Significance
Human rhinoviruses (HRVs) cause the common cold and exacerbate chronic pulmonary diseases. Their single-stranded RNA genome is protected by an icosahedral capsid and must be released into the host cell cytosol for translation and replication. Using X-ray and cryo-EM analyses, we identified structural alterations that take place in the virus architecture during infection. In acidic endosomes in vivo and in our experimental conditions, the native virion is converted into the expanded, porous uncoating intermediate A-particle. This is accompanied by altered RNA–protein contacts at the inner capsid wall, leading to major changes in RNA conformation that result in a well-organized RNA layer. These rearrangements suggest that the RNA–protein interactions prepare RNA and facilitate its subsequent egress via a well-ordered mechanism.
Keywords: genome uncoating, X-ray analysis, 3D cryo-EM, picornavirus
Abstract
During infection, viruses undergo conformational changes that lead to delivery of their genome into host cytosol. In human rhinovirus A2, this conversion is triggered by exposure to acid pH in the endosome. The first subviral intermediate, the A-particle, is expanded and has lost the internal viral protein 4 (VP4), but retains its RNA genome. The nucleic acid is subsequently released, presumably through one of the large pores that open at the icosahedral twofold axes, and is transferred along a conduit in the endosomal membrane; the remaining empty capsids, termed B-particles, are shuttled to lysosomes for degradation. Previous structural analyses revealed important differences between the native protein shell and the empty capsid. Nonetheless, little is known of A-particle architecture or conformation of the RNA core. Using 3D cryo-electron microscopy and X-ray crystallography, we found notable changes in RNA–protein contacts during conversion of native virus into the A-particle uncoating intermediate. In the native virion, we confirmed interaction of nucleotide(s) with Trp38 of VP2 and identified additional contacts with the VP1 N terminus. Study of A-particle structure showed that the VP2 contact is maintained, that VP1 interactions are lost after exit of the VP1 N-terminal extension, and that the RNA also interacts with residues of the VP3 N terminus at the fivefold axis. These associations lead to formation of a well-ordered RNA layer beneath the protein shell, suggesting that these interactions guide ordered RNA egress.
Human rhinoviruses (HRVs) cause the common cold. Although seldom severe, this disease is widespread and frequent in man; HRVs thus have considerable economic impact due to expenditure on medication and lost working days. More than 150 serotypes belong to the genus Enteroviruses (EVs) of the Picornaviridae family, which includes serious human and animal pathogens. In addition to phylogenetic classification into species A, -B, and -C, HRVs are divided into a minor receptor group (12 HRV-A) that bind low-density lipoprotein receptors (LDLRs), and a major receptor group (more than 89 HRV-A and -B serotypes) that use intercellular adhesion molecule 1 (ICAM-1) for cell entry (1). HRV-C binds an unknown receptor (2).
The EV icosahedral shell is built from four viral proteins (VP1–4) that encase a single-stranded (+)–sense RNA genome. Sixty copies each of these four polypeptides assemble on a T = 1 (pseudo T = 3) lattice, ∼30 nm in diameter. VP1, VP2, and VP3 are surface-exposed; the small myristoylated VP4 is internal. In the mature virion, the N-terminal extensions of VP1, VP2, and VP3, together with the entire VP4, interact in an intricate network beneath the shell (Fig. S1) (3, 4).
In the cytosol, the viral RNA is translated into a ∼250 kDa precursor polyprotein that is processed by viral proteinases. Assembly of the viral shell involves immature pentamers built from VP0, VP1, and VP3. VP2 and VP4 arise late in infection through VP0 cleavage, concomitant with RNA encapsidation. In addition to mature virions, native empty capsids (NECs) of HRV2, HRV14, and equine rhinovirus (5), and presumably of other EVs, are assembled in the infected cell. They might be direct precursors of native virions, a capsid protein reservoir (6), and/or the end-product of an abortive assembly process (7). NECs attach to the receptor and undergo conformational changes similar to those of the native virus, except that they retain VP4, as it remains connected to VP2 (3, 8).
All EVs are thought to undergo a similar sequence of events leading to infection. After binding their respective receptors, they are endocytosed. In poliovirus (PV), receptor attachment catalyzes uncoating, but in some HRVs the acidic pH in endosomes is an additional trigger for the structural changes needed for RNA exit (9, 10). In minor group HRVs, low pH alone induces these changes (11). As shown for HRV2 (12), the acid-triggered beta-propeller switch of the LDLR assists rhinovirus infection. Once the virion is in the late endosomal compartment, it dissociates from its receptor and is simultaneously transformed into the A-particle, which has an expanded shell, lacks VP4, and is more hydrophobic than native virus and NEC due to surface exposure of the amphipathic VP1 N-terminal extension (13). The A-particle can bind directly to the endosomal membrane for RNA translocation (14), leaving behind the empty B-particle.
Recent work on PV caught in the act of releasing its genome shows the RNA exiting through channels near the twofold axes (15). The X-ray structure of the HRV2 B-particle showed the details of the structural rearrangements that lead to the end-product of uncoating (16). A hinge movement around the hydrophobic pocket in VP1 induces a coordinated displacement of VP2 and VP3, resulting in capsid expansion and the opening of channels in the shell. Similar alterations were observed in EV-71 when the X-ray structures of native virus and expanded NECs were compared (17).
By solving the 3D cryo–electron microscopy (3D cryo-EM) structures of native HRV2 and its A-particle (the intermediate between native virus and empty capsid) produced by incubation in acidic buffer that mimics the endosomal environment, we identified important changes in the interactions between RNA and the protein shell. We confirm these RNA–protein contacts in the A-particle in our 6.4 Å X-ray structure. The well-ordered RNA layer close to the inner capsid face is stabilized by numerous contacts; this framework might facilitate exit of the genome in a highly ordered, coordinated manner.
Results
Cryo-EM Structures of HRV2 Native and Subviral Particles.
A-particles were prepared from purified HRV2 by incubation at pH 5, applied to holey carbon grids, vitrified, and visualized in the electron microscope. The sample before (Fig. 1A) and after acidification (Fig. 1B) contained similar proportions of empty particles. Negative stain EM and capillary electrophoresis (18) confirmed that no native virus remained in the acidified sample. The four types of particles (full and empty without and with acidification) were selected manually and a 3D reconstruction (3DR) was obtained for each set (Fig. 1 C–F).
Fig. 1.
Cryo-EM images and 3DR of HRV2 and subviral particles. (A) Cryo–electron micrograph of a representative preparation of purified HRV2 (2.1 μm underfocus; first zero of the contrast transfer function (CTF) at 20.5 Å). The blue asterisk indicates a native (full) virus and the pink asterisk a NEC. (B) Cryo–electron micrograph of the sample in A after exposure to pH 5 (2.7 μm underfocus; first zero of the CTF at 23 Å). The green asterisk indicates a (full) A-particle and the orange asterisk an (empty) B-particle. Some broken virions are also observed. (Scale bar, 50 nm.) (C) 3DR of NEC, (D) native HRV2, (E) A-particle, and (F) B-particle. Upper row, the radially color-coded outer surfaces viewed along a twofold axis of symmetry contoured at 2.5 σ above the mean density; lower row, central sections from the 3DR (left half) and 25 Å–thick slabs (right half) contoured at 1 σ above the mean density to highlight the RNA density locations. Darker shading indicates higher density. In D (Upper), the prominent structural features on the capsid exterior are indicated: (i) the star-shaped mesa at the fivefold axis, (ii) the three-bladed propeller at the threefold axis, and (iii) the canyon surrounding the mesa. In D and E, red and black arrows indicate the two- and fivefold axes (lower, right half), respectively. Note the oval pores at the twofold axes in the acid-exposed particles (E and F) and the differences in RNA conformation in native virus (D) and the A-particle (E).
Based on a 0.5 Fourier shell correlation (FSC) threshold, the resolution was 8.2 Å for native HRV2 and 10.9 Å for NEC. For full (A-) and empty (B-) particles in the acidified sample, we obtained a resolution of 8.8 and 9.9 Å, respectively (Fig. S2). Diameter was determined from spherically averaged radial density plots of the 3DR (Fig. S3); it was identical for native HRV2 and NEC (304 Å; Fig. S3A). This finding and the SDS/PAGE detection of varying amounts of VP0 in our HRV2 preparations (not shown) confirmed the empty capsids as NECs (5, 17). A- and B-particles in the acidified sample were 316 Å in diameter (Fig. S3B). This ∼4% size increase relative to native virions and NECs concurs with X-ray and cryo-EM studies of empty shells from HRV2 and other EVs as obtained after incubation at 50 °C to 56 °C (19–21).
To assess RNA content, the datasets were analyzed for internal density of individual particles within a radius of 113 Å and 120 Å for native and acidified samples, respectively. The bimodal Gaussian distribution was almost identical in both cases. This suggests that the (expanded) empty and full particles generated by acid pH derived mainly from NEC and native HRV2, respectively (Fig. S4). These results indicate that low pH alone is insufficient to trigger conversion of A- into B-particles.
The capsids of the four particle types shared the typical topological features of EVs; the star-shaped “mesas” at the fivefold axes, the canyons surrounding these mesas, and the three-bladed propellers at the threefold axes (Fig. 1). Most evident were the similarities between native virus and NEC, on the one hand (Fig. 1 C and D), and the A- and B-particles, on the other (Fig. 1 E and F). The A- and B-particles were highly porous compared with the native virion. The largest pores were found at the twofold axes between two VP2 molecules (pores I), which were also observed in the X-ray structure of B-particles produced by heating (16); additional pores were observed at the canyon floor at the quasithreefold axis (II) and at the base of the mesa (III) (Fig. S5A). Pores II were partially occluded by a conspicuous density on the outer surface of the A-particle capsid, not observed on the B-particle capsid. This well-defined density constituted the major difference after subtraction of the B-particle map from the A-particle map (Fig. S5 B, green, C, and D). In light of previous studies of the related PV (22) and Coxsackievirus A 16 (CAV16) (23), this density at the quasithreefold axis probably corresponds to the externalized VP1 N terminus.
The transverse central sections of the 3D maps emphasize the internal differences, drawing attention to the density corresponding to the genomic RNA (Fig. 1 C–F, Lower) involved in visible contacts with the protein shell (Fig. 1 D and E, Lower). As predicted, RNA was absent from NECs (Fig. 1C) and from B-particles in the acidified sample (Fig. 1F). In native virions, RNA–protein interactions were limited to the twofold axes (Fig. 1D, red arrows). In the A-particle, these were maintained and new ones formed at the fivefold axes (Fig. 1E, black arrows). The rearrangements approximate the RNA layer and the inner wall of the shell (Movie S1).
X-Ray Structure of the HRV2 A-Particle.
We prepared crystals from virus incubated at pH 5. Diffraction data were collected up to 6.4 Å, and the structure was solved by molecular replacement, starting with the phases that correspond to the HRV2 B-particle structure (16) [retrieved from Protein Data Bank (PDB) ID code 3TN9] (Table S1). After density modification using 15-fold noncrystallographic symmetry averaging and solvent flattening, the averaged maps showed that A- and B-particles were almost identical, in accordance with the cryo-EM results; the only notable differences were found at the VP2 N terminus (residues 46–55) and the VP3 F–G loop (residues 149–162) (Fig. S6). Again concurring with the cryo-EM data, the X-ray map showed extra density corresponding to partially ordered RNA and RNA–protein contacts at the two- and fivefold axes (see next section).
Switch of RNA–Capsid Contacts After Conversion of Native HRV2 into the A-Particle.
Comparison of cryo-EM 3DR of native HRV2 with the cryo-EM and X-ray structures of the A-particle highlighted the distinct interactions of capsid proteins with packaged RNA (Fig. 1 D and E and Fig. 2 A–C). In native HRV2, contacts occurred only at the twofold axes (Fig. 2A). Consistent with X-ray data of native HRV2 (4), VP2 Trp38 contacted RNA nucleotide(s) (Fig. 2D, green arrow); this residue is strictly conserved in all HRVs (1) and in various EVs (24). In addition, we identified previously unreported RNA interactions near the twofold axes, mediated by the VP1 N-terminal region (Fig. 2D, blue arrow). No protein–RNA interactions were seen at the fivefold axes (Fig. 2E), and VP4 appeared to act as a “repellent surface”.
Fig. 2.
RNA–protein contacts in native HRV2 and A-particles. (A and B) Slabs 23 Å thick of (A) native HRV2 and (B) A-particle viewed along an icosahedral twofold axis, with the respective docked X-ray coordinates of native HRV2 and of the B-particle X-ray structures. (C) The 6.5 Å X-ray structure of the A-particle oriented as in A and B. Close-ups of insets D–I are shown. (D and E) Slabs 23 Å thick (parallel to but displaced ∼11 Å from the central section) of native HRV2 including the (D) two- and (E) fivefold axes (the outer surface of the capsid faces upward). Envelopes of protein shell and RNA density are depicted as gray and red mesh, respectively. Note the contacts at the twofold axis between the inner capsid surface and the RNA layer outer surface, mediated by VP2 (Trp38, green arrow) and VP1 (Asn21 and Ser22, blue arrow), displayed as stick models. VP3 (red) and VP4 (yellow) are shown. (F and G) Similar ∼20 Å–thick slabs of the A-particle at the (F) two- and (G) fivefold axes. Contacts mediated by VP2 Trp38 are maintained, although slightly displaced (green arrow); at the fivefold axis, N-terminal VP3 residues interacting with RNA are indicated (red arrows). (H and I) Similar slabs of the A-particle X-ray structure. In addition to the VP2 Trp38-mediated contact (green arrow), Ser44 is involved in direct contacts with RNA density (open green arrow); at the fivefold axis, VP3 Gly1 and Gln20 contact the RNA density.
Both cryo-EM 3DR and the X-ray structure of the A-particle (Fig. 2 B and C) showed that the VP2 Trp38-mediated interactions at the twofold axes were maintained, although slightly displaced relative to their original position in native HRV2 (Fig. 2 F and H, green arrows). In addition, VP2 Ser44 contacted the RNA in the X-ray A-particle map (Fig. 2H, open green arrow). Because the VP1 N-terminal extension was externalized, VP1-mediated interactions were absent in the A-particle (Fig. 2 F and H).
The VP3 β-annulus, which was essentially identical in native virus and A-particle (compare Fig. 2E with Fig. 2 G and I), was exposed at the inner capsid surface after loss of VP4. It now contacted the RNA (Fig. 2 G and I, red arrows). Direct interactions with Gly1 and Gln20 were observed in the crystal structure (Fig. 2I). The Gly1–Leu2–Pro3 segment is strictly conserved in all HRVs (1). All RNA–protein interactions in the A-particle gave rise to a well-structured RNA layer near the inner capsid surface (Fig. 2 B and C). The VP3-mediated RNA contacts at the fivefold axes have not been defined in other EV uncoating intermediates, presumably because of experimental differences; here we used near-physiological conditions to trigger formation of A-particles.
Interactions Mediated by N-Terminal VP1 Sequences.
Comparison of radial density profiles of native HRV2 and NECs showed that the protein shell (radius ∼110–152 Å) had a peak of extra density for the native virus, within the 115–126 Å radius (Fig. S3A, arrow). Calculation of a difference map located this major structural difference to the inner capsid surface close to the twofold axes (Fig. 3 A, blue and B–D). Fitting the X-ray coordinates of native HRV2 (PDB ID code 1FPN) into native and NEC cryo-EM maps identified most of the density lacking in the NEC map to the VP1 N-terminal region and the adjacent VP2 loop Ala47–Pro53 (Fig. 3 A and B). The disorder of these regions in NECs is consistent with their ordering in native HRV2 (4), where the VP1 Glu52–Gln57 segment interacts with the VP2 Leu42–Lys52 region (Fig. 3 E, C, and D). Their ordered state appeared to correlate with encapsidated RNA that interacts with the VP1 N terminus (Fig. 3A, striped areas).
Fig. 3.
Structural comparison of native HRV2 and NEC. (A) Difference map calculated by subtracting NEC from the native HRV2 protein shell. The resulting difference map (blue) is superimposed onto the corresponding region of the NEC inner surface (pink), viewed along an icosahedral twofold axis (black oval). Note that the VP1 N-terminal segment and an adjacent VP2 loop match well the difference density. The contact surface between VP1 N-terminal extensions and RNA is depicted as striped area; minor differences [for example, some VP3 (red) and VP2 (green) loops that do not match the cryo-EM density] were ignored. Two quasithreefold axes are indicated (gray triangles). (B) Region equivalent to A, showing the interior of the NEC cryo-EM map (white surface) with the native HRV2 X-ray structure fitted: VP1 (blue), VP2 (green), and VP3 (red). The VP1 N-terminal segment (Leu15–Thr61) and the adjacent VP2 loop (Ala47–Pro53) are indicated and are clearly not included within the density map of NEC (white surface). (C and D) Transverse sections, 1.89 Å thick, from the 3D maps of NEC (C) and native HRV2 (D), parallel to but displaced by 13.2 Å from the central section, viewed along a twofold axis (darker, denser). The regions corresponding to the densities of the VP1 N terminus and the adjacent VP2 segment are indicated (arrows). For the map of native HRV2 (D), the relative density of this region was ∼73% of the total capsid density and for the NEC map (C) ∼9%, indicating the absence of protein and RNA. (E) Close-up of the VP1 N-terminal segment interacting with the adjacent VP2 loop (from ref. 4), corresponding to the region outlined in B. Residues mentioned in the text are displayed as sticks. VP1 Gln57 and Glu52 form a hydrogen bond and a salt bridge with VP2 Ser44 and Lys52, respectively, and VP1 Ile20 and Val56 make up a hydrophobic cluster including VP2 Ile50; finally, VP1 Arg54 is hydrogen bonded with the VP2 main chain (Leu42).
Native HRV2 showed previously unreported interactions with RNA near the twofold axis mediated by the VP1 N terminus. Fitting of the native X-ray coordinates suggested that this region corresponds to the Asn21–Ser22 tract (Fig. 2D, blue arrow). The cross-section of interacting areas in these VP1-mediated contacts was larger than those mediated by VP2. As for the X-ray structure of native HRV2, the VP1 N-terminal extension is folded as a long arm that bends back over itself, resulting in roughly antiparallel strands (Fig. 3B); Asn21–Ser22 (which interact with RNA) and Glu52–Gln57 (which interact with the VP2 loop) are thus positioned almost in front of each other, forming a ternary network (Fig. 3).
discussion
Members of the genus EV, such as HRV, assemble from protein precursors into a robust (but dynamic) capsid stabilized by an internal network of interactions of the N-terminal extensions of the major capsid proteins VP1–VP3 (Fig. S1) (4). In addition to native virions, NECs are assembled from VP1, VP0 (the precursor of VP2 and VP4), and VP3. Their structural analysis has been valuable for understanding viral assembly and uncoating processes (3, 25).
Our cryo-EM data for native HRV2 indicate that the VP1 N-terminal segment is directly involved in interactions with RNA; the Asn21–Ser22 tract, positioned in front of the Glu52–Gln57 segment, interacts with the encapsidated RNA (Figs. 2D and 3A). These previously unidentified RNA–VP1 contacts (4) suggest that during HRV2 capsid assembly, the packaged RNA acts as a scaffold that promotes ordering of N-terminal extensions (not only for VP1) and increases virion stability. Whereas the VP2 Trp38-mediated interaction with RNA is conserved in all EVs analyzed so far (4, 17), interactions with VP1 (and other capsid proteins) vary in other EVs; for example, in EV71, VP1 Gln30 and VP3 Gln48 interact with nucleotides.
Our preparation of purified HRV2 contained almost 20% NEC, allowing their direct structural comparison with native virus. The major NEC fraction has identical antigenic makeup and receptor binding as full virions (5, 26) and the same size (Fig. S3). Nonetheless, the inner surfaces vary, and difference mapping showed clear conformational disparities (Fig. 3). Whereas VP1 N-terminal sequences (residues Leu15–Thr61) and the VP2 loop Ala47–Pro53 are well ordered in the native virion, both are disordered in NEC; this leads to an overall loss of contacts and suggests that the protein network is maintained only in the presence of the RNA. The X-ray structure of empty PV shows similar disorder in capsid-internal regions (27). The disorder of the VP1 N-terminal segment and the VP2 loop is not associated with capsid expansion in HRV2 (there must be additional disordered segments in NEC capsid proteins not apparent in our analysis, including all of VP4). EV71 can expand spontaneously (17), indicating differences among EVs.
In contrast to the EV species A–D, the three HRV species are acid labile; after entry into late endosomes, HRV2 undergoes conformational modifications and gives rise to A-particles. These intermediates are expanded, lack VP4, and have channels that cross the protein shell and surface-exposed amphipathic VP1 N-terminal extensions. Extrusion of the genomic RNA into the host cytoplasm converts them into (empty) B-particles. Our X-ray and cryo-EM 3D structures of A-particles are probably a faithful representation of the uncoating intermediate generated during infection, as they were obtained by acidification, as occurs in the endosome, rather than by heating. Comparison of the X-ray structures shows that except for minor differences in the VP2 N-terminal region (residues 46–55) and in the VP3 F–G loop (residues 149–162), A- and B-particle shells are identical. This indicates that the presence of the RNA in the expanded particle induces neither positive nor negative strain. In the native virion, however, it participates in the maintenance of the N-terminal capsid protein network through interaction with the VP1 N-terminal segment and the VP2 loop (see Interactions Mediated by N-Terminal VP1 Sequences). This important structural role is observed in many other RNA viruses (28). The acid-triggered conversion into the A-particle not only breaks these connections, but also results in a major restructuring of the RNA; whereas its contacts with Trp38 are maintained, new contacts are established with the now-accessible β-tubes built of the five intertwined copies of VP3 at the fivefold axes. As a consequence, before its release into host cytosol, the RNA constitutes a well-ordered layer, closely associated with the capsid inner surface through interactions with VP2 and VP3 in the A-particle state. The recently described structure of the CAV16 A-particle (23) shows that RNA–protein contacts are roughly conserved at the two- and fivefold axes, although the interacting residues cannot be discerned precisely from these ∼6 Å X-ray maps (Fig. S7). In both structures, the gap between the RNA outer layer and the capsid inner surface is ∼15 Å.
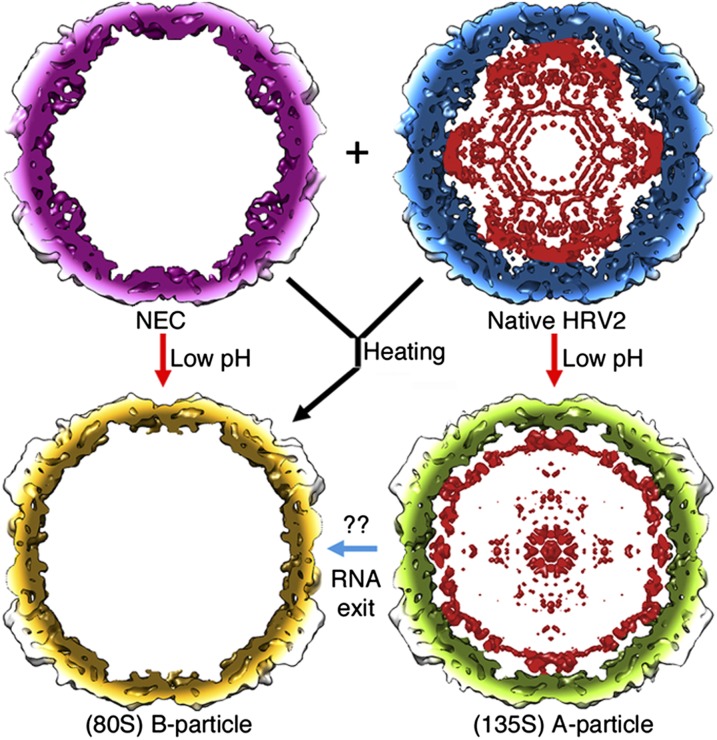
Conformational changes associated with HRV2 uncoating were described in detail from near-atomic resolution X-ray analysis of B-particles obtained by heating (16); the acid environment in late endosomes must trigger release of the pocket factor to allow the hinge movement in VP1. This movement induces the concerted displacement of VP2 and VP3, forcing reorganization of the protomer interfaces that lead to capsid expansion and irreversible formation of channels that act as gateways for externalization of protein segments (VP4 and VP1 N termini) and viral RNA (16). These conformational changes appear to be similar to those described for EV71 and PV (17, 29). We found that incubation of a mixture of ∼80% native HRV2 and 20% NEC at acid pH resulted in a similar ratio of (now expanded) full and empty particles (Fig. 1). The bimodal Gaussian distributions of RNA content of the individual particles were also similar (Fig. S4). This suggests that acidification transformed native HRV2 into the A-particle stage and NEC into B-particles. Conversion of A- (and native HRV2) into B-particles at low pH is thus very inefficient in vitro, indicating that low pH does not suffice for productive uncoating (18). This process might be catalyzed in vivo by insertion of the amphipathic exposed N-terminal VP1 helices into membranes and/or by a specific ionic milieu (30, 31) (Fig. 4).
Fig. 4.
Relationship between HRV2 expansion/uncoating and low pH/heat-induced changes. HRV2 capsids are represented as 25 Å slabs, colored as in Fig. 1, viewed along an icosahedral twofold axis. Virions were purified as a mixture of empty (NEC) and full (native HRV2) particles; their simultaneous coexistence indicates that ordering of the VP1 N-terminal segment is promoted by ordered RNA segments that participate in the internal network. RNA helps to stabilize the triple RNA–VP1–VP2 sandwich, in which RNA and the VP2 segment (residues ∼40–50) form a clamp around the VP1 N terminus. When the pH is lowered to 5.0 for 15 min (red arrows), NECs expand to the B-particle conformation and the native HRV2 to A-particles; direct conversion of native HRV2 into B-particles (or through the A-particle stage) is minor. Major morphological changes include reduction in capsid wall thickness, pore formation, and VP4 and VP1 N terminus externalization. These conformational changes indicate that capsid expansion depends on low pH and that the genome has no role in this process. After heating to 56 °C for 10–12 min (black arrow), NEC and native HRV2 are transformed into B-particles (16). RNA exit [i.e., conversion of A- into B-particles (blue arrow)] might require lipid bilayers and/or specific ion gradients; indicated as “??”).
These and previous findings (32) imply that heating and acidification should no longer be considered equivalent triggers for HRV2 uncoating (Fig. 4); whereas heating of native HRV2 results in B-particles (expansion plus uncoating), simple acidification gives rise to A-particles (expansion only). Our present structural data also strongly suggest that the transition of the VP2 segment (including residues ∼40–50) from ordered to disordered conformation is implicated in expansion and/or uncoating. The VP2 segment participates in 60 triple RNA–VP1–VP2 interactions critical for the correct positioning of the VP1 N terminus in the native HRV2 capsid, relieving stress on the VP1 N terminus and allowing its externalization through one of the newly formed pores. Externalization of VP4 and the VP1 N terminus, together with capsid expansion, implies more space and thus less strain on the RNA. The VP3-interacting regions might control the more relaxed state of the RNA molecule—for example, to avoid tangling and provide a 3D framework for ordered RNA exit. In this model, the contacts with the inner capsid surface would be dynamic, acting like rails that conduct the RNA strands toward the release channel.
Materials and Methods
Production of Native Virus and Subviral Particles.
HRV2 was produced and purified as described (33), and the virus pellet was dispersed in 50 mM Na borate buffer (pH 7.4). To generate A-particles, HRV2 (5–7 mg/mL) in the same buffer was brought to pH 5 with 1.4 vol 50 mM Na acetate, pH 5 [15 min, room temperature (RT)], reneutralized with 1.2 vol 100 mM Na borate buffer, pH 8.3, and incubated (1 h, RT).
Cryo-EM.
Samples (4 μL) were applied to Quantifoil glow-discharged grids in a humidified chamber, blotted, and plunged into liquid ethane following standard procedures (34). Micrographs were recorded in low-dose conditions (∼20 e−/Å2) in a FEI Tecnai F30 Polara cryo–electron microscope (300 kV) with C2 and objective aperture of 70 and 100 μm. Images were acquired between 0.8 and 3.5 μm underfocus with a Gatan Ultrascan 4000 4k CCD camera, using Leginon automatic image acquisition software (35). CCD frames were recorded at detector magnification of 79,372x (1.89 Å/pixel sampling rate).
Image Processing.
Images were processed using Xmipp (36). Automatic particle picking was used to select individual images for NEC, native HRV2, and A- and B-particles. Defocus was determined with CTFfind (37). Particle origin and orientation determined with Xmipp via iterative projection matching using the HRV2 X-ray structure (4) low pass-filtered to 30 Å as the starting map. For the slightly larger A-particles, we used a size-scaled low-resolution HRV2 map. Resolution was assessed by FSC between independent half-dataset maps, applying a correlation limit of 0.5. After the independent refinements, 2,223, 7,946, 15,170, and 2,976 particles were included in the respective NEC, native HRV2, and A- and B-particle 3DRs. Amplitude decay was calculated using spatial frequency components from cryo-EM maps and the HRV2 X-ray map (PDB ID code 1FPN) for NEC, native HRV2, and the 80S HRV2 X-ray map (PDB ID code 3TN9) for A- and B-particles. The decay profile of the cryo-EM maps was adjusted to match the profile of the X-ray maps, the fitted function was applied to the cryo-EM maps in the frequency range from 230 Å to the maximum resolution achieved, and a soft low-pass filter was applied.
For difference map calculations, spherically averaged radial density profiles were calculated, normalized, and scaled to match the fit between the profiles of cryo-EM maps and atomic models. The two 3DRs were subtracted and rendered at 3 σ above the mean density; at that contour level, the difference density is rendered similarly on the parental 3D model. Statistical significance of the difference densities was evaluated by measurement of the average density centered on designated spots in the two (raw) 3D maps used in the subtraction. These spots corresponded to the major differences shown in Fig. 3A (densities in blue). Average densities computed from cubes (with a side dimension of 7.6–11.4 Å) are referred to as an estimation of occupancy relative to capsid density (in which maximum occupancy is given).
Crystallization and Data Collection.
Crystals were obtained by the vapor diffusion method in hanging drops at 20 °C by mixing equal volumes of 135S particles (2 mg/mL) and a reservoir solution containing 0.5–0.7 M Na acetate in Na phosphate buffer with pH ranging from 7.5 to 9.0, containing 5% glycerol (vol/vol). Crystals were transferred to a cryoprotective solution with 20% (vol/vol) glycerol in the crystallization buffer, and flash-cooled in liquid nitrogen. A dataset up to 6.5 Å resolution was collected using synchrotron radiation at the swiss light source (SLS), Villigen, Switzerland (beamline PXI) using a Pilatus 6M detector. Diffraction images were processed with XDS and internally scaled with SCALA (Table S1).
Structure Determination and Refinement.
Crystals of HRV2 135S particles belong to space group I222 and have unit cell parameters of a = 311.9 Å, b = 357.8 Å, c = 386.7 Å. The crystal asymmetric unit has 15 protomers. Initial phases were obtained after a rigid body refinement using the coordinates of the HRV2 80S particle structure (PDB ID code 3TN9) with Refmac5 (38). After 10 cycles of rigid body refinement, the R factor was 36% for data in the 121–6.5 Å resolution shell. Cycles of 15-fold noncrystallographic averaging and solvent flattening with the DM program (39) were used to improve map quality. Averaging and solvent masks were created using NcsMask around an expanded model of the 80S capsid to cover the region where the RNA contacts the viral shell. Model rebuilding was initiated using Coot (40), followed by refinement with Refmac5 using data in the resolution shell 121–6.5 Å. Graphics and movies were produced using University of California–San Francisco Chimera and Pymol (41).
Structural Analysis.
Uro fitting (42) was carried out on entire NEC, native HRV2, and A- and B-particle cryo-EM maps with X-ray structures of native HRV2 (4) and the B-particle (16) asymmetric units. VP1, VP2, and VP3 were initially treated as a single rigid body and the initial docking coordinates were refined, treating each subunit as an independent rigid body. As VP4 is near VP1 in the native HRV2 capsid, VP1 movements during docking were imposed on VP4. Correlation coefficients between the native HRV2 X-ray map and the cryo-EM density map were 83.4% and 89.1% for native HRV2 and NEC, respectively. Similar calculations for the B-particle X-ray map and the cryo-EM density maps of A- and B-particle capsids gave correlation coefficients of 91.6% and 94.6%.
Supplementary Material
Acknowledgments
We thank I. Gösler for preparing the virus, T. Marlovits for advice, G. Resch for help with the Polara microscope, H. Kowalski for critical reading of the manuscript and helpful comments, and C. Mark for editorial assistance. D.B. and A.P.-H. are particularly thankful to S. Hafenstein and R. S. Sinkovic for help and advice in the early phase of the project, and K. Djinovic-Carugo in the initial crystallization trials. Part of the data processing was performed on the Vienna Scientific Cluster (Project 70172 to D.B.), and X-ray data were obtained at the SLS (beamline PX1) Villigen, Switzerland (BioStruct Project 2669). L.V.-A. is the recipient of a Formació de Investigadors (FI) fellowship from the Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR). This work was supported by grants from the Spanish Ministry of Economy and Competitivity (BIO2011-24333 to N.V. and BFU2011-25902 to J.R.C.), the National Institutes of Health Intramural Research Program and the Center for Information Technology (to B.L.T.), and the Austrian Science Foundation Project 18693-B09. Short stays in Madrid were made possible by funding through “Acciones Integradas” (ES 03/2010 to D.B. and AT2009-0041 to N.V.) awarded by the Austrian Exchange Service [Östereichischer Austauschdienst (OEAD)].
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The 3D reconstructions have been deposited in the Electron Microscopy Data Bank, www.ebi.ac.uk/pdbe/emdb [accession nos. EMD-2106 (native empty capsids), EMD-2107 (native human rhinovirus 2; HRV2), EMD-2109 (A-particle), and EMD-2108 (B-particle)]. The X-ray coordinates and structure factors for the HRV2 A-particles have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4L3B).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312128110/-/DCSupplemental.
References
- 1.Palmenberg AC, et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324(5923):55–59. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau SK, et al. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45(11):3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basavappa R, Gómez-Yafal A, Hogle JM. The poliovirus empty capsid specifically recognizes the poliovirus receptor and undergoes some, but not all, of the transitions associated with cell entry. J Virol. 1998;72(9):7551–7556. doi: 10.1128/jvi.72.9.7551-7556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdaguer N, Blaas D, Fita I. Structure of human rhinovirus serotype 2 (HRV2) J Mol Biol. 2000;300(5):1179–1194. doi: 10.1006/jmbi.2000.3943. [DOI] [PubMed] [Google Scholar]
- 5.Korant BD, Lonberg-Holm K, Noble J, Stasny JT. Naturally occurring and artificially produced components of three rhinoviruses. Virology. 1972;48(1):71–86. doi: 10.1016/0042-6822(72)90115-8. [DOI] [PubMed] [Google Scholar]
- 6.Ansardi DC, Morrow CD. Amino acid substitutions in the poliovirus maturation cleavage site affect assembly and result in accumulation of provirions. J Virol. 1995;69(3):1540–1547. doi: 10.1128/jvi.69.3.1540-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nugent CI, Johnson KL, Sarnow P, Kirkegaard K. Functional coupling between replication and packaging of poliovirus replicon RNA. J Virol. 1999;73(1):427–435. doi: 10.1128/jvi.73.1.427-435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lonberg-Holm K, Noble-Harvey J. Comparison of in vitro and cell-mediated alteration of a human Rhinovirus and its inhibition by sodium dodecyl sulfate. J Virol. 1973;12(4):819–826. doi: 10.1128/jvi.12.4.819-826.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer N, Prchla E, Schwab M, Blaas D, Fuchs R. Human rhinovirus HRV14 uncoats from early endosomes in the presence of bafilomycin. FEBS Lett. 1999;463(1-2):175–178. doi: 10.1016/s0014-5793(99)01610-5. [DOI] [PubMed] [Google Scholar]
- 10.Nurani G, Lindqvist B, Casasnovas JM. Receptor priming of major group human rhinoviruses for uncoating and entry at mild low-pH environments. J Virol. 2003;77(22):11985–11991. doi: 10.1128/JVI.77.22.11985-11991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neubauer C, Frasel L, Kuechler E, Blaas D. Mechanism of entry of human rhinovirus 2 into HeLa cells. Virology. 1987;158(1):255–258. doi: 10.1016/0042-6822(87)90264-9. [DOI] [PubMed] [Google Scholar]
- 12.Konecsni T, et al. Low pH-triggered beta-propeller switch of the low-density lipoprotein receptor assists rhinovirus infection. J Virol. 2009;83(21):10922–10930. doi: 10.1128/JVI.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fricks CE, Hogle JM. Cell-induced conformational change in poliovirus: Externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilek G, et al. Liposomal nanocontainers as models for viral infection: Monitoring viral genomic RNA transfer through lipid membranes. J Virol. 2011;85(16):8368–8375. doi: 10.1128/JVI.00329-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy HC, Bostina M, Filman DJ, Hogle JM. Catching a virus in the act of RNA release: A novel poliovirus uncoating intermediate characterized by cryo-electron microscopy. J Virol. 2010;84(9):4426–4441. doi: 10.1128/JVI.02393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garriga D, et al. Insights into minor group rhinovirus uncoating: The X-ray structure of the HRV2 empty capsid. PLoS Pathog. 2012;8(1):e1002473. doi: 10.1371/journal.ppat.1002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, et al. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol. 2012;19(4):424–429. doi: 10.1038/nsmb.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss VU, et al. Characterization of rhinovirus subviral A particles via capillary electrophoresis, electron microscopy and gas-phase electrophoretic mobility molecular analysis: Part I. Electrophoresis. 2012;33(12):1833–1841. doi: 10.1002/elps.201100647. [DOI] [PubMed] [Google Scholar]
- 19.Xing L, Casasnovas JM, Cheng RH. Structural analysis of human rhinovirus complexed with ICAM-1 reveals the dynamics of receptor-mediated virus uncoating. J Virol. 2003;77(11):6101–6107. doi: 10.1128/JVI.77.11.6101-6107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewat EA, Blaas D. Cryoelectron microscopy analysis of the structural changes associated with human rhinovirus type 14 uncoating. J Virol. 2004;78(6):2935–2942. doi: 10.1128/JVI.78.6.2935-2942.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belnap DM, et al. Molecular tectonic model of virus structural transitions: The putative cell entry states of poliovirus. J Virol. 2000;74(3):1342–1354. doi: 10.1128/jvi.74.3.1342-1354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J, et al. An externalized polypeptide partitions between two distinct sites on genome-released poliovirus particles. J Virol. 2011;85(19):9974–9983. doi: 10.1128/JVI.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren J, et al. Picornavirus uncoating intermediate captured in atomic detail. Nat Commun. 2013;4:1929. doi: 10.1038/ncomms2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadfield AT, et al. The refined structure of human rhinovirus 16 at 2.15 A resolution: Implications for the viral life cycle. Structure. 1997;5(3):427–441. doi: 10.1016/s0969-2126(97)00199-8. [DOI] [PubMed] [Google Scholar]
- 25.Curry S, et al. Dissecting the roles of VP0 cleavage and RNA packaging in picornavirus capsid stabilization: The structure of empty capsids of foot-and-mouth disease virus. J Virol. 1997;71(12):9743–9752. doi: 10.1128/jvi.71.12.9743-9752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korant BD, Lonberg-Holm K, Yin FH, Noble-Harvey J. Fractionation of biologically active and inactive populations of human rhinovirus type 2. Virology. 1975;63(2):384–394. doi: 10.1016/0042-6822(75)90311-6. [DOI] [PubMed] [Google Scholar]
- 27.Basavappa R, et al. Role and mechanism of the maturation cleavage of VP0 in poliovirus assembly: Structure of the empty capsid assembly intermediate at 2.9 A resolution. Protein Sci. 1994;3(10):1651–1669. doi: 10.1002/pro.5560031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneemann A. The structural and functional role of RNA in icosahedral virus assembly. Annu Rev Microbiol. 2006;60:51–67. doi: 10.1146/annurev.micro.60.080805.142304. [DOI] [PubMed] [Google Scholar]
- 29.Hogle JM. A 3D framework for understanding enterovirus 71. Nat Struct Mol Biol. 2012;19(4):367–368. doi: 10.1038/nsmb.2276. [DOI] [PubMed] [Google Scholar]
- 30.Bostina M, et al. Single particle cryoelectron tomography characterization of the structure and structural variability of poliovirus-receptor-membrane complex at 30 A resolution. J Struct Biol. 2007;160(2):200–210. doi: 10.1016/j.jsb.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubeck D, Filman DJ, Hogle JM. Cryo-electron microscopy reconstruction of a poliovirus-receptor-membrane complex. Nat Struct Mol Biol. 2005;12(7):615–618. doi: 10.1038/nsmb955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harutyunyan S, et al. Viral uncoating is directional: Exit of the genomic RNA in a common cold virus starts with the poly-(A) tail at the 3′-end. PLoS Pathog. 2013;9(4):e1003270. doi: 10.1371/journal.ppat.1003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann E, Moser R, Snyers L, Blaas D, Hewat EA. A cellular receptor of human rhinovirus type 2, the very-low-density lipoprotein receptor, binds to two neighboring proteins of the viral capsid. J Virol. 2003;77(15):8504–8511. doi: 10.1128/JVI.77.15.8504-8511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luque D, et al. Infectious bursal disease virus capsid assembly and maturation by structural rearrangements of a transient molecular switch. J Virol. 2007;81(13):6869–6878. doi: 10.1128/JVI.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carragher B, et al. Leginon: An automated system for acquisition of images from vitreous ice specimens. J Struct Biol. 2000;132(1):33–45. doi: 10.1006/jsbi.2000.4314. [DOI] [PubMed] [Google Scholar]
- 36.Scheres SH, Núñez-Ramírez R, Sorzano CO, Carazo JM, Marabini R. Image processing for electron microscopy single-particle analysis using XMIPP. Nat Protoc. 2008;3(6):977–990. doi: 10.1038/nprot.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol. 2003;142(3):334–347. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 38.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53(Pt 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 39.Cowtan K, Main P. Miscellaneous algorithms for density modification. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 4):487–493. doi: 10.1107/s0907444997011980. [DOI] [PubMed] [Google Scholar]
- 40.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 41.Pettersen EF, et al. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 42.Siebert X, Navaza J. UROX 2.0: An interactive tool for fitting atomic models into electron-microscopy reconstructions. Acta Crystallogr D Biol Crystallogr. 2009;65(Pt 7):651–658. doi: 10.1107/S0907444909008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin J, et al. Structure of the Fab-labeled breathing state of native poliovirus. J Virol. 2012;86(10):5959–5962. doi: 10.1128/JVI.05990-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.