale. The indirect colorimetric cell viability assay (MTS) showed a strong inhibition of undifferentiated hPK (UhPK) proliferation by the extinction of both Orai1 and STIM1 proteins (Fig. 3A). These data were confirmed by the direct cell counting following 48 h of cell transfections with siRNA against Orai1 and STIM1 (Fig. 3B).
Fig. 3.
The role of Orai1 and STIM1 in proliferation and migration of human keratinocytes. (A) A time course of UhPK cell (kept in 0.07 mM Ca2+) growth revealed by indirect colorimetric MTS assay reflecting the number of viable cells. Cells were transfected with siRNA against Orai1 (gray squares), STIM1 (black triangles), compared with siCtrl (white circles). Data represented are mean ± SEM; *P < 0.05; **P < 0.01, n = 3. (B) Relative cell number of UhPK (kept in 0.07 mM Ca2+) cells after 48 h of transfection with siRNA against Orai1 (gray column), STIM1 (black column), compared with siCtrl (white column). Data represented are mean ± SEM; **P < 0.01, n = 3. (C) A representative experiment of three independent experiments of wound-healing assay of UhPK (kept in 0.07 mM Ca2+) cells transfected for 24 h with siRNA against Orai1 versus siCtrl (Upper), and UhPK cells treated with DMSO, 10 µM FAKi 14, and 50 µM BAPTA-AM for the indicated period (Lower). Pictures were taken every 24 h during 3 d. (D) Cell migration assay using Transwell chambers of UhPK (kept in 0.07 mM Ca2+) cells transfected with 60 nM of siRNA against Orai1, STIM1 versus siCtrl as well as FAKi 14, BAPTA-AM, and Z-LLY-FMK (10 µM) versus DMSO. Cells were allowed to migrate for 24 h from the time of transfection. Data shown are mean ± SEM; *P < 0.05; **P < 0.01, n = 3 done in quadruplicate. (E) The immunostaining of phosphorylated FAK397 protein in human skin sections. The small black box corresponds to the magnified skin area. (Scale bar: 50 µm.) (F) A representative immunoblotting showing the level of autophosphorylation of FAK397 compared with overall FAK in UhPK cells treated with 60 nM siRNA against siOrai1 and siSTIM1 for 24 h. siFAK has been used as a positive control. (G) A quantification histogram of FAK397 autophosphorylation in UhPK cells (of a representative immunoblotting shown in F). Data represented are mean ± SEM; **P < 0.01, n = 3. (H) The autophosphorylation of FAK397 in UhPK cells treated with FAKi 14 and BAPTA-AM; DMSO was used as a control.
Further, the issue of cell migration was addressed. We have used two methods: wound healing and Transwell assays. The down-regulation of Orai1 protein by siRNA has decreased UhPK cell ability to migrate and to “heal the wound” (Fig. 3C, Upper). In the other series of experiments, we inhibited FAK with 10 µM FAKi 14 inhibitor for the indicated period (Fig. 3C, Lower). This inhibition arrested the cell migration. To determine whether the intracellular calcium ([Ca2+]i) may have a role, we blocked the [Ca2+]i with the cell-permeable Ca2+-chelator BAPTA-AM (50 µM). The effect of chelating [Ca2+]i was also prominent and arrested hPK cell migration in the time scale of 3 d (Fig. 3C, Lower). Thus, Orai1, as well as [Ca2+]i, is extremely important for hPK cell migration.
The use of a Transwell assay enabled us to distinguish proliferating and migrating cells. This assay showed a significant inhibition of UhPK migration to the lower chamber of the Transwell system when Orai1 and STIM1 proteins are down-regulated (Fig. 3D). The same effects were observed when FAK was inhibited by FAKi 14, or [Ca2+]i is chelated by BAPTA-AM. In addition, Z-LLY-FMK, a specific inhibitor of calcium-dependent protease calpain, a protease well-known to cleave FAK, at 10 µM successfully blocked hPK migration, confirming its important role in focal adhesion turnover.
We have further studied the expression of FAK in human skin sections and the level of FAK autophosphorylation. The expression of FAK, especially its tyrosin-397 autophosphorylated form (pFAK397), crucial for its activation and involvement in focal adhesion turnover, was predominantly located in the basal layer as for Orai1 and STIM1 (Fig. 3E). The independent knockdowns of both Orai1 and STIM1 significantly diminished the quantity of autophosphorylated FAK397 protein, compared with the total FAK protein (Fig. 3F). The efficiency of transfection, as well as the quantification of blots, is shown in Fig. 3G. The other channels, such as TRPC1 and TRPV6, were also studied and did not show any significant decrease in FAK397 protein (Fig. S1B). Further, we have checked whether the inhibition of FAK autophosphorylation could have a role by inhibiting FAK with its inhibitor, 10 µM FAKi 14 or by sequestrating [Ca2+]i with the cell-permeable chelator BAPTA-AM. The level of pFAK397 protein was significantly attenuated by these agents (Fig. 3H), both of which decreased the quantity of pFAK397 protein whereas the total FAK was unchanged (see quantifications in Fig. S1C). Therefore, STIM1/Orai1-mediated Ca2+ influx is an important controller of FAK autophosphorylation and focal adhesion turnover.
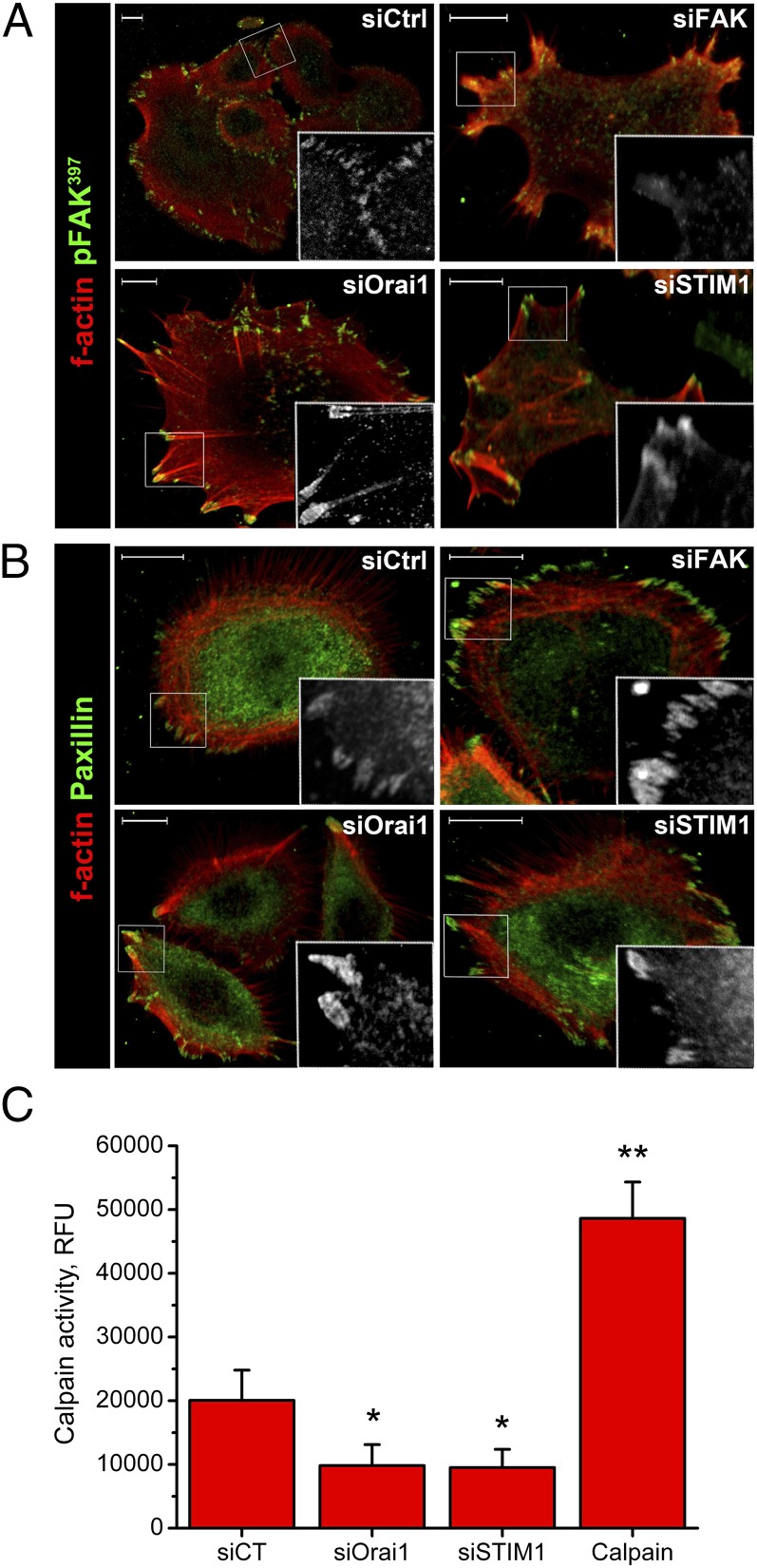
Finally, because the inhibitor of calpain, Z-LLY-FMK, successfully blocked keratinocyte migration, we have studied the role of Orai1 and STIM1 proteins in calpain activity using the fluorometric calpain activity assay kit from Merck Chemicals Ltd. The activity of endogenous calpains was decreased upon Orai1 and STIM1 knockdowns (Fig. 4C), suggesting the possible activation of calcium-dependent calpain proteases downstream of the Orai1 channel.
Fig. 4.
Involvement of Orai1 and STIM1 in focal adhesion turnover. Immunostaining of F-actin (red) and pFAK (green, A) or paxillin (green, B) of UhPK cells (kept in 0.07 mM Ca2+). UhPK cells were transfected with siOrai1, siSTIM1, siFAK, and siCtrl for 24 h before fixation. Small white boxes correspond to the magnified area of the image where pFAK (A) or paxillin (B) are shown in gray scale. (Scale bars: 10 µm.) (C) Calpain activity in UhPK cells treated with 60 nM siRNA against siOrai1 and siSTIM1 for 24 h. Cell lysates were obtained and assayed according to the fluorometric calpain activity kit (Merck Chemicals Ltd.) and compared with a provided positive control (Calpain).
Orai1 and STIM1 Regulate Focal Adhesion Turnover and Undifferentiated hPK Polarized Motility.
The reorganization of the cell cytoskeleton is necessary to exercise cell movement in space. As a part of this complex process, the formation and disassembly of focal-adhesion contacts is a prerequisite for the dynamics of this process. The turnover of these focal adhesions has been monitored using confocal microscopy (Fig. 4 A and B). Two proteins crucial for cell movement, pFAK397 and paxillin, were stained together with F-actin, a polymerized form of G-actin. The knockdown of Orai1 and STIM1 proteins provoked the aggregation of pFAK397 protein and inhibited focal adhesion contacts turnover, as well as stimulated stress-fiber formation compared with a total extinction of focal adhesion contacts when FAK protein was inhibited by siRNA (Fig. 4A). The knockdown of Orai1 and FAK yielded a constricted cell morphology and stress-fiber formation (Fig. 4B). The other protein important for focal adhesion contacts formation, paxillin, was also aggregated. These data demonstrate the important role of Orai1 and STIM1 in focal-adhesion turnover crucial for cell migration.
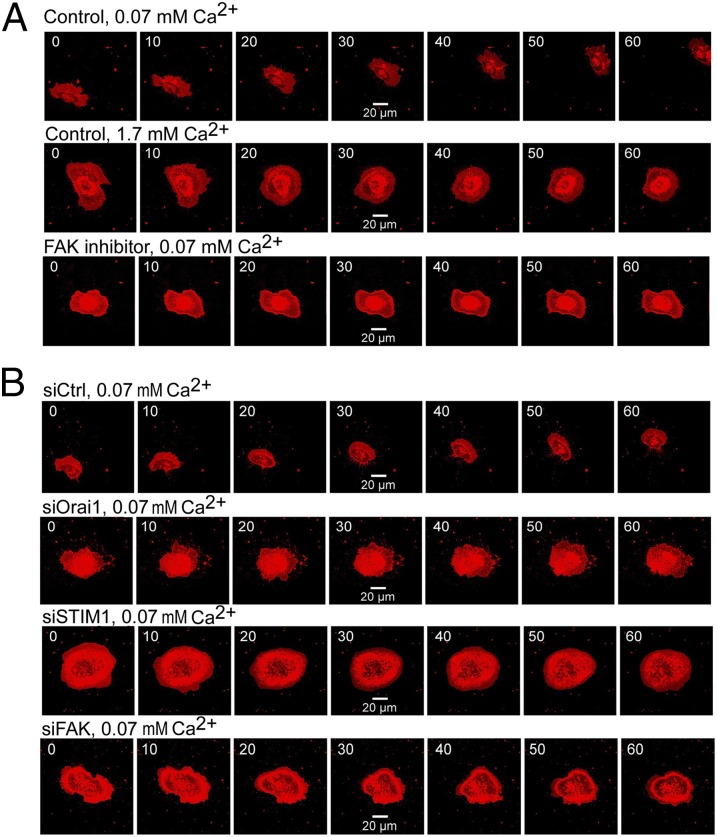
Because the focal adhesion turnover is necessary for keratinocyte migration, we have studied the role of Orai1 and STIM1 in polarized motility of undifferentiated hPK (Fig. 5). In control experiments using confocal microscopy and hPK staining with CellMask, we have shown that differentiated hPK lack polarized motility and cell migration in general (Movies S1 and S2). These effects were mimicked by the use of the FAK inhibitor in UhPK (Fig. 5A and Movie S3). The successful knockdown of Orai1 (Movie S4), STIM1 (Movie S5), and FAK (Movie S6) proteins in UhPK completely arrested cell migration of UhPK, implying the important role of these proteins in polarized motility of human keratinocytes (Fig. 5B).
Fig. 5.
Polarized motility of human keratinocytes affected by Orai1 and STIM1. The motility of hPK cells (kept in 0.07 mM Ca2+) was monitored during 1 h using confocal microscopy imaging of the hPK cells stained with CellMask for 30 min. (A) Polarized motility of UhPK cells (kept in 0.07 mM Ca2+) and DhPK cells (kept in 1.7 mM Ca2+) compared with UhPK cells pretreated with 10 µM FAK inhibitor 14 for 30 min. (B) Polarized motility of UhPK cells (kept in 0.07 mM Ca2+) transfected with siRNA against Orai1, STIM1, and FAK versus siCtrl.
The Role of the EGF-Dependent Pathway and SOCE in Keratinocyte Polarized Motility.
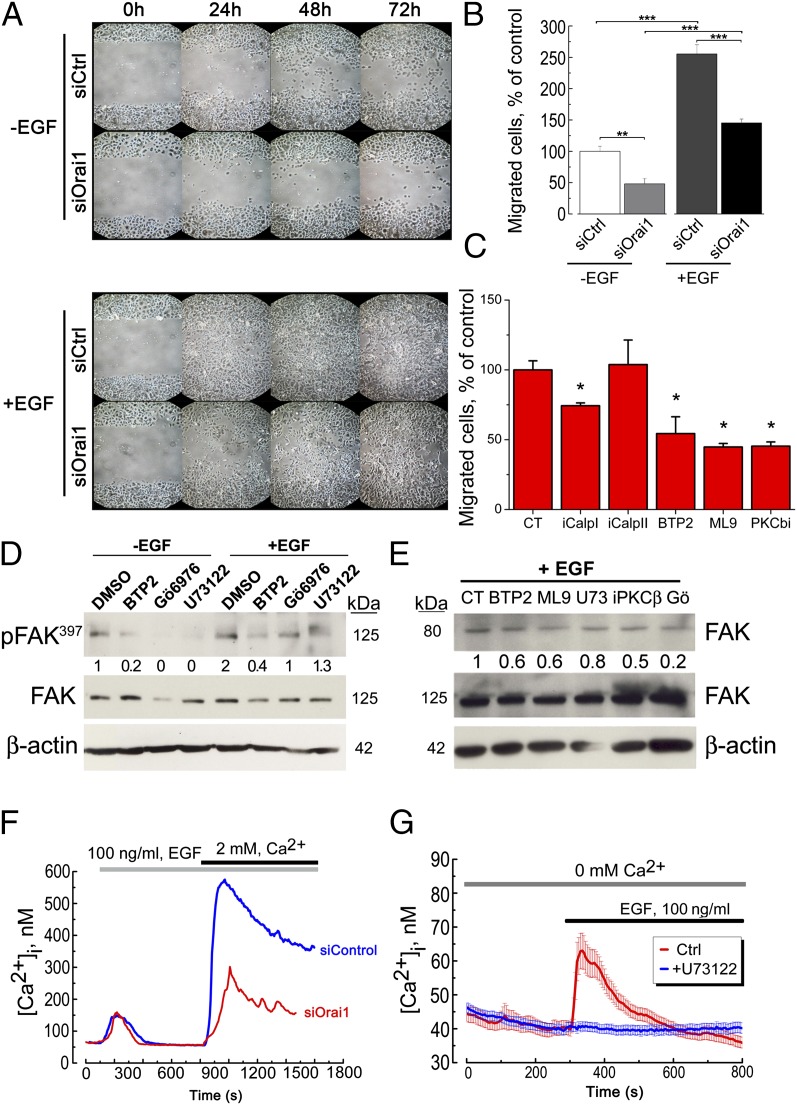
To have an insight into the possible mechanisms leading to migration/polarized motility via the Orai1 channel, we have investigated the possible role of endothelial growth factor (EGF) signaling in activation of hPK cell migration. Our data show that EGF stimulates the migration in the wound-healing assay whereas siOrai1 pretreatment of cells inhibits these effects (Fig. 6A). The same protocol has been performed in Transwell assays, and the same data have been obtained (Fig. 6B), suggesting the pivotal role of EGF in keratinocyte migration and the important contribution of Orai1 channels in this pathway. To prove the role of Orai1 channels and SOCE in this signaling, a panel of pharmacological inhibitors was used in the study. The migration studies using Transwell assays have been preformed using the pharmacological inhibitors against calpain I (iCalpI, 10 nM), calpain II (iCalpII, 1 µM), Orai1 (BTP2, 1 µM), STIM1 (ML9, 10 µM), and PKCβ (iPKCβ, 100 nM). All of them, except for the inhibitor of calpain II protease, diminished the rate of keratinocyte migration (Fig. 6C). Moreover, the other inhibitors of SOCE, such as 2-APB, La3+, and Gd3+, and the very recently appeared inhibitors of Orai1 selectivity filter, GSK-7975A and GSK-5503A (20), were used and showed the significant decrease in hPK cell migration (Fig. S1D). Further, because the autophosphorylation of FAK at the tyrosine 397 residue is crucial for its activation and involvement in focal adhesion turnover, we further studied the effects of pharmacological inhibition of PKC and SOCE pathway on FAK397 autophosphorylation. Indeed, the inhibition of Orai1 (BTP2, 1 µM), calcium-dependent PKC (Gö6976, 1 µM), and PLC (U73122, 1 µM) decreased the level of FAK397 autophosphorylation induced by EGF (Fig. 6D). As for the migration, the other inhibitors of SOCE, such as 2-APB, La3+, and the very recently appeared inhibitors of Orai1 selectivity filter, GSK-7975A and GSK-5503A, were tested and showed a decrease in pFAK397 level (Fig. S1E). EGF also induced the cleavage of FAK, yielding an 80-kDa fragment, the result of FAK degradation by proteases such as calpain and therefore enhanced its turnover (Fig. 6E). The effects of pharmacological inhibition of FAK degradation under the inhibition of Orai1 (BTP2, 1 µM), STIM1 (ML9, 10 µM), two PKC inhibitors (iPKCβ, 0.1 µM and Gö6976, 1 µM), and PLCγ (U73122, 1 µM) are shown in Fig. 6E. Moreover, the quantity of 80-kDa fragment of the cleaved FAK was diminished following both siOrai1 and siSTIM1 treatments, but not those of siTRPC1 and siTRPV6 (Fig. S1B). The expression of EGFR and paxillin was also monitored as a control that the observed effects are solely due to the intracellular messengers downstream to the EGFR, and the number of structural proteins such as paxillin does not change during the treatments (Fig. S1F).
Fig. 6.
Calcium influx via Orai1 activates FAK and triggers migration. (A) A representative experiment of three independent experiments of a wound-healing assay of UhPK (kept in 0.07 mM Ca2+) cells treated with 100 ng/mL EGF (+EGF) for the indicated time or without EGF (−EGF), and transfected with either siOrai1 or siCtrl. (B) Cell-migration assay using Transwell chambers of UhPK (kept in 0.07 mM Ca2+) treated with 100 ng/mL EGF (+EGF) or without EGF (−EGF), and transfected with either siOrai1 or siCtrl. Cells were allowed to migrate for 24 h. Data shown are mean ± SEM; **P < 0.01, ***P < 0.001; n = 3 in quadruplicate. (C) Cell-migration assay using Transwell chambers of UhPK (kept in 0.07 mM Ca2+) treated with DMSO (CT), 10 nM iCalpI, 1 µM iCalpII, 1 µM BTP2, 10 µM ML9, and 100 nM iPKCβ. Data shown are mean ± SEM; *P < 0.05. (D) FAK397 autophosphorylation studies using UhPK cells treated with 100 ng/mL EGF (+EGF) for 30 min or without EGF (−EGF), and pretreated with 1 µM BTP2, 1 µM Gö6976, 1 µM U73122 for 45 min. Numbers below correspond to the relative quantity of FAK397 compared with the total FAK, and the loading control β-actin. (E) The effects of pharmacological inhibition of Orai1 (BTP2, 1 µM), STIM1 (ML9, 10 µM), PLCγ (U73122, 1 µM), and two PKC inhibitors (iPKCβ, 0.1 µM and Gö6976, 1 µM) on FAK cleavage under the EGF stimulation (+EGF) yielding the expected cleaved form of FAK at around 80 kDa. Numbers below correspond to the relative quantity of the cleaved FAK (80 kDa) compared with the FAK full-length (125 kDa), and the loading control β-actin. (F) Store-operated calcium entry in UhPK cells induced by 100 ng/mL EGF and its inhibition by siOrai1. A representative experiment of five independent experiments, n = 30 cells per experiment. (G) Store-operated calcium entry in UhPK cells induced by 100 ng/mL EGF and its inhibition by PLC inhibitor U73122, 1 µM.
Finally, EGF by itself was capable of inducing SOCE, and this SOCE induced by EGF was successfully inhibited by siRNA against Orai1, suggesting the direct role of this channel in EGF-induced SOCE (Fig. 6F). Further, the PLC inhibitor U73122 (10 µM, 30 min) completely inhibited ER Ca2+ release induced by EGF (Fig. 6G). In an additional series of experiments, we inhibited the expression of FAK with its respective siRNA, and we observed no difference in SOC current, suggesting that FAK signaling is definitely downstream of Orai1 signaling (Fig. S1G).
Keratinocyte Physiology and Epidermis Barrier Are Impaired in orai1−/− Mice.
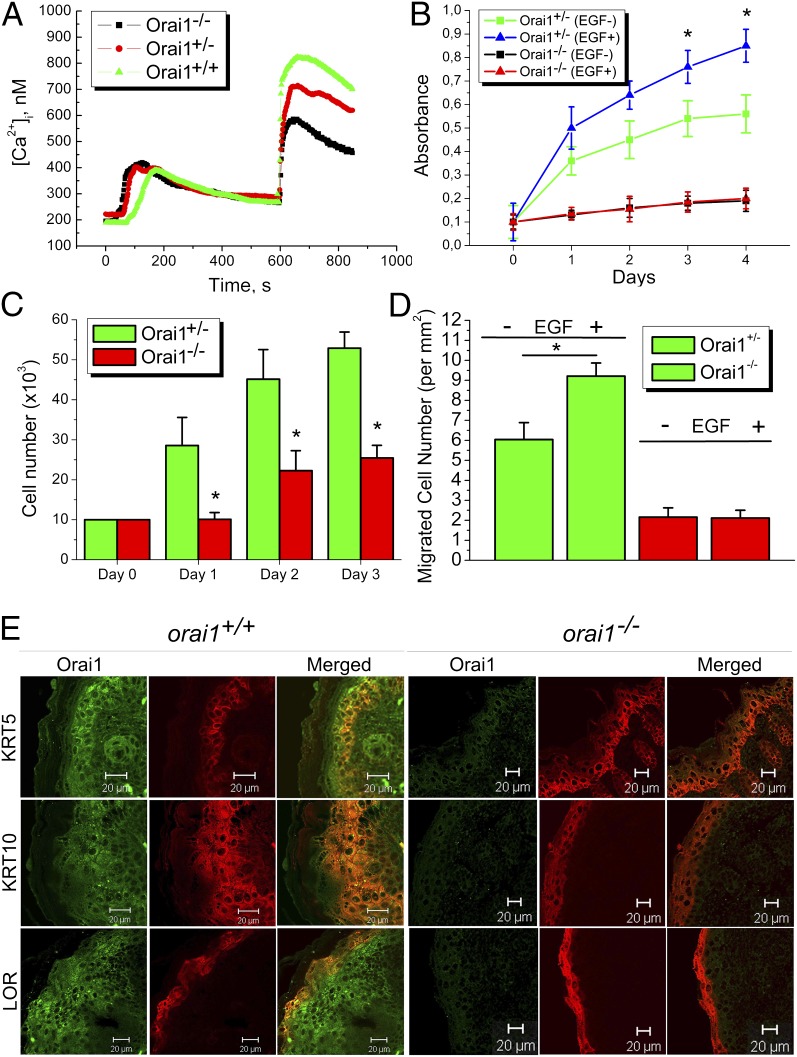
To confirm the importance of our findings obtained in vitro, we exploited the recently created orai1−/− mouse model (17). SOCE induced by 1 µM TG is smaller in keratinocytes from orai1−/− mice than in control ones from orai1+/+ or orai1+/− (Fig. 7A). The proliferation rate is remarkably affected (Fig. 7 B and C). The ability of keratinocytes to migrate was assessed using the Transwell assay and showed a significant decrease in the number of migrating cells obtained from orai1−/− mice compared with control ones from orai1+/+ mice irrespective of whether EGF was used or not (Fig. 7D). We have also studied the epidermis in orai1−/− mice compared with orai1+/+ mice, using immunostaining procedures and confocal microscopy (Fig. 7E). Immunostaining using anti-KRT5, KRT10, and loricrin (LOR) in orai1−/− mice showed the impaired expression of differentiation markers such that the marker of basal cell KRT5, the marker of early differentiation KRT10, and the late differentiation LOR, are equally expressed in all layers (Fig. 7E). Conversely, the epidermis of orai1+/+ mice had the well-distinguished classical expression of KRT5 in basal and suprabasal layer, the increasing expression of KRT10 above the suprabasal layer, and the expression of LOR in the terminal cornified layer. We also observed the disintegration and detaching of the stratum corneum layer in orai1−/− mice, with a significant decrease in both skin and epidermis thickness.
Fig. 7.
Keratinocyte physiology in Orai1 KO mice. (A) Capacitative calcium entry induced by 1 µM TG in keratinocytes from orai1+/+, orai1+/−, and orai1−/− mice. (B) Proliferation of keratinocytes from orai1+/− and orai1−/− mice treated with 100 ng/mL EGF (EGF+) or vehicle (EGF−), measured using either WTS assay (C) or direct cell counting (D). Cell migration assay using Transwell chambers from orai1+/− and orai1−/− mice treated with EGF (EGF+) or vehicle (EGF−). (E) Skin epidermis from orai1+/+ and orai1−/− mice stained with anti-Orai1, anti-KRT5, anti-KRT10, and anti-LOR antibodies.
Discussion
In the current study, we report several major findings: (i) we have identified in human epidermis an Orai1 protein whose expression is predominantly confined to the basal layer of epidermis; (ii) the Orai1 channel is a principal source of endogenous SOCE in human keratinocytes; (iii) Orai1 is involved in proliferation and migration, and keratinocyte differentiation is under the negative control of the Orai1 channel; and (iv) the Orai1 channel mediates polarized motility in keratinocytes both basal and induced by EGF by supplying calcium via SOCE used to (i) activate PKCβ, which enhances focal adhesion contact formation via stimulation of pFAK397 autophosphorylation and association of pFAK397 and paxillin with actin filaments, and (ii) activate a calcium-dependent protease calpain participating in FAK degradation and therefore accelerating FAK turnover, both of them promoting polarized cell migration. Finally, the keratinocytes from Orai1 KO mice exhibit remarkably decreased migration, proliferation, and impaired differentiation, yielding impaired epidermis formation.
In the present study, we have shown that Orai1 is one of the most important pore-forming entities in the plasma membrane and, together with STIM1 as a signal transducer from the ER, represents the major molecular component of SOCE in human primary keratinocytes: siRNA-mediated knockout of any of them strongly diminishes ISOC in keratinocytes. The translocation of STIM1 to the keratinocyte plasma membrane following agonist stimulation was shown for HaCaT cells (21); however, the molecular mechanisms and the role of Orai1/STIM1 coupling remained obscure. Although the role of Orai1/STIM1-mediated SOCE in the HaCaT keratinocyte cell line has recently been described (18), it does not address the physiological role of this channel in skin. In fact, the HaCaT cell line is an artificial cell line of spontaneously immortalized keratinocytes (22). This aneuploid cell line has a transformed phenotype in vitro and altered and unlimited growth potential, conserving through its ability to differentiate. This cell line was initially used during the 1990s by many teams around the world in signal-transduction studies; however, later, the phenotype and the physiology of these cells were shown to be different to human primary keratinocytes and therefore were considered not suitable for studies in skin physiology (22–27). Moreover, the data of Numaga-Tomita and Putney (18) on calcium switch-induced differentiation involve the expression of only one marker of differentiation, KRT1, which cannot reflect differentiation per se, especially bearing in mind that the expression of this protein is Ca2+-dependent (28).
Because in our experiments Orai1 expression was found to decrease in differentiated cells following calcium switch, we hypothesized that down-regulation of ISOC, which accompanies the transition to the differentiated state, is associated with the reduction of Orai1 levels. And indeed, our results allow us to conclude that the transition to the differentiated hPK phenotype is associated with the loss/decrease of Orai1 expression and is under its negative control.
The principal question concerns the differential role of two calcium entries in keratinocytes: SOCE and massive constitutive entry. A number of calcium-permeable channels have been shown to be expressed in hPK (29) especially those belonging to the Transient Receptor Potential (TRP) family. From this TRPC family, TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 were shown to be expressed and functional in hPK, and all of them were shown to be involved in SOCE (27, 29–31). Interestingly, although the short-term transient increase in [Ca2+]i is mostly attributed to be proproliferative, for two of them, TRPC1 and TRPC4, which where shown to be SOCs, a role in SOCE and keratinocyte differentiation was shown (27, 29, 32, 33). Among all of them, a member of the vanilloid family of TRP channels, TRPV6, has been shown to be expressed and to positively correlate with the calcium gradient (a stepwise increase in both intra- and extracellular calcium from stratum basale to stratum corneum) (34–36) and differentiation in the skin, providing a massive calcium entry into keratinocytes that is necessary to induce differentiation (37, 38). The Orai1 channel and Orai1-mediated SOCE are likely to control the onset of differentiation. Indeed, the existence of lipid rafts/caveolae as microdomains and initiation of calcium signaling therefrom are well-known (39, 40). The formation of STIM1–Orai1 complexes in those rafts was already demonstrated for SOCE (41). Although the disruption of lipid rafts resulted in an increase of the proliferative capacity of human keratinocytes, their reintegration led to the expression of the late differentiation marker, KRT2 (42). It remains intriguing just how these two types of entries may coexist and whether they are interrelated because the decrease in SOCE and Orai1 expression is accompanied by the increase in constitutive calcium entry and TRPV6 expression following the gradient of calcium and differentiation (37).
We have demonstrated the important role of the Orai1 channel in UhPK proliferation. A role of the Orai1 channel and SOCE in cell proliferation has been suggested for endothelial cells (13), lymphocytes (14), airway smooth muscle (43), and others. The increase in Orai1 and STIM1 expression, and therefore SOCE in general is a prerequisite to stimulate proliferation because the use of SOC inhibitors like SKF-96365, NiCl2, or BTP2 abrogated the observed effects, as well as the selective knockdown of Orai1 and STIM1 (43). The increase in [Ca2+]i induced by SOCE was shown to activate the proliferation-associated calcineurin-transcription factor NFAT pathway (14, 44). Further studies will be needed to correlate the molecular mechanisms of Orai1-promoted proliferation and the calcium gradient in the skin.
In normal epidermis, keratinocytes migrate upward from the basal layer as they undergo terminal differentiation, yet they also have the capacity for lateral movement during wound healing. It was shown that keratinocytes in low-calcium medium were laterally more motile than keratinocytes in normal medium (45). During calcium-induced stratification, hPK cells moved upward from the basal layer by gliding over their neighbors and forming contacts with other suprabasal cells (3). Of central importance, mostly for keratinocytes, is the ability to balance proliferation and differentiation, control adhesion to an underlying substratum, and, when necessary, remodel this substratum and migrate in a directed fashion. These functions are particularly central to the mitotically active epidermal cell hPK, which must continually undergo proliferation, detachment, and differentiation in the course of homeostasis, and upon injury, proliferate and migrate toward and repair the wound.
We have demonstrated the involvement of Orai1 channels in keratinocyte polarized motility and migration. Indeed, the coordinated and dynamic regulation of adhesions is central for cell migration in both normal and pathological processes (46). Cell migration is initiated by forming protrusions, which are stabilized by integrin-mediated adhesions that establish structural and signaling linkages between the extracellular matrix and the actin cytoskeleton. A prominent component involved in this regulation is FAK (47). FAK promotes cell migration by its capacity to orchestrate signals between integrin and growth factor receptors (47). Downstream of integrin or growth factor stimulation, FAK is phosphorylated at Tyr-397, which is an important binding site for Src family kinases (48). There are numerous reports that the control of FAK phosphorylation and cell morphology is a key factor involved in the formation of focal contacts that regulate cell attachment and detachment required for cell migration (49). Previous studies have demonstrated a critical role for FAK as a regulator of adhesion dynamics (50, 51), as well as SOCE in the control of NFAT translocation to regulate cell migration (19). In the present work, we have shown that the depletion of the ER by the EGF-triggered pathway, followed by the activation of Orai1 channels and consequently SOCE, may lead to autophosphorylation of FAK at Tyr-397, thus stimulating keratinocyte migration. Nevertheless, the mechanisms by which FAK regulates the assembly–disassembly of focal adhesions remain to be elucidated.
EGF is an important growth factor that elicits cellular signaling through an EGF receptor and affects cell migration in many cell types (52). When EGF binds to EGFR, the receptor dimerizes and is autophosphorylated, which subsequently leads to the downstream activation of mitogen-activated protein kinase (MAPK), phosphatidyl 3-kinase (PI3-kinase), and phospholipase C pathways (53, 54). It has also been shown that EGFR signaling acts through the TRPV3 channel regulating keratinocyte cornification (55). In our studies, we used EGF as a natural regulator for the processes studied, and we have shown that EGF signaling in keratinocytes involves SOCE mediated by Orai1 channels.
Some reports demonstrated that phosphorylation of FAK was increased in EGF-stimulated cell spreading and migration (56). Others have shown that EGFR-mediated signaling induced the dephosphorylation of FAK during cell motility (57). Thus, most investigators have examined either FAK phosphorylation or dephosphorylation stimulated by EGFR-mediated signaling during cell migration and wound healing. It was reported that EGFR-induced signaling pathways are necessary for cell migration (58). Thus, EGF is involved antagonistically in both FAK phosphorylation and dephosphorylation with different mechanisms in a cell (59), and there is still a limited understanding of the mechanism linking a cell attachment–detachment process and EGF stimulation.
On the other hand, evidence has emerged supporting the role of the calpain family of intracellular calcium-dependent proteases in regulating cell migration (60, 61). Calpains have been proposed to regulate migration, at least in part through their ability to modulate the dynamics of adhesions (62). Numerous calpain targets have been identified, some of which are proteins that are present in focal adhesions, including talin, paxillin, and FAK (63). It has been proposed that calpain cleavage may play a role in terminating FAK signaling by attenuating its kinase activity (64). It seems likely that different signaling pathways may be activated by the same ligand such as EGF because it has been shown that, in undifferentiated keratinocytes, IFN-inducible protein-9 activates μ-calpain downstream of a PLC-β3-mediated calcium flux whereas EGF uses the same ERK to M-calpain cascade that it triggers to converge at the disassembly of adhesion plaques (65). In our studies, we have confirmed that the selective inhibition of calpain, most probably calpain I, arrests UhPK migration, suggesting the crucial role of this pathway in focal adhesion turnover and cell motility mediated by Orai1 activation upstream of FAK. Thus, it is likely that proteolysis of these substrates contributes to the regulation of adhesion dynamics and cell migration.
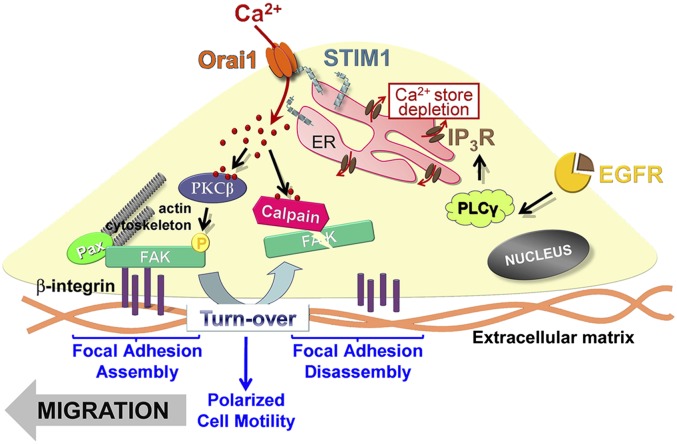
We can summarize our findings on the role of Orai1/STIM in keratinocyte migration: the activation of EGFR induces the stimulation of PLCγ and consequently the depletion of calcium in the endoplasmic reticulum, which induces the redistribution of the calcium sensor STIM1 (Fig. 8). The latter, in turn, transactivates the Orai1 channel and induces store-operated calcium entry. This store-operated calcium entry promotes both the autophosphorylation of FAK via activation of calcium-dependent PKCβ and the degradation of FAK by calcium-dependent µ-calpain protease, enhancing focal adhesion turnover and thereby stimulating polarized cell motility of human keratinocytes.
Fig. 8.
The role of Orai1 in keratinocyte polarized motility. The activation of EGFR induces the activation of PLCγ and consequently the depletion of calcium in the endoplasmic reticulum, which stimulates the calcium sensor STIM1. The latter, in turn, transactivates the Orai1 channel and induces store-operated calcium entry. This store-operated calcium entry promotes both the autophosphorylation of FAK397 via activation of calcium-dependent PKCβ and the degradation of Focal Adhesion Kinase by calcium-dependent µ-calpain protease, enhancing focal adhesion turnover and thereby both stimulating polarized cell motility of human keratinocytes.
Our data obtained in humans are strongly supported by our orai1−/− mouse model studies. Although the absence of Orai1 protein in keratinocytes affects the SOCE in these cells, it may be compensated by other store-operated channels. In addition, the proliferation of keratinocytes and particularly polarized migration, both basal and induced, are significantly decreased. The consequences of orai1−/− deletion lead to impaired epidermis/barrier formation as a result of aberrant keratinocyte structural protein expression, suppressed proliferation, and, eventually, migration—all of them crucial for normal skin homeostasis.
Materials and Methods
Cell Culture and Human Skin Sections.
Human Primary Keratinocytes (hPK) were purchased from Invitrogen. Cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2 in a keratinocyte serum-free medium (K-SFM) supplemented with 5 µg/mL recombinant epidermal growth factor (EGF) and 50 µg/mL bovine pituitary extract (BPE), containing 0.07 mM Ca2+. The medium was changed three times a week, and cultures were split by treating the cells with 0.25% (vol/vol) trypsin (in PBS) for 5 min at 37 °C before confluency. Throughout the text, the terms UhPK and DhPK are used. They imply that hPKs kept in 0.07 mM Ca2+ are called undifferentiated (UhPK) because their phenotype corresponds to undifferentiated proliferating cells from stratum basale, or hPK cells treated with 1.7 mM Ca2+ for at least 3 d, which provokes an irreversible differentiation process and keratinocytes have to be considered as differentiated (DhPK).
Orai1−/− Animals and Gene Targeting.
Gene targeting of the Orai1 gene was performed by homologous recombination in B6/3 embryonic stem (ES) cells derived from C57BL/6 mice (TaconicArtemis GmbH). Chimeric mice with targeted Orai1 alleles were generated by blastocyst injection of heterozygous Orai1neo/+ ES cell clones, as previously described (17). All mice were maintained in specific pathogen-free barrier facilities at the La Jolla Institute for Allergy and Immunology (LIAI) and were used in accordance with protocols approved by the Center for Animal Resources and LIAI. All mice were genotyped by PCR. PCR-ready genomic DNA was extracted from tail biopsies using the QuickExtractDNA Extraction Solution (Epicentre) and then amplified with the primers WT Forward 5′-GGGTGTGGCGTATGCAAATAACCT-3′, WT Reverse 5′-ACTCGAGCCGGTCTCCC-3′, KO Reverse 5′-TCGTACCACCTTCTTGGGACTTGA-3′ (IDT), and Taq Choice (Applied Biosystems).
Mouse Primary Keratinocyte Culture.
Mouse primary keratinocytes were isolated by dissecting the skin from orai1−/− mice using a standard protocol. The removed skin was washed in PBS completed with Penicillin/Streptavidin 1% (Gibco) and then fragmented and incubated in a vol/vol solution of Collagenase I (8 mg/mL) and Dispase (5 mg/mL) (Gibco) for 3 h at 37 °C. Then Trypsin is added during 15 min, and the mixture is filtered using a 40-µm filter, centrifuged at 1,000 × g for 5 min, and finally resuspended in a Defined Epidermal keratinocyte Medium CnT-07, PCT (CellNTec) and incubated at 37 °C and 5% CO2 for a week. Then, cell medium was replaced by CnT-57, PCT (CellNTec) and changed every 3 d.
siRNA Cell Transfection.
All siRNAs were synthesized by Eurogentec France SASU and are indicated in Table S1. Cells were transfected with 60 nM siRNA using 6 µl of Hyperfect transfection reagent (Qiagen) following the manufacturer’s instructions. The efficiency of cell transfections with the siRNAs for each particular target has been validated using real-time quantitative PCR.
Immunohistochemistry.
Paraffinized human skin sections were obtained following breast-reduction surgery after informed patient consent and the approval of the ethical committee of the St. Vincent Hospital of Lille. Paraffin-embedded skin sections were subjected to conventional deparaffinization followed by antigen retrieval using citrate buffer at 95 °C in a water bath. After saturation in the solution containing 1% BSA and 0.05% Triton X-100 in PBS-gelatin, the skin sections were incubated with the specific rabbit polyclonal anti-ORAI1 antibody (ProScience; 1/200), mouse monoclonal anti-STIM1 (BD Biosciences; 1/200), mouse monoclonal anti-KRT 10 (Chemicon International; 1/500), anti-Involucrin (Sigma-Aldrich ; 1/500), anti-Filaggrin (Abcam; 1/500), and anti-phospho-FAK (pTyr397) (Sigma-Aldrich; 1/200) overnight at 4 °C. Goat polyclonal anti-rabbit and anti-mouse peroxidase-conjugated secondary antibodies (Chemicon International; 1/200) were used. After revelation with diaminobenzidine (Sigma-Aldrich), images were analyzed using a Zeiss Axioskope microscope (Carl Zeiss) and Leica Image Manager software (Leica Geosystems AG Heinrich).
Immunocytochemistry.
Cells grown on glass coverslips were washed once with PBS and fixed in 3.5% paraformaldehyde in PBS. PBS-glycine (30 mM) + BSA (50 µl/mL) was used as a saturation buffer, and permeabilization was obtained using 0.1% Triton X-100. The cells were washed again in PBS and subjected to F-actin staining by the Phalloidin-tetramethylrhodamine B isothiocyanate (Fluka-Sigma-Aldrich) for 30 min at room temperature (1/1000). Then, a conventional immunostaining procedure was used for the detection of focal adhesion proteins: mouse monoclonal anti-paxillin (Abcam; 1/200) and rabbit polyclonal anti-phospho-FAK (pTyr397) (Abcam; 1/400). Alexa Fluor 546 or 488 goat anti-rabbit or anti-mouse IgGs (Molecular Probes; ¼,000) were used as secondary antibodies. Fluorescence was analyzed on a Carl Zeiss Laser Scanning Systems LSM 510 connected to a Zeiss Axiovert 200 M with a ×63 1.4 numerical aperture oil immersion lens at room temperature.
Quantitative Real-Time PCR.
The quantitative real-time PCR of Orai1, STIM1, FLG, and HPRT mRNA transcript was done using MESA GREEN qPCR MasterMix Plus for SYBR Assay (Eurogentec) on the Bio-Rad CFX96 Real-Time PCR Detection System. The sequences of primers are indicated in Table S2. The HPRT (hypoxanthine-guanine phosphoribosyltransferase) gene was used as an endogenous control to normalize variations in the RNA extractions, the degree of RNA degradation, and variability in RT efficiency. To quantify the results, the comparative threshold cycle method ∆∆Ct and CFX Manager Software v2.0 were used.
Western Blotting.
hPK cells were treated with an ice-cold lysis buffer containing 10 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, 10 mM MgCl, 1 mM PMSF, 1% Nonidet P-40, and protease inhibitor mixture from Sigma. The lysates were centrifuged at 15,000 × g and 4 °C for 20 min, mixed with a sample buffer containing 125 mM Tris⋅HCl, pH 6.8, 4% SDS, 5% b-mercaptoethanol, 20% glycerol, and 0.01% bromophenol blue, and boiled for 5 min at 95 °C. The total protein samples were subjected to 8–10% SDS/PAGE and transferred to a PVDF membrane by semidry Western blotting (Bio-Rad Laboratories). The membrane was blocked in a 5% milk TNT buffer (Tris⋅HCl, pH 7.4, 140 mM NaCl, and 0.05% Tween 20) for 1 h, and then probed using a specific rabbit polyclonal anti-FAK (Abcam; 1/400) and anti-phospho-FAK (pTyr397) (Sigma-Aldrich; 1/1000) and anti-β-actin (Lab Vision; 1/1000) antibodies. The bands on the membrane were visualized by enhanced chemiluminescence (Pierce Biotechnologies). A densitometric analysis was performed using a Bio-Rad image acquisition system (Bio-Rad Laboratories).
Electrophysiology and Solutions.
Macroscopic currents were recorded from hPK cells in the whole-cell configuration of the patch-clamp technique using a computer-controlled EPC-9 amplifier (HEKA Electronic), as previously described. Voltage ramps (−100 to +100 mV) of 250 ms were recorded every 2 s for IP3 and 5 s for TG immediately after gaining access to the cell from a holding of 0 mV. The currents were normalized based on the cell capacitance. Leak currents were subtracted by subtracting an initial ramp current (before SOC development) from all subsequent ramp currents.
The composition of the extracellular solution for patch-clamp recording was (in mM): 120 NaCl, 10 CaCl2, 2 MgCl2, 5 glucose, and 10 Hepes, pH 7.4 (adjusted with TEA-OH), osmolarity 310 mOsm/kg adjusted with d-mannitol. The patch pipettes were filled with the basic intracellular pipette solution (in mM): 120 Cs-methane sulfonate, 10 CsCl, 10 Hepes, 10 BAPTA [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid], and 6 MgCl2 (pH adjusted to 7.4 with CsOH and osmolarity 295 mOsm/kg adjusted with d-mannitol). The necessary supplements in the desired concentrations were added to the experimental solutions directly from the appropriate stock solutions, dissolved in water, ethanol, or dimethyl sulfoxide. All chemicals were purchased from Sigma-Aldrich. The experiments were carried out at room temperature.
Cell-Proliferation Assays.
hPK cells were harvested and plated at the initial density of 3,000 cells per well in 96-well plates (Poly-Labo). Cells were transfected, and the number of viable cells was determined every 24 h using the CellTiter 96 Aqueous nonradioactive cell proliferation assay (Promega Corp.). Cell count was also used to determine the proliferation rate of hPK 48 h after transfection with siRNA using a Malassez counting chamber. Cells were trypsinized from six-well plates, and cells from each well were counted eight times.
Migration Assay.
Cells were seeded onto the top of Transwell cell-culture inserts with 8.0-μm pore size (Falcon) at the density of 20,000 per well (24-well format) in noncomplemented K-SFM. Cells were stimulated to migrate across the filters by complemented K-SFM as a chemoattractant in the assay chambers beneath the inserts. After 24 h of incubation at 37 °C, nonmigratory cells were removed from the top of the filter by scraping whereas cells that had migrated through the filter pores to the lower side of the inserts then were fixed in methanol and stained with Hoechst (5 mg/L in PBS). Cells on the lower side of the inserts were counted using a Leica DMIRB. Data are expressed as means of four wells ± SEM.
Wound-Healing Assay.
hPK cells were seeded and allowed to reach subconfluency, and then cells were transfected with the siRNAs. After 24 h, a 1,000-μL pipette tip was used to scrape across the dish, and the resulting wound was washed with PBS. Bright field images were captured along the wound using a Nikon TS100 microscope (×100), and then a series of pictures were taken at 24 h, 48 h, and 72 h after wounding. The images were then analyzed using Adobe Photoshop CS5.
Cell Motility.
hPKs were transfected with the corresponding siRNA for 24 h or treated with the FAKi 14 (Tocris Bioscience; 10 µM) for 30 min. Cell membrane was stained for 30 min with CellMask, and cell movements were observed by taking images every 36 s using a Carl Zeiss Laser Scanning Systems LSM 510 connected to a Zeiss Axiovert 200 M.
Data Analysis.
For each type of experiment, the data were accumulated from at least three measurements. Electrophysiological data were analyzed offline using HEKA (HEKA Electronic) and Origin 7.0 (Microcal Software) software. Results were expressed as mean ± SEM, where appropriate. N equals the number in the series of experiments, and n equals the number of cells used in the study. ANOVA was used for statistical comparison of the differences, and P < 0.05 was considered significant. In the graphs, (*) and (**) denote statistically significant differences with P < 0.05 and P < 0.01, respectively.
Supplementary Material
Acknowledgments
We thank Dr. Adriana Mihalache and Prof. Pierre Gosset for immunostaining expertise. We also thank Dr. David House from GlaxoSmithKline for the provision of GSK-7975A and GSK-5503A compounds. We thank the Department of Research and Development of Johnson and Johnson France for the PhD funding of M.V. and M.R., as well as for the scientific collaboration with our laboratory. D.G. was supported by the State Fund for Fundamental Research (F 46.2/001). The studies on keratinocytes from Orai1−/− mice were funded in part by National Institutes of Health Grants AI084167 and AI40127 (to A.R. and P.G.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310394110/-/DCSupplemental.
References
- 1.Pillai S, Bikle DD, Mancianti ML, Cline P, Hincenbergs M. Calcium regulation of growth and differentiation of normal human keratinocytes: Modulation of differentiation competence by stages of growth and extracellular calcium. J Cell Physiol. 1990;143(2):294–302. doi: 10.1002/jcp.1041430213. [DOI] [PubMed] [Google Scholar]
- 2.Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109(3):1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heenen M, De Graef C, Galand P. Kinetics of the calcium induced stratification of human keratinocytes in vitro. Cell Prolif. 1992;25(3):233–240. doi: 10.1111/j.1365-2184.1992.tb01398.x. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie JC. Ordered structure of the stratum corneum of mammalian skin. Nature. 1969;222(5196):881–882. doi: 10.1038/222881a0. [DOI] [PubMed] [Google Scholar]
- 5.Barritt GJ. Does a decrease in subplasmalemmal Ca2+ explain how store-operated Ca2+ channels are opened? Cell Calcium. 1998;23(1):65–75. doi: 10.1016/s0143-4160(98)90075-6. [DOI] [PubMed] [Google Scholar]
- 6.Smyth JT, et al. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14(10):2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 8.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 9.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 10.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312(5777):1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338(6112):1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443(7108):226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullaev IF, et al. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103(11):1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: Store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42(2):145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Ng SW, di Capite J, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem. 2008;283(46):31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 16.Stiber J, et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10(6):688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwack Y, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28(17):5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Numaga-Tomita T, Putney JW. Role of STIM1- and Orai1-mediated Ca2+ entry in Ca2+-induced epidermal keratinocyte differentiation. J Cell Sci. 2013;126(Pt 2):605–612. doi: 10.1242/jcs.115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jans R, et al. Lysophosphatidic acid promotes cell migration through STIM1- and Orai1-mediated Ca2+(i) mobilization and NFAT2 activation. J Invest Dermatol. 2013;133(3):793–802. doi: 10.1038/jid.2012.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derler I, et al. The action of selective CRAC channel blockers is affected by the Orai pore geometry. Cell Calcium. 2013;53(2):139–151. doi: 10.1016/j.ceca.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross K, Whitaker M, Reynolds NJ. Agonist-induced calcium entry correlates with STIM1 translocation. J Cell Physiol. 2007;211(3):569–576. doi: 10.1002/jcp.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boukamp P, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warskulat U, Brookmann S, Reinen A, Häussinger D. Ultraviolet B radiation induces cell shrinkage and increases osmolyte transporter mRNA expression and osmolyte uptake in HaCaT keratinocytes. Biol Chem. 2007;388(12):1345–1352. doi: 10.1515/BC.2007.140. [DOI] [PubMed] [Google Scholar]
- 24.Bellei B, et al. Ultraviolet A induced modulation of gap junctional intercellular communication by P38 MAPK activation in human keratinocytes. Exp Dermatol. 2008;17(2):115–124. doi: 10.1111/j.1600-0625.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- 25.Sun T, et al. An integrated systems biology approach to understanding the rules of keratinocyte colony formation. J R Soc Interface. 2007;4(17):1077–1092. doi: 10.1098/rsif.2007.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marionnet AV, Chardonnet Y, Viac J, Schmitt D. Differences in responses of interleukin-1 and tumor necrosis factor alpha production and secretion to cyclosporin-A and ultraviolet B-irradiation by normal and transformed keratinocyte cultures. Exp Dermatol. 1997;6(1):22–28. doi: 10.1111/j.1600-0625.1997.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 27.Beck B, et al. TRPC channels determine human keratinocyte differentiation: New insight into basal cell carcinoma. Cell Calcium. 2008;43(5):492–505. doi: 10.1016/j.ceca.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhu S, et al. C/EBPbeta modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol Cell Biol. 1999;19(10):7181–7190. doi: 10.1128/mcb.19.10.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu CL, Chang W, Bikle DD. Phospholipase cgamma1 is required for activation of store-operated channels in human keratinocytes. J Invest Dermatol. 2005;124(1):187–197. doi: 10.1111/j.0022-202X.2004.23544.x. [DOI] [PubMed] [Google Scholar]
- 30.Beck B, et al. TRPC7 is a receptor-operated DAG-activated channel in human keratinocytes. J Invest Dermatol. 2006;126(9):1982–1993. doi: 10.1038/sj.jid.5700352. [DOI] [PubMed] [Google Scholar]
- 31.Cai S, Fatherazi S, Presland RB, Belton CM, Izutsu KT. TRPC channel expression during calcium-induced differentiation of human gingival keratinocytes. J Dermatol Sci. 2005;40(1):21–28. doi: 10.1016/j.jdermsci.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Cai S, et al. Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflugers Arch. 2006;452(1):43–52. doi: 10.1007/s00424-005-0001-1. [DOI] [PubMed] [Google Scholar]
- 33.Fatherazi S, et al. Evidence that TRPC4 supports the calcium selective I(CRAC)-like current in human gingival keratinocytes. Pflugers Arch. 2007;453(6):879–889. doi: 10.1007/s00424-006-0156-4. [DOI] [PubMed] [Google Scholar]
- 34.Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab. 2012;7(4):461–472. doi: 10.1586/eem.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu CL, et al. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J Invest Dermatol. 2012;132(10):2350–2359. doi: 10.1038/jid.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurasawa M, Maeda T, Oba A, Yamamoto T, Sasaki H. Tight junction regulates epidermal calcium ion gradient and differentiation. Biochem Biophys Res Commun. 2011;406(4):506–511. doi: 10.1016/j.bbrc.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 37.Lehen’kyi V, et al. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J Biol Chem. 2007;282(31):22582–22591. doi: 10.1074/jbc.M611398200. [DOI] [PubMed] [Google Scholar]
- 38.Lehen’kyi V, et al. Acceleration of keratinocyte differentiation by transient receptor potential vanilloid (TRPV6) channel activation. J Eur Acad Dermatol Venereol. 2011;25(Suppl 1):12–18. doi: 10.1111/j.1468-3083.2010.03894.x. [DOI] [PubMed] [Google Scholar]
- 39.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45(6):625–633. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berridge MJ. Calcium microdomains: Organization and function. Cell Calcium. 2006;40(5-6):405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Jardin I, Salido GM, Rosado JA. Role of lipid rafts in the interaction between hTRPC1, Orai1 and STIM1. Channels (Austin) 2008;2(6):401–403. doi: 10.4161/chan.2.6.7055. [DOI] [PubMed] [Google Scholar]
- 42.Spörl F, et al. Real-time monitoring of membrane cholesterol reveals new insights into epidermal differentiation. J Invest Dermatol. 2010;130(5):1268–1278. doi: 10.1038/jid.2009.412. [DOI] [PubMed] [Google Scholar]
- 43.Zou JJ, Gao YD, Geng S, Yang J. Role of STIM1/Orai1-mediated store-operated Ca²⁺ entry in airway smooth muscle cell proliferation. J Appl Physiol (1985) 2011;110(5):1256–1263. doi: 10.1152/japplphysiol.01124.2010. [DOI] [PubMed] [Google Scholar]
- 44.Bobe R, et al. SERCA2a controls the mode of agonist-induced intracellular Ca2+ signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol. 2011;50(4):621–633. doi: 10.1016/j.yjmcc.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magee AI, Lytton NA, Watt FM. Calcium-induced changes in cytoskeleton and motility of cultured human keratinocytes. Exp Cell Res. 1987;172(1):43–53. doi: 10.1016/0014-4827(87)90091-7. [DOI] [PubMed] [Google Scholar]
- 46.Ridley AJ, et al. Cell migration: Integrating signals from front to back. Science. 2003;302(5651):1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 47.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112(Pt 16):2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 48.Schaller MD, Parsons JT. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994;6(5):705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 49.van de Water B, Nagelkerke JF, Stevens JL. Dephosphorylation of focal adhesion kinase (FAK) and loss of focal contacts precede caspase-mediated cleavage of FAK during apoptosis in renal epithelial cells. J Biol Chem. 1999;274(19):13328–13337. doi: 10.1074/jbc.274.19.13328. [DOI] [PubMed] [Google Scholar]
- 50.Webb DJ, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6(2):154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 51.Schober M, et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol. 2007;176(5):667–680. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17(10):1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 53.Sharma GD, Ottino P, Bazan NG, Bazan HE. Epidermal and hepatocyte growth factors, but not keratinocyte growth factor, modulate protein kinase Calpha translocation to the plasma membrane through 15(S)-hydroxyeicosatetraenoic acid synthesis. J Biol Chem. 2005;280(9):7917–7924. doi: 10.1074/jbc.M408852200. [DOI] [PubMed] [Google Scholar]
- 54.Wong WS. Inhibitors of the tyrosine kinase signaling cascade for asthma. Curr Opin Pharmacol. 2005;5(3):264–271. doi: 10.1016/j.coph.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Cheng X, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141(2):331–343. doi: 10.1016/j.cell.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pichard V, et al. Adhesion, actin cytoskeleton organisation and the spreading of colon adenocarcinoma cells induced by EGF are mediated by alpha2beta1 integrin low clustering through focal adhesion kinase. Histochem Cell Biol. 2001;116(4):337–348. doi: 10.1007/s004180100324. [DOI] [PubMed] [Google Scholar]
- 57.Lu Z, Jiang G, Blume-Jensen P, Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001;21(12):4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moissoglu K, Schwartz MA. Integrin signalling in directed cell migration. Biol Cell. 2006;98(9):547–555. doi: 10.1042/BC20060025. [DOI] [PubMed] [Google Scholar]
- 59.Kim SH, Kim SH. Antagonistic effect of EGF on FAK phosphorylation/dephosphorylation in a cell. Cell Biochem Funct. 2008;26(5):539–547. doi: 10.1002/cbf.1457. [DOI] [PubMed] [Google Scholar]
- 60.Huttenlocher A, et al. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272(52):32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 61.Franco SJ, Huttenlocher A. Regulating cell migration: Calpains make the cut. J Cell Sci. 2005;118(Pt 17):3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 62.Bhatt A, Kaverina I, Otey C, Huttenlocher A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J Cell Sci. 2002;115(Pt 17):3415–3425. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- 63.Franco S, Perrin B, Huttenlocher A. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp Cell Res. 2004;299(1):179–187. doi: 10.1016/j.yexcr.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 64.Cooray P, et al. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem J. 1996;318(Pt 1):41–47. doi: 10.1042/bj3180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Satish L, Blair HC, Glading A, Wells A. Interferon-inducible protein 9 (CXCL11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of mu-calpain. Mol Cell Biol. 2005;25(5):1922–1941. doi: 10.1128/MCB.25.5.1922-1941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.