Significance
Human adenoviruses encode Early region 3 (E3) proteins that manipulate the host immune response to establish an infection or to persist longer. To date, only a few E3 functions from a single adenovirus species (C) have been characterized, all of which act directly on infected cells. Here we describe a secreted E3 protein that is uniquely expressed by species D adenoviruses. This protein targets noninfected leukocytes using a cell surface phosphatase as a receptor. We provide evidence that this interaction suppresses leukocyte activation and effector functions, implying that species D adenoviruses can affect the host distant from the site of infection.
Keywords: subversion of leukocytes, E3 protein shedding, Ad19a/Ad64, epidemic keratoconjunctivitis
Abstract
The E3 transcription unit of human adenoviruses (Ads) encodes immunomodulatory proteins. Interestingly, the size and composition of the E3 region differs considerably among Ad species, suggesting that distinct sets of immunomodulatory E3 proteins may influence their interaction with the human host and the disease pattern. However, to date, only common immune evasion functions of species C E3 proteins have been described. Here we report on the immunomodulatory activity of a species D-specific E3 protein, E3/49K. Unlike all other E3 proteins that act on infected cells, E3/49K seems to target uninfected cells. Initially synthesized as an 80- to 100-kDa type I transmembrane protein, E3/49K is subsequently cleaved, with the large ectodomain (sec49K) secreted. We found that purified sec49K exhibits specific binding to lymphoid cell lines and all primary leukocytes, but not to fibroblasts or epithelial cells. Consistent with this binding profile and the molecular mass, the sec49K receptor was identified as the cell surface protein tyrosine phosphatase CD45. Antibody-blocking studies suggested that sec49K binds to the membrane proximal domains present in all CD45 isoforms. Functional studies showed that sec49K can suppress the activation and cytotoxicity of natural killer cells as well as the activation, signaling, and cytokine production of T cells. Thus, we have discovered an adenovirus protein that is actively secreted and describe immunomodulatory activities of an E3 protein uniquely expressed by a single Ad species.
Human adenoviruses (Ads) comprise more than 60 different types classified into seven species, designated A–G (1, 2). Ads can establish acute as well as persistent infections (3). Ad-induced diseases are generally mild or even subclinical in immunocompetent individuals, but tend to be severe or even fatal in immunocompromised patients, demonstrating the significant impact of the immune system on Ad disease. Generally, disease patterns differ among Ad species. Interestingly, some Ads of species D, including Ad8, Ad37, Ad19a [recently renamed Ad64 (2)], and the more recently sequenced Ad53, Ad54, and Ad56 (2, 4, 5), cause a distinct disease, epidemic keratoconjunctivitis (EKC) (6). Surprisingly, this specific pathogenesis in the eye contrasts with the rather ubiquitous receptor profile of EKC-causing Ads (7, 8), and thus is likely linked to selective events occurring postattachment, such as differential triggering of innate and adaptive immune responses (3, 9, 10). Thus, differential immunomodulatory functions encoded in early transcription unit 3 (E3) may play an important role in disease (9, 11). However, an immunomodulatory E3 function of species D Ads has yet to be described. Further insight into such functions also would be important for the application of species D Ads as vectors for vaccination and gene therapy in humans, given that these have a number of favorable features (12).
Previous studies of species C E3 proteins in vitro and in vivo revealed several molecular mechanisms by which Ads can evade recognition and elimination by the host immune system. For example, E3/19K retains MHC class I molecules and MHC class I-related chain A and B in the endoplasmic reticulum of infected cells, thereby suppressing recognition by cytotoxic T lymphocytes (13–15) and activation of natural killer (NK) cells (16, 17). Other common E3 proteins (E3/10.4K–14.5K) down-regulate various apoptosis receptors from the cell surface or affect TNF-α–induced signaling (9, 18, 19). All of these common/species C E3 proteins target functions of infected cells. Interestingly, the coding capacity of the E3 region exhibits substantial species-specific variations, and some E3 genes seem to be unique to a particular species (3, 11). Overall, E3 represents one of the most divergent regions of Ads (20, 21). Although some E3 proteins of species B, D, and E have been characterized biochemically (3, 22–24), no immunomodulatory function has been assigned to E3 proteins of Ads other than species C or to species-specific E3 proteins.
We previously identified a gene in the E3 region of the EKC-causing Ad19a/Ad64, designated E3/49K, that is absent in Ads of other species (25). The sequence of the extracellular/luminal domain suggested three internal repeats, named conserved region 1–3 (CR1–3), which may form an Ig-like fold. The corresponding protein was expressed by all species D Ads tested (26) and thus may be implicated in their pathogenesis. E3/49K is a highly glycosylated type I transmembrane protein migrating with an apparent molecular weight (mr) of 80–100 kDa and as such is by far the largest E3 protein. It is localized in the Golgi/trans-Golgi network, in early endosomes, and in the late phase of infection in lysosomes as well (24).
Here we demonstrate that E3/49K is cleaved, and that its large ectodomain is secreted. This is a unique processing pathway for E3 proteins and to date E3/49K represents the only actively secreted or shed Ad protein known. Rather than infected cells, secreted E3/49K targets selectively noninfected leukocytes via binding to the protein phosphatase CD45, and can suppress functions of both NK cells and T cells.
Results
Proteolytic Processing and Secretion of E3/49K.
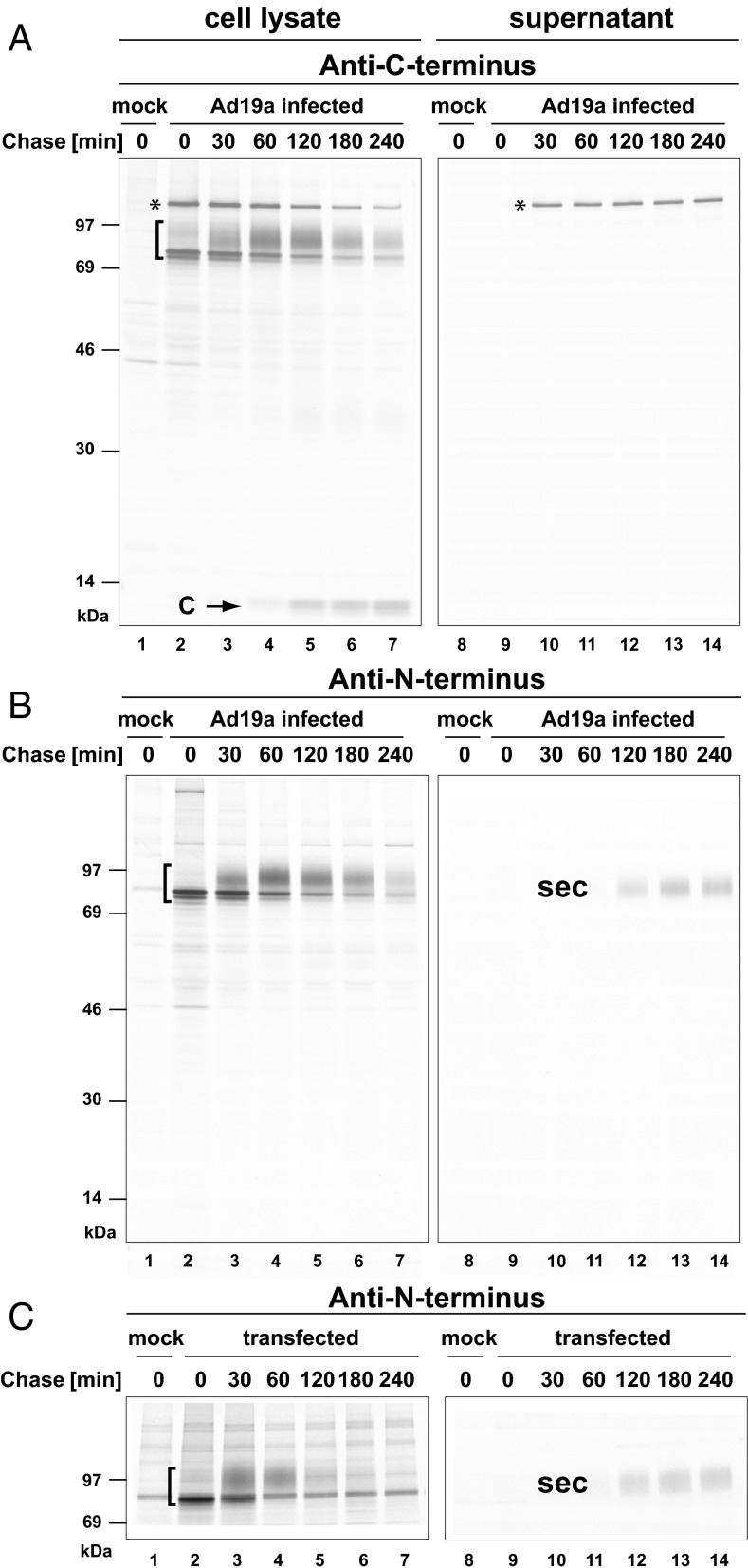
While monitoring the processing of E3/49K, we previously noted the emergence of 10- to 13-kDa C-terminal fragments (24). Whether the protein is further degraded or the putative large N-terminal cleavage fragment is secreted remained unclear, however. To address this question, we raised a rabbit antiserum, R48, against the bacterially expressed, refolded N-terminal (ecto)domain of E3/49K (Fig. S1). In pulse chase experiments, this antiserum detected similar high molecular weight species of E3/49K as the antiserum against the C terminus of E3/49K (compare the bracketed areas in the left panels of Fig. 1 A and B), but not the low molecular weight fragments (C; 10–13 kDa) (see Fig. 1 A and C). This finding confirms the previous hypothesis that E3/49K is cleaved into a large N-terminal fragment of ∼90 kDa and 10- to 13-kDa fragments derived from the C terminus.
Fig. 1.
The Ad19a E3/49K protein is proteolytically processed and secreted from Ad19a infected cells and cells stably expressing E3/49K. A549 cells infected with Ad19a for 16 h (A and B) and A549 cells stably expressing E3/49K (C) were metabolically labeled for 30 min with 200 µCi/mL [35S]-methionine and chased with medium containing nonradioactive methionine for the indicated times. Subsequently, E3/49K was immunoprecipitated from cell lysates and supernatants with rabbit antibodies R25050 and R48 directed against the C terminus (A) and N terminus, respectively (B and C). The ∼120-kDa protein species (asterisk) coprecipitating unspecifically at late stages of infection using the C-terminal specific serum corresponds to the abundant hexon protein. It was previously shown that it is not visible in the early phase of infection or in transfected cells (24).
Strikingly, when the supernatant was probed with R48 directed to the N-terminal domain, 49K-specific material was visualized after 120 min of chase, demonstrating that the N-terminal fragment is indeed secreted (Fig. 1B, lanes 11–14; sec). Proteolytic processing and secretion also were observed in 49K-transfected cells (Fig. 1C), demonstrating that proteolytic processing of E3/49K is independent of viral infection and can be executed by cellular proteases.
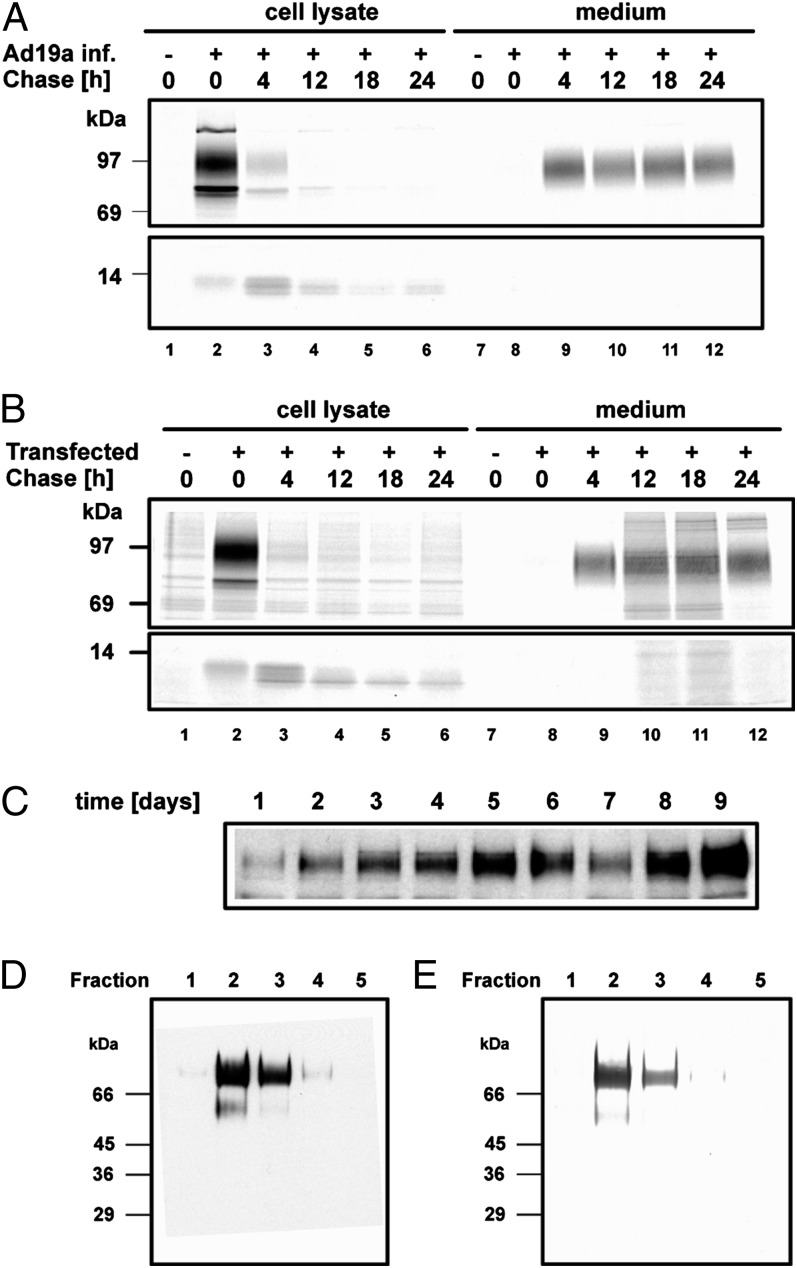
Several lines of evidence suggest that the secreted E3/49K (sec49K) is very stable. First, sec49K was detectable in pulse-chase experiments using extended chase times of up to 24 h with both infected and transfected cells (Fig. 2 A and B). Second, sec49K accumulated in the supernatant of transfected cells over a period of 9 d, as shown by Western blot analysis (Fig. 2C). Third, functional active protein (see below) could be detected in sterile supernatants even after years of storage at 4 °C. This high stability of sec49K is consistent with a potentially important function in the extracellular environment.
Fig. 2.
Secreted Ad19a E3/49K protein has a long half-life in the supernatant of Ad19a-infected cells and cells stably expressing E3/49K, and can be purified to homogeneity. (A and B) A549 cells infected with Ad19a for 9 h (A) and A549 cells stably expressing E3/49K (B) were metabolically labeled for 1 h with [35S]-methionine and then chased with medium containing nonradioactive methionine for the indicated times. E3/49K was precipitated from lysates using the antiserum directed to the C terminus, whereas sec49K was precipitated with serum R48 against the N-terminal domain. (C) Supernatants of A549 cells stably transfected with the E3/49K gene were collected over a period of 1–9 d after placement in medium without FCS. Cellular debris was removed by centrifugation, and 15 µL of supernatant was directly analyzed by Western blot analysis using antiserum R48. (D and E) Sec49K was purified using affinity chromatography with mAb 4D1 coupled to protein G Sepharose beads. Sec49K was eluted from the columns collecting 500-µL fractions. Then 15 µL of each fraction was analyzed by SDS-PAGE, followed by Western blot analysis with antiserum R48 (D) or silver staining (E).
Monoclonal Antibodies Recognizing the E3/49K Ectodomain and Purification of sec49K.
To enable purification of sec49K and its detailed functional analysis, we generated mAbs against the E3/49K ectodomain by immunizing rats with a purified and refolded N-terminal fragment of E3/49K (Fig. S1 A and B). Two mAbs, 4D1 and 1E6, were selected for further study. The use of mAb 4D1 in FACS analysis clearly showed that 49K is present not only in the endoplasmic reticulum, the Golgi apparatus, and endosomes (24), but also on the cell surface of infected cells (12) and transfected cells.
To purify sec49K for functional studies, we coupled mAb 4D1 to protein G- or N-hydroxysuccinimide (NHS)-Sepharose columns. As a reliable, noninfectious source for sec49K, we used culture supernatant from E3/49K-expressing cell clones derived from A549 cells (24) and 293 cells. Supernatant from these cells was applied to the 4D1 affinity column, and bound sec49K was eluted. Western blot analysis of eluted fractions using rabbit antiserum R48 showed that the majority of sec49K migrated as a major species of 78–90 kDa and usually a minor species of 50–60 kDa (Fig. 2D) that may represent a degradation product. Remarkably, silver staining of the same fractions (Fig. 2E) revealed only protein species of identical molecular weight as those detected by 49K-specific Western blot analysis (Fig. 2D); thus, we concluded that sec49K was apparently purified to homogeneity and so could be used for functional tests. In similar way, A549 supernatant was mock-purified as a negative control. To exclude the possibility that the purification scheme might have affected functional activity, we also used crude or concentrated supernatant in some experiments.
Sec49K Binds Selectively to Lymphoid Cell Lines and All Primary Leukocytes.
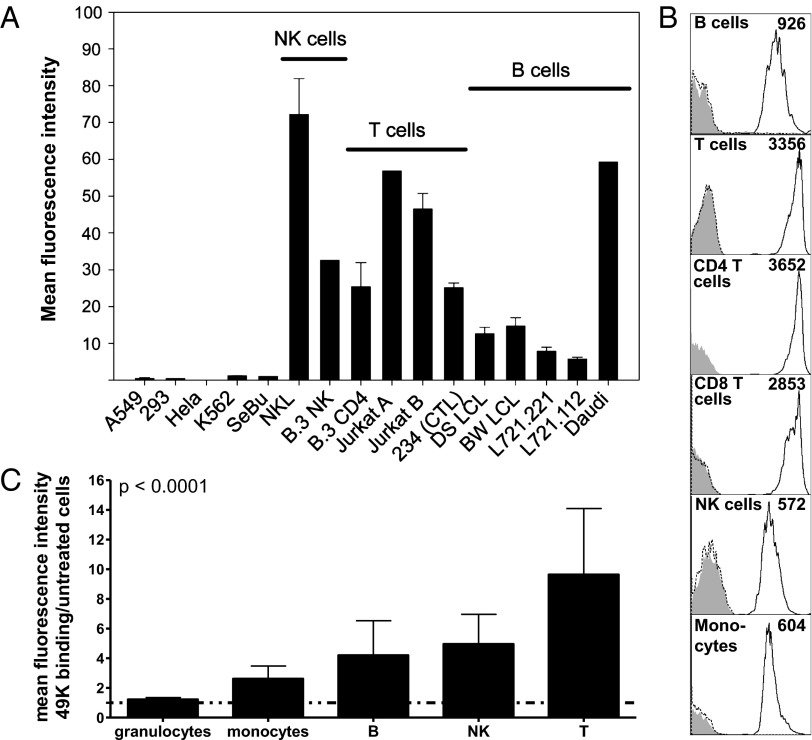
Considering that most E3 proteins are involved in immune evasion and E3/49K is secreted, we hypothesized that unlike all other E3 gene products characterized to date that directly modulate functions of infected cells, sec49K may target surrounding cells, most likely cells associated with the immune system. This hypothesis is supported by the presence of an unusual putative Ig domain in E3/49K (24, 27). Thus, we tested a panel of lymphoid and nonlymphoid cell lines for their capacity to bind purified sec49K. Sec49K binding was observed for all human lymphoid cell lines tested, irrespective of whether they were derived from NK cells (NKL, B.3NK; NK-92C1), T lymphocytes [Jurkat, B.3CD4, 234 (CTL)], or B lymphocytes (Daudi) or represented other types of EBV-transformed lymphoblastoid cell lines (L721.221, L721.112, BW LCL, DS LCL; T2) (Fig. 3A). In contrast, no significant binding was detected for human cell lines derived from various other tissues, including A549 (lung epithelial carcinoma), 293 (embryonal kidney cell line), HeLa (cervix carcinoma), K562 (erythroleukemia), and SeBu (primary human fibroblasts) (19, 24). Thus, sec49K seems to selectively bind to lymphoid cell lines.
Fig. 3.
Secreted E3/49K binds specifically to lymphocyte-derived cell lines and primary lymphocytes. (A) Binding of sec49K to different human cell lines and primary fibroblasts (SeBu) of lymphoid or nonlymphoid origin was determined by incubating ∼200 ng of purified sec49K with cells for 1 h at 4 °C, followed by incubation with rat mAb 4D1 and FITC-labeled anti-rat IgG. Data are mean ± SEM compiled from three independent experiments except for B.3NK, Jurkat E6.1, and Daudi (one experiment each). (B) Isolated PBMCs were incubated with purified sec49K (black line), mock-purified material from untransfected A549 cells (dotted line), or medium alone (gray shading) for 1 h on ice. After washing, cells were stained with rat mAb 4D1 against E3/49K, followed by PE-conjugated goat anti-rat IgG and FITC- or Cy-chrome–conjugated mAbs against various lymphocyte subsets indicated (CD19 for B cells, CD3+CD4/8 for T cells, CD16/56 for NK cells, CD14 for monocytes). The mean value of 49K-specific fluorescence of the lymphocyte subpopulation is indicated. The data are for one representative experiment out of four experiments. (C) Sec49K and BSA (control) were added to heparinized blood for 1 h. Sec49K binding to lymphocyte subpopulations was detected by costaining with lymphocyte/monocyte-specific markers or gating on granulocytes, respectively. Sec49K binding is depicted as the ratio of mean fluorescence intensities of 49K-specific mAb 4D1 in the presence and absence of sec49K. Data are derived from the analysis of blood from five different donors (mean ± SD). Statistical analysis of sec49K binding between different leukocyte subpopulations was performed by repeated-measures ANOVA. Comparison of the mean fluorescent intensities in the presence or absence of 49K on each individual subpopulation by the two-tailed paired t test indicates that the difference in staining is statistically significant for all leukocyte subpopulations (granulocytes, P = 0.019; monocytes, P = 0.002; B cells, P = 0.028; NK cells, P = 0.009; T cells, P = 0.008).
To ensure that this binding pattern is not an artifact of transformation of the cells, we tested primary human peripheral blood mononuclear cells (PBMCs) for 49K binding activity (Fig. 3B). As shown in the figure, significant 49K binding was detectable only after previous incubation with purified sec49K (black line), but not with mock-purified material from A549 (dotted line) or medium alone (gray histogram). Most importantly, sec49K bound to all leukocyte populations examined, albeit with an apparent hierarchy. Staining was strongest for T cells, followed by B cells, NK cells, and then monocytes. Separate analysis of CD4 and CD8 T-cell populations revealed greater 49K binding for CD4 T cells compared with CD8 T cells. This binding pattern was reproduced by direct incubation of sec49K and control with whole blood, with sec49K demonstrating significantly different binding to the various leukocyte subpopulations (P < 0.0001) (Fig. 3C). Moreover, whole-blood analysis provided evidence that sec49K binds to granulocytes as well, but to the weakest degree of all leukocyte subpopulations (Fig. 3C). Taken together, these data suggest the presence of a common receptor for sec49K that seems differentially expressed on all leukocytes.
E3/49K Coprecipitates High Molecular Weight Proteins in the Jurkat T-Cell Line.
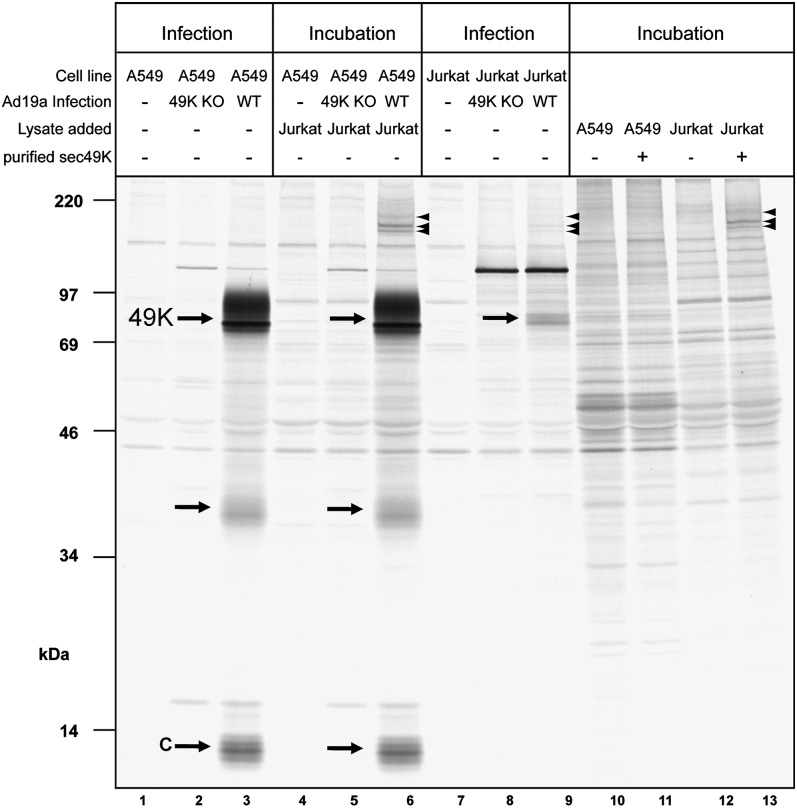
To identify proteins interacting with sec49K in lymphocytes, we exposed various sources of 49K to lysates from Jurkat T cells expressing the sec49K receptor (Fig. 3A), to possibly allow coprecipitation. Lysate of A549 cells that did not bind sec49K in the FACS assay served as a negative control. In our first approach, A549 and Jurkat cells were mock-infected or infected with WT Ad19a or the 49K-deficient Ad19a virus (49K KO) (12) and then labeled with [35S]-methionine. As expected, E3/49K was precipitated only from lysates of A549 and Jurkat cells after infection with WT Ad19a (Fig. 4, WT; compare lanes 1–3 and 7–9). Close inspection revealed three apparently specific coprecipitating protein species of 172, 181, and 196 kDa (arrowheads) in 49K receptor-positive Jurkat cells on infection with WT Ad19a (Fig. 4, lane 9), but not in mock-infected cells (−) or 49K KO infected cells.
Fig. 4.
Ad19a E3/49K interacts with high molecular weight protein species in the T lymphocyte cell line Jurkat, but not in the epithelial cell line A549. Then 2 × 106 A549 (lanes 1–6) or 4 × 106 Jurkat cells (lanes 7–9) cells were mock-infected (−) or infected at a multiplicity of infection of 10 with Ad19a (WT) or an Ad19a mutant (49K KO), in which 49K expression was specifically abolished by inducing a frameshift (12). Cells were labeled 6 h and 18 h postinfection respectively, for 1.5 h and then lysed. In some cases (lanes 4–6), 0.5 mL of Jurkat lysate was added to 0.5 mL of A549 lysates as indicated, whereas in other samples (lanes 1–3 and 7–9), 0.5 mL of lysis buffer was added instead. For coprecipitation with purified sec49K, 600 ng of purified sec49K was added, followed by incubation for 1 h at 4 °C before immunoprecipitation. Immunoprecipitation was performed with antibodies directed against the C terminus (lanes 1–9) or N terminus (lanes 10–13). Arrows indicate full-length E3/49K (49K) and E3/49K-derived species, and arrowheads indicate the three protein species specifically binding to E3/49K in Jurkat cell lysates.
Consistent with the low efficiency of Ad infection in lymphocytes (28) E3/49K was poorly expressed in Jurkat cells (Fig. 4, compare lanes 3 and 9). Thus, we took two other approaches to enhance coprecipitation of potential E3/49K interaction partners. First, lysates of WT Ad19a-infected A549 cells (containing high amounts of E3/49K; see lane 3 or lane 6) or mock-infected A549 cells were mixed with metabolically labeled Jurkat cell lysates containing the putative receptor (Fig. 4, lanes 4–6). Second, purified sec49K was added to lysates of mock-infected Jurkat cells, or A549 cells as a control (Fig. 4, lanes 11 and 13). Strikingly, in the presence of receptor-positive Jurkat cell lysates, protein species with the same high apparent molecular mass (mr) as seen on infection of Jurkat cells were specifically coprecipitated with E3/49K (Fig. 4, lanes 6 and 13). Corresponding bands were not detected in the absence of E3/49K (lanes 4 and 5 or lanes 10 and 12) or in receptor-negative A549 cell lysates on the addition of purified sec49K (Fig. 4, lane 11). We conclude that E3/49K specifically interacts with high molecular weight proteins in sec49K receptor-positive Jurkat cells.
Identification of the Leukocyte Common Antigen CD45 as the Cellular E3/49K Receptor.
Based on the binding profile of sec49K and the mr of the sec49K receptor, we hypothesized that the receptor might be the leukocyte common antigen CD45 or a leukocyte-specific integrin (CD11a/CD18). Analysis of CD18− Jurkat and SKW leukemia cells revealed no significant effect on sec49K binding (Fig. S2). In contrast, J45.01 Jurkat cells that had lost most CD45 expression after negative selection (29) exhibited only minimal binding of purified sec49K, providing the first evidence that CD45 might be the sec49K receptor.
To verify these findings, we tested the independently derived Jurkat cell line J-AS-1 (30), which lacked all detectable CD45 isoforms on expression of an antisense construct (Fig. 5A, Upper). Indeed, these cells were unable to bind sec49K, whereas retransfectants of J-AS-1 expressing either the CD45RO or CD45RABC isoform (Fig. 5A, Upper) regained 49K-binding capacity (Fig. 5A, Lower). This confirmed CD45 as the sec49K receptor. Further confirmation was obtained with HPB-acute lymphoblastic leukemia (HPB-ALL) cells for which CD45-negative variants were sorted and retransfected with certain CD45 isoforms. Again a strict correlation between CD45 expression and sec49K binding was observed (Fig. S3). Moreover, although the expression levels of the CD45RO and CD45RABC isoforms varied substantially, both isoforms clearly have the capacity to bind sec49K.
Fig. 5.
Sec49K binds to the cell surface phosphatase CD45. (A) Jurkat and its CD45− derivative J-AS-1, as well as two J-AS-1 transfectants expressing the CD45R0 and CD45RABC isoform, respectively, were incubated with medium (Upper) or sec49K (Lower). After washing, cells were stained for CD45 using mAb HI30 (black histograms) followed by Alexa Fluor 488-labeled goat anti-mouse IgG (Invitrogen). In parallel, cell-bound sec49K was detected by mAb 4D1, followed by Alexa Fluor 488-labeled goat anti-rat IgG (gray histograms). The background staining obtained with the secondary reagents only is shown in white. The results represent one of three similar experiments. (B) 293T cells were transiently transfected with expression constructs for CD45RO, CD45RABC, or control Ad2 E3 DNA as indicated on the right. At 40 h later, cells were incubated with either BSA or sec49K, after which CD45 expression and sec49K binding were monitored using single staining (α49K; 4D1) or double staining with Tricolor-labeled anti-CD45 mAb HI30 (α49K+CD45) and mAb 4D1. The data represent one of three similar experiments. (C) Ad19a E3/49K coimmunoprecipitates CD45 expressed in Jurkat cells. Jurkat cell lysates (J) were incubated with cell lysates of 293 cells (293) and 293 cells stably expressing E3/49K (K35; 0.5 mg each). Immunoprecipitations (IP) were performed using 1 μg of mAb MEM28 recognizing CD45 (CD45) or 4 μg mAb 4D.1 recognizing E3/49K (49K). The precipitated proteins were evaluated by Western blot analysis (WB) with the same antibodies. The data are from one of two representative experiments.
To unequivocally demonstrate that CD45 alone is sufficient for sec49K binding, we transiently transfected 293T cells that otherwise showed no binding activity for sec49K (Fig. 5B, control) with cDNA expression vectors encoding CD45RO and CD45RABC isoforms (31) and then performed binding tests with sec49K. As shown in Fig. 5B (Middle and Bottom), after transfection and incubation with sec49K, but not with control BSA, a large proportion of 293T cells comparable to those expressing CD45RO and CD45RABC (column 2) stained for E3/49K (column 3). Moreover, dual-color FACS staining with anti-E3/49K and CD45PE-Cy5 (α49K+CD45) clearly demonstrated that only CD45+ cells bound E3/49K (diagonal population in Fig. 5B, Right). Sec49K binding also was quantitatively correlated with CD45 expression, with cells with higher CD45 expression binding more sec49K.
Evidence that CD45 and E3/49K Interact Physically.
To visualize a physical interaction between CD45 and E3/49K, we mixed lysates of CD45+ Jurkat cells with lysates of the E3/49K+ cell line 293K35 (K35) and either immunoprecipitated (IP) CD45 and then assessed the presence of E3/49K in the precipitate by Western blot analysis using E3/49K-specific Abs (Fig. 5C, lanes 1 and 2), or immunoprecipitated E3/49K and then used CD45-specific Abs for Western blot analysis (Fig. 5C, lanes 3 and 4). Coprecipitation of 49K was observed only when CD45 was precipitated from mixed lysates of Jurkat and 293K35 containing both CD45 and E3/49K, not when Jurkat lysates were mixed with lysates from E3/49K− 293 cells (Fig. 5C, compare lanes 1 and 2). Similarly, CD45 was detectable in immunoprecipitates of E3/49K only when Jurkat and K35 lysates were mixed (Fig. 5C, compare lanes 4 and 3). Thus, we conclude that E3/49K physically interacts with CD45.
E3/49K Interacts with the Membrane Proximal Domain of CD45 Present in All Isoforms.
We next investigated whether we could identify the domain of CD45 with which sec49K binds. For this, we incubated Jurkat cells with purified sec49K before FACS analysis with a panel of CD45-specific mAbs directed to different parts of CD45 (Fig. 6A). The pan-CD45–specific mAbs GAP8.3, HI30, MEM28, and AICD45.2 recognize all isoforms of CD45 and thus are likely to bind the membrane proximal cysteine-rich domain or the fibronectin type III (FNIII) domains that are common to all isoforms, whereas UCHL1 recognizes specifically the CD45RO isoform represented by amino acids N-terminal from the four common core domains. MEM56 recognizes CD45RA, and MEM55 and MEM143 recognize CD45RB-containing isoforms, including the longest isoform (Fig. 6B). Previous binding of purified sec49K, as demonstrated by corresponding 4D1 staining in each experiment, consistently affected all pan-CD45–specific antibodies (Fig. 6A). We found only a modest 15–20% inhibition for HI30 and MEM28, but GAP8.3 and AICD45.2 binding was compromised more severely, showing a consistent ∼30–50% reduction. In contrast, sec49K binding did not substantially inhibit the recognition of Abs directed to the more distal domains present in RO, RA, and RB isoforms. Rather, their binding actually increased (relative to the negative control mAb W6/32 directed to HLA), which is particularly obvious for mAb MEM55, indicating conformational changes on complex formation with sec49K. The results of these blocking studies are consistent with the foregoing transfection data and binding studies, indicating that sec49K binds to the membrane-proximal domains present in all CD45 isoforms.
Fig. 6.
(A) Previous incubation of CD45+ Jurkat cells with sec49K selectively reduces binding of pan-CD45–specific mAbs. Jurkat cells were incubated with purified sec49K or BSA for 1 h on ice. After washing, cells were stained with a panel of mAbs directed to various isoforms of CD45. As a negative control, the impact of sec49K on the binding of mAb W6/32 against HLA was measured. The mean binding ± SEM calculated from at least four independent experiments is depicted relative to the mean value obtained on incubation with BSA. Binding of sec49K was confirmed by parallel staining with mAb 4D1. (B) Schematic view of the structure of CD45 isoforms R0, RB, and RABC, adapted from (37).
Sec49K Inhibits NK Cell-Mediated Lysis and Expression of Activation Markers.
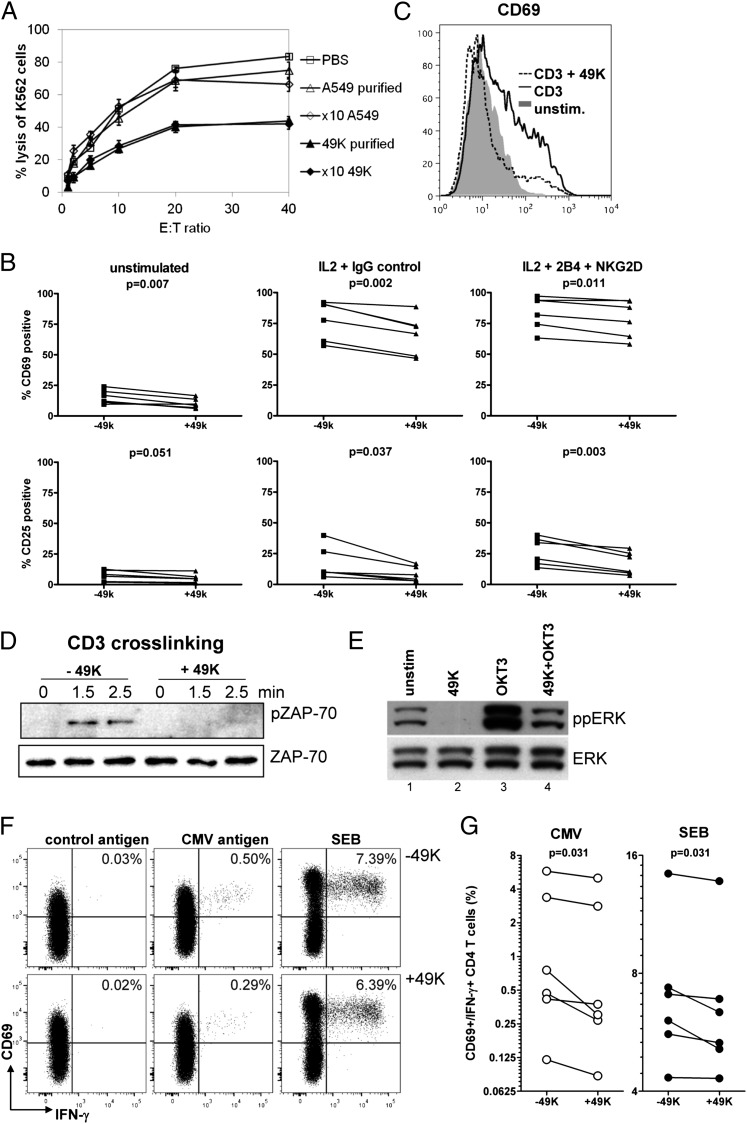
In the 49K-binding assays (Fig. 3A), the NK cell line NKL exhibited the highest mean fluorescence intensity values, suggesting high expression of the putative sec49K receptor. To test whether the binding of sec49K might interfere with NK cell functions, we measured NK cell-mediated lysis of the MHC class I-negative K562 cells in the presence and absence of purified sec49K (Fig. 7A). Sec49K inhibited NKL cell-mediated cytotoxicity over a range of effector-to-target (E:T) ratios compared with mock-purified material from A549 cells or PBS. A similar inhibition was seen when concentrated supernatant from 49K-expressing transfectants and A549 cells were compared, excluding the possibility that the inhibition is related to the purification process. Another NK cell line, NK-92, was also specifically affected by purified or concentrated sec49K, and a substantial, dose-dependent inhibition of cytotoxicity was noted with freshly isolated primary NK cells (Fig. S4). Interestingly, exclusive expression of E3/49K in its membrane-integrated form on Jurkat target cells (which are unable to cleave the molecule and do not produce sec49K) or on A549 cells had no effect on NK cell-mediated killing (Fig. S5).
Fig. 7.
Sec49K impairs activation, signaling, and functions of NK cells and T cells. (A) 51Cr- labeled K562 target cells were exposed to NKL cells at the indicated E:T ratios for 4 h in the presence of purified sec49K or 10-fold concentrated supernatant (×10 49K), along with mock-purified material from untransfected A549 cells (A549 purified), 10-fold concentrated supernatant (×10 A549), or PBS as a control. Data are mean ± SEM of triplicates. One of two similar experiments is shown. (B) Effect of sec49K on NK activation markers. Primary NK cells from five healthy donors were stimulated overnight with IL-2 or IL-2+anti-2B4+anti-NKG2D or left unstimulated, and the expression of the activation markers CD69 and CD25 was determined. Statistical analysis was assessed by a paired two-tailed t test; P values are indicated. (C) Sec49K also suppresses the up-regulation of CD69 in Jurkat T cells on CD3 cross-linking. A typical experiment out of two is shown. (D) Sec49K affects tyrosine phosphorylation of ZAP-70. Jurkat cells were preincubated with or without sec49K (+49K) and anti-CD3 mAb (OKT3) on ice before CD3 cross-linking was induced with goat anti-mouse IgG by incubation at 37 °C for the indicated times. ZAP-70 was immunoprecipitated and evaluated by Western blot analysis using antibodies against phosphorylated ZAP-70 (pZAP-70) or total ZAP-70. One of three similar experiments is depicted. (E) Jurkat T cells were incubated with anti-CD3 mAbs (OKT3, lanes 3 and 4) or mock- treated (lanes 1 and 2) without or with sec49K. After initiation of cross-linking by anti-IgG, total ERK (ERK) and tyrosine-phosphorylated ERK (ppERK) was detected by Western blot analysis (SI Materials and Methods). (F) PMBCs of a CMV seropositive control were preincubated for 30 min in the presence or absence of sec49K, and then stimulated with a whole CMV antigen lysate, a control lysate, or SEB. Numbers indicate the percentage of antigen-specific CD4 T cells that were quantified based on CD69 and IFN-γ induction. (G) Results from six CMV seropositive controls. The presence of 49K led to a significant reduction in the percentage of IFN-γ–positive CMV-specific CD4 T cells (by 22.5%; interquartile range, 12.6–47.1%) and SEB-reactive CD4 T cells (by 4.7%; interquartile range, 2.2–14.0%). Statistical analysis was performed using the two-tailed Wilcoxon matched-pairs test; P values are indicated.
We also examined the effect of sec49K on primary NK cells by monitoring the activation-induced expression of CD25 (IL-2 receptor α chain) and CD69 in the presence and absence of sec49K using different stimulation protocols. Sec49K significantly reduced the number of cells displaying these activation markers when stimulated with IL-2 alone (Fig. 7B, Center) or with IL-2 plus antibodies against the major activating NK receptor NKG2D and the coreceptor 2B4 (Fig. 7B, Right). A significant reduction in the immediate activation marker CD69 was noted even without stimulation. Taken together, these findings suggest that only the secreted form of E3/49K reduces NK cell activation and target cell killing, and thus has an immunomodulatory function.
Sec49K Suppresses CD69 Expression and Disturbs Signaling and Cytokine Production in T Lymphocytes.
To investigate potential mechanisms of 49K action on T cells, we stimulated Jurkat cells by CD3 cross-linking and monitored the expression of the activation marker CD69 and phosphorylation events. Similar to the effect seen in NK cells, sec49K inhibited up-regulation of the activation marker CD69 after CD3 stimulation of Jurkat T cells (Fig. 7C). Moreover, the addition of purified sec49K markedly suppressed or delayed phosphorylation of the TCR target protein ZAP-70 (Fig. 7D), as well as phosphorylation of the downstream target ERK-1/2 (Fig. 7E). In the absence of CD3 stimulation (Fig. 7E, lanes 1 and 2), sec49K prevented ERK phosphorylation, whereas triggering with anti-CD3 Abs (OKT3) in the presence of 49K (49K+OKT3) drastically reduced phosphorylation of ERK (Fig. 7E, lanes 3 and 4). These data demonstrate that sec49K can interfere with activating signaling pathways in NK and T cells and thereby possibly inhibit the function of these cells.
To investigate potential effects of sec49K on antigen-specific T-cell functions, PMBCs from CMV-seropositive controls were stimulated with a whole CMV antigen lysate or Staphylococcus aureus enterotoxin B (SEB), and CD69+ cytokine-producing CD4 T cells were analyzed by intracellular cytokine staining. As shown for a typical sample in Fig. 7F and by analysis of all samples in Fig. 7G, preincubation of PBMCs with sec49K led to a significant decrease in the percentage of both CMV- and SEB-reactive CD4 T cells producing IFN-γ. This also held true for CMV-specific CD4 T cells producing TNF-α, whereas only a trend toward a decrease was observed for TNF-α–positive CD4 T cells after SEB stimulation, and no effect was noted for T cells producing IL-2 (Fig. S6). Taken together, these findings indicate that binding of sec49K directly inhibits T-cell functionality, which is most pronounced for cells producing IFN-γ.
Discussion
In this study, we have uncovered a unique processing pathway and function for Ad E3 proteins. E3/49K of Ad19a, which is initially synthesized as a type I transmembrane protein, was cleaved, resulting in a ∼90-kDa soluble N-terminal fragment that is secreted. Apart from Ad proteins that are released on death of the infected cells [e.g., fiber (32)], this is to our knowledge the only adenovirus protein known to be actively secreted. Considering the size of the smaller nonglycosylated C-terminal fragments (24), cleavage may occur some 20- to 50-aa N terminal of the transmembrane domain (TMD), but the precise site and the compartment in which cleavage occurs remain unclear, although the timing and cell surface exposure suggest that it occurs on the cell surface or in an endosomal compartment.
The abundantly glycosylated, secreted form of E3/49K, sec49K, is highly stable in the supernatant of infected or transfected cells, which is compatible with a role for this protein in body fluids in vivo. Unlike the other E3 proteins described thus far that all act on the infected cell, E3/49K seems to be directed primarily at surrounding/uninfected cells. This implies that E3 proteins can have long-range effects very distant from the site of infection. We cannot rule out the possibility that the transmembrane form of E3/49K or the C-terminal fragment may have additional functional activities acting on the infected cell, although we did not observe any inhibition of killing when cells expressing only the membrane form were used as target cells for primary NK cells (Fig. S5). In line with a role distant from the site of infection, we showed that the secreted form, sec49K, binds selectively to leukocytes using the leukocyte common antigen CD45 as a sec49K receptor. CD45 is a large cell surface-exposed protein tyrosine phosphatase that removes an inhibitory phosphate group from Src-family kinases. Because Src-family kinases are critically involved in immunoreceptor signaling in all leukocytes, CD45 plays an important role in signaling pathways, leading to activation and differentiation of T cells, B cells, NK cells, and macrophages (33–36). Differential splicing of at least three exons encoding the A, B, and C parts of the CD45 extracellular domain gives rise to different isoforms with mr ranging between 180 and 220 kDa and between 391 and 552 amino acids in the extracellular domain. This extracellular part assumes a rod-like structure consisting of three membrane-proximal FNIII domains followed N-terminal by a cysteine-rich domain and amino acids encoding the isoforms RO, RA, RB, RC, RABC, or other combinations (Fig. 6B) (31, 33, 37). We demonstrate here that sec49K has the capacity to bind the R0 and the RABC isoforms, suggesting that binding is independent of amino acids encoded by exons A, B, and C. This was confirmed by showing that previous binding of sec49K to CD45 significantly affected binding only of mAbs directed to the common domains. Thus, we conclude that sec49K binds to all CD45 isoforms, most likely by attaching to the membrane-proximal FNIII and/or cysteine-rich domains.
Binding of sec49K to CD45 impaired the activation and cytotoxic activity of NK cells. Sec49K also interfered with activation and cytokine production of primary T cells. The foregoing effects were correlated with impaired signal transduction in Jurkat T cells affecting tyrosine phosphorylation of the target proteins ZAP-70 and ERK known to be involved in NK- and T-cell activation. A similar suppression of ERK signaling was seen in murine CD45-deficient NK cells, whereas cytotoxicity was less affected (38). The exact mechanism of sec49K activity remains to be determined. Sec49K binding might modulate the phosphatase activity of CD45, either by inducing dimerization (33) or by interfering with its organization in lipid rafts (39).
To date, a physiological ligand for the large CD45 ectodomain remains elusive. Although a number of molecules were proposed (e.g., CD22 on B cells or the apoptosis-inducing lectin, galectin 1), the interactions turned out to not be CD45-specific (33, 34, 37). It was recently reported that the human cytomegalovirus (HCMV) protein pUL11 of the RL11 family fused to the Fc domain of human Ig also is able to attach to CD45, causing impaired T-cell proliferation and cytokine production and overall T-cell paralysis (40). Based on sequence comparisons, a structural relationship between E3/49K and the HCMV RL11 family was suggested by Davison et al. (27). By altering the boundaries of the E3/49K CR1 repeat domain (25), these authors identified a homologous domain in other E3 proteins as well as in the HCMV RL11 family. Here we report a striking functional relationship between pUL11 and E3/49K as well, in that they both target the same protein, CD45. However, although we found an influence of sec49K on T-cell receptor signaling and production of IFN-γ and TNF-α after antigen-specific stimulation of CD4 T cells, we did not detect any significant effect on CTL lysis. In this respect, our findings are reminiscent of effects demonstrated by certain anti-CD45 mAbs showing a differential inhibition of NK cell lysis but not CTL lysis (41). We cannot rule out the possibility that effects on T cells as seen for pUL-11 might require cross-linking of sec49K; however, it should be noted that pUL6, another member of the CMV RL11 family, does not bind CD45 and does not show these effects on T cells (40), and other family members have demonstrated Fc receptor activity (42, 43). Thus, differential functions are to be expected from members containing the RL11/CR1 domain.
There is strong evidence that the extracellular portion of CD45, particularly the cysteine-rich and FNIII domains, proposed here as sec49K attachment sites, are under strong positive selection during evolution, and it has been hypothesized that CD45 evolution may be driven by pathogens (37, 44, 45). Taken together, the HCMV data and our studies on adenovirus 19a E3/49K lend further weight to this hypothesis.
It is intriguing that Ad19a initially generates a membrane-anchored protein to eventually produce a cleaved secreted molecule. Thus, the smaller 12- to 13-kDa fragment, comprising the cytoplasmic tail, the TMD, and some 30 amino acids adjacent to the TMD, could have an additional function. In support of this idea, the small E3/49K-derived fragments exhibit a relatively long half-life of 5–6 h and may actually accumulate during the course of infection. We have evidence that the YxxΦ and LL motifs in the cytoplasmic tail are important for trafficking of E3/49K. Thus, these motifs and membrane integration may be required to direct E3/49K to the relevant processing compartment, or may point to an additional role of the C-terminal fragment in an intracellular compartment. Therefore, a dual function of E3/49K cannot be ruled out. It is not uncommon for cellular secreted proteins to be initially synthesized as membrane-anchored precursor forms, such as growth factors, cytokines, and amyloid precursor proteins. However, this is not a widely documented property of viruses. Instead, a number of viruses produce proteins in two alternative reading frames; for example, Ebola synthesizes both a secreted form and a transmembrane form of its glycoprotein, targeting neutrophils and endothelial cells, respectively (46).
By demonstrating sec49K binding to CD45 and its functional effects on NK cells as well as T-cell signaling and cytokine production, we have discovered an immunomodulatory function for a species-specific E3 protein that is not present in the common species C Ads. It will be very interesting to determine whether this unique E3 function is common in species D Ads or limited to those species D Ads that cause EKC, a severe eye disease that is highly contagious and presents with typical subepithelial corneal infiltrates. This would implicate sec49K in EKC pathogenesis. All species D Ads have a 49K gene and also express a corresponding protein (26); however, the protein sequences and mr of these 49K-like proteins differ considerably, particularly in the extracellular domain comprising sec49K (26). Thus, it is possible that not all E3/49K proteins are cleaved and produce a secreted product. Future studies requiring the generation of new antibodies against external domains of various E3/49K molecules of non-EKC Ads should clarify this important question.
Materials and Methods
Cloning and Molecular Biology Methods.
Cloning of the Ad19a E3/49K gene into the pSG5 expression vector and generation of A549 cells constitutively expressing E3/49K (A549K27S) have been described previously (24). E3/49K transfectants of 293 cells were generated as described previously (47).
Cells, Viruses, and Infection.
The lung epithelial carcinoma cell lines A549 (ATCC CCL-185), 293 cells (ATCC CRL-1573), 293T cells (ATCC CRL-11268) and HeLa cells were maintained in DMEM as described previously (24). Unless noted otherwise, all lymphoid cell lines, Jurkat E6-1 and the CD45 “negative” variant J45.01 (29), J77 (SVT 35), and K562 were maintained in RPMI, 10% (vol/vol) FCS, and penicillin/streptomycin (complete RPMI). The Jurkat derivative J-AS-1 rendered CD45 negative by expression of an antisense construct and CD45+ transfectants, CD45RO and CD45RABC, were cultured as described previously (30). Culture conditions of human NK cell lines are provided in SI Materials and Methods. Ad19a/Ad64, referred to here as Ad19a for consistency with earlier work, was grown and titered essentially as described previously (26). Typically, cells were infected with 5–10 plaque forming units per cell for 1.5 h.
Transient Transfection.
The 293T cells were transfected using polyethyleneimine (PEI) with 4 μg of LCA.1 and LCA.6 expression vectors encoding CD45RO and CD45RABC, respectively (31), or as a negative control with pBS-EcoRVc, encoding the Ad2 E3 region (48). Cells were seeded at 24 h before transfection, incubated with the PEI-DNA mixture for 40 h, washed with PBS, harvested using EDTA, and then resuspended in FACS buffer. Each set of transfected cells was incubated with either BSA or sec49K as described below for the FACS binding assay and stained for sec49K using mAb 4D1 followed by Alexa Fluor 488-coupled goat anti-rat IgG (Invitrogen) and Tricolor (PE-Cy5)-coupled pan-CD45–specific mAb HI30 (Invitrogen). Dual color plots from 10,000 cells were recorded.
MAbs and Antisera.
The antiserum R48 directed against the N-terminal domain of E3/49K was produced by immunizing rabbits with the 49K His-tag fusion protein (N49K-His) essentially as described previously (26, 47). N49K-His was also used as immunogen to raise rat mAbs. Two hybridoma clones (4D1 and 1E6) that produce IgG2a Ig isotypes recognizing native and denatured E3/49K were subcloned at least twice by limiting dilution. Unless stated otherwise, mAb 4D1 was used. Details of N49K-His production and purification and the generation of mAbs, along with a list of the other antibodies used, are provided in SI Materials and Methods.
Purification of SecE3/49K.
Sec49K was purified from supernatants of cell lines A549K27S and 293K35 stably expressing the E3/49K protein using affinity columns containing immobilized anti-E3/49K mAb 4D1. As negative control, supernatants of untransfected A549 and 293 cells were processed in the same way using separate affinity columns (SI Materials and Methods).
FACS Binding Assay for sec49K.
Typically 600,000 cells were incubated for 1 h at 4 °C with purified sec49K (100–200 ng), 1–3 μL of 50-fold concentrated supernatant (Vivaspin with a molecular weight cutoff of 30K or 50K) or sec49K-containing supernatant from transfected cells in 200 μL. As negative controls, cells were incubated with medium alone, medium containing an equivalent amount of BSA and/or accordingly processed supernatant from untransfected A549 or 293 cells. After washing, cells were stained with 1 μg of 4D1 followed by Alexa Fluor 488-coupled goat anti-rat IgG (Invitrogen) essentially as described previously (17, 19, 47). Staining of PBMCs is described in SI Materials and Methods.
Metabolic Labeling, Immunoprecipitation, SDS/PAGE, Silver Staining, and Western Blot Analysis.
Metabolic labeling of cells, immunoprecipitation, and SDS-PAGE were performed as described previously (13, 24). For silver staining of polyacrylamide gels a commercial kit (BIORAD) was used. Proteins were blotted as described previously (49). E3/49K was detected with either R48 or mAb 4D1. Detection of phosphorylated ZAP-70 and ERK is described in detail in SI Materials and Methods.
NK Cell-Mediated Lysis.
NK cell-mediated lysis was quantified using NKL and K562 target cells in a standard 4-h chromium-51 (51Cr; GE Healthcare) release assay essentially as described previously (50). NKL cells (clone 234) were preincubated with purified sec49K or 10× concentrated supernatants of E3/49K+ cells or control supernatant (derived from A549, or BSA) for 30 min at room temperature and then added to 51Cr-labeled K562 target cells at varying E:T ratios.
Activation Markers on Fresh NK Cells.
Human NK cells were purified from PBMCs using the Dynabeads Untouched Human NK Cell kit (Invitrogen) and rested overnight (2 × 106 cells/mL) in medium. Subsequently, NK cells were incubated overnight in 96-well flat-bottom plates coated with 10 µg/mL of anti-MHCI (W6/32) as IgG control; anti-2B4 (C1.7; Beckman Coulter) or anti-NKG2D (149810, R&D Systems) with or without sec49K (∼100 ng/mL) as described previously (50, 51). Recombinant IL-2 (100 U/mL) was added to the indicated samples. After overnight incubation, cells were harvested and stained with anti-CD69PE (1:50; Biolegend) or anti-CD25FITC (1:25; BD Biosciences) and analyzed by FACS.
Antigen-Specific Stimulation of CD4 T Cells.
First, 5 × 106/mL PBMCs from six CMV-seropositive healthy controls were preincubated for 30 min with or without purified sec49K (∼250 ng/mL). Then 2 × 106 PBMCs were stimulated for 6 h in a total volume of 400 µL of RPMI-1% glutamine-1% antibiotics-0.5% human serum albumin with 32 µL/mL CMV lysate and control lysate (Virion) or with 2.5 µg/mL S. aureus Enterotoxin B (Sigma-Aldrich) in the presence of 1 µg/mL costimulatory antibodies anti-CD28 and anti-CD49d as described previously (52). After 2 h, 10 µg/mL brefeldin A was added to accumulate cytokines intracellularly. After 6 h, PBMCs were treated with 2 mM EDTA for 15 min, fixed using 4% paraformaldehyde and stained as described previously (52) using antibodies against CD4, CD69, IFN-γ, TNF-α, and IL-2 (all from BD Biosciences). Cells were analyzed on a FACSCanto II flow cytometer (BD Biosciences) using FlowJo software (Tree Star). Antigen-specific T-cell frequencies were calculated after subtraction of reactive T cells observed after control stimulation.
Supplementary Material
Acknowledgments
We thank Andrea Osterlehner and Candida Guckelmus for excellent technical assistance; Vaclav Horejsi, Peter Cresswell, and Reinhard Schwinzer for kindly providing antibodies or hybridomas; Art Weiss, Dennis Alexander, Rainer Haas, and David Rothstein for providing cells and DNA constructs; Gavin Wilkinson, Michele Calos, Juan Martin-Serrano, and Nick Holmes for helpful advice with NK cell assays, advice on the PEI method, provision of 293T cells, and discussions of the CD45 structure, respectively; and Keith Leppard for critically reading the manuscript. This work was supported by the Biotechnology and Biological Sciences Research Council (Grant BB/D002877/1) to H-G.B. and the Deutsche Forschungsgemeinschaft (Grant BU 642/1, to H-G.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 19976.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312420110/-/DCSupplemental.
References
- 1.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy: Classification and Nomenclature of Viruses. Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2012. [Google Scholar]
- 2.Zhou X, et al. Analysis of human adenovirus type 19 associated with epidemic keratoconjunctivitis and its reclassification as adenovirus type 64. Invest Ophthalmol Vis Sci. 2012;53(6):2804–2811. doi: 10.1167/iovs.12-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wold WSM, Horwitz MS. Adenoviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, editors. Fields Virology. 5th Ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2395–2436. [Google Scholar]
- 4.Kaneko H, et al. Nucleotide sequence variation in the hexon gene of human adenovirus type 8 and 37 strains from epidemic keratoconjunctivitis patients in Japan. J Gen Virol. 2009;90(Pt 9):2260–2265. doi: 10.1099/vir.0.010934-0. [DOI] [PubMed] [Google Scholar]
- 5.Walsh MP, et al. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS ONE. 2009;4(6):e5635. doi: 10.1371/journal.pone.0005635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chodosh J, Miller D, Stroop WG, Pflugfelder SC. Adenovirus epithelial keratitis. Cornea. 1995;14(2):167–174. [PubMed] [Google Scholar]
- 7.Arnberg N. Adenovirus receptors: Implications for tropism, treatment and targeting. Rev Med Virol. 2009;19(3):165–178. doi: 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson EC, et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med. 2011;17(1):105–109. doi: 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]
- 9.Burgert H-G, et al. Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol. 2002;269:273–318. doi: 10.1007/978-3-642-59421-2_16. [DOI] [PubMed] [Google Scholar]
- 10.Gregory SM, Nazir SA, Metcalf JP. Implications of the innate immune response to adenovirus and adenoviral vectors. Future Virol. 2011;6(3):357–374. doi: 10.2217/fvl.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Windheim M, Hilgendorf A, Burgert H-G. Immune evasion by adenovirus E3 proteins: Exploitation of intracellular trafficking pathways. Curr Top Microbiol Immunol. 2004;273:29–85. doi: 10.1007/978-3-662-05599-1_2. [DOI] [PubMed] [Google Scholar]
- 12.Ruzsics Z, et al. Transposon-assisted cloning and traceless mutagenesis of adenoviruses: Development of a novel vector based on species D. J Virol. 2006;80(16):8100–8113. doi: 10.1128/JVI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgert H-G, Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- 14.Sester M, Ruzsics Z, Mackley E, Burgert H-G. The transmembrane domain of the adenovirus E3/19K protein acts as an ER retention signal and contributes to intracellular sequestration of MHC class I molecules. J Virol. 2013;87(11):6104–6117. doi: 10.1128/JVI.03391-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horwitz MS, Efrat S, Christen U, von Herrath MG, Oldstone MB. Adenovirus E3 MHC inhibitory genes but not TNF/Fas apoptotic inhibitory genes expressed in beta cells prevent autoimmune diabetes. Proc Natl Acad Sci USA. 2009;106(46):19450–19454. doi: 10.1073/pnas.0910648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McSharry BP, et al. Adenovirus E3/19K promotes evasion of NK cell recognition by intracellular sequestration of the NKG2D ligands major histocompatibility complex class I chain-related proteins A and B. J Virol. 2008;82(9):4585–4594. doi: 10.1128/JVI.02251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sester M, et al. Conserved amino acids within the adenovirus 2 E3/19K protein differentially affect downregulation of MHC class I and MICA/B proteins. J Immunol. 2010;184(1):255–267. doi: 10.4049/jimmunol.0902343. [DOI] [PubMed] [Google Scholar]
- 18.McNees AL, Gooding LR. Adenoviral inhibitors of apoptotic cell death. Virus Res. 2002;88(1-2):87–101. doi: 10.1016/s0168-1702(02)00122-3. [DOI] [PubMed] [Google Scholar]
- 19.Elsing A, Burgert H-G. The adenovirus E3/10.4K-14.5K proteins down-modulate the apoptosis receptor Fas/Apo-1 by inducing its internalization. Proc Natl Acad Sci USA. 1998;95(17):10072–10077. doi: 10.1073/pnas.95.17.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davison AJ, Benko M, Harrach B. Genetic content and evolution of adenoviruses. J Gen Virol. 2003;84(Pt 11):2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- 21.Robinson CM, Seto D, Jones MS, Dyer DW, Chodosh J. Molecular evolution of human species D adenoviruses. Infect Genet Evol. 2011;11(6):1208–1217. doi: 10.1016/j.meegid.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frietze KM, Campos SK, Kajon AE. Open reading frame E3-10.9K of subspecies B1 human adenoviruses encodes a family of late orthologous proteins that vary in their predicted structural features and subcellular localization. J Virol. 2010;84(21):11310–11322. doi: 10.1128/JVI.00512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson CM, et al. The E3 CR1-gamma gene in human adenoviruses associated with epidemic keratoconjunctivitis. Virus Res. 2011;160(1-2):120–127. doi: 10.1016/j.virusres.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Windheim M, Burgert H-G. Characterization of E3/49K, a novel, highly glycosylated E3 protein of the epidemic keratoconjunctivitis-causing adenovirus type 19a. J Virol. 2002;76(2):755–766. doi: 10.1128/JVI.76.2.755-766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deryckere F, Burgert H-G. Early region 3 of adenovirus type 19 (subgroup D) encodes an HLA-binding protein distinct from that of subgroups B and C. J Virol. 1996;70(5):2832–2841. doi: 10.1128/jvi.70.5.2832-2841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blusch JH, et al. The novel early region 3 protein E3/49K is specifically expressed by adenoviruses of subgenus D: Implications for epidemic keratoconjunctivitis and adenovirus evolution. Virology. 2002;296(1):94–106. doi: 10.1006/viro.2002.1404. [DOI] [PubMed] [Google Scholar]
- 27.Davison AJ, et al. Homology between the human cytomegalovirus RL11 gene family and human adenovirus E3 genes. J Gen Virol. 2003;84(Pt 3):657–663. doi: 10.1099/vir.0.18856-0. [DOI] [PubMed] [Google Scholar]
- 28.Körner H, Burgert H-G. Down-regulation of HLA antigens by the adenovirus type 2 E3/19K protein in a T-lymphoma cell line. J Virol. 1994;68(3):1442–1448. doi: 10.1128/jvi.68.3.1442-1448.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koretzky GA, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci USA. 1991;88(6):2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenney DW, Onodera H, Gorman L, Mimura T, Rothstein DM. Distinct isoforms of the CD45 protein-tyrosine phosphatase differentially regulate interleukin 2 secretion and activation signal pathways involving Vav in T cells. J Biol Chem. 1995;270(42):24949–24954. doi: 10.1074/jbc.270.42.24949. [DOI] [PubMed] [Google Scholar]
- 31.Streuli M, Morimoto C, Schrieber M, Schlossman SF, Saito H. Characterization of CD45 and CD45R monoclonal antibodies using transfected mouse cell lines that express individual human leukocyte common antigens. J Immunol. 1988;141(11):3910–3914. [PubMed] [Google Scholar]
- 32.Walters RW, et al. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110(6):789–799. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- 33.Hermiston ML, Xu Z, Weiss A. CD45: A critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21(1):107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 34.Trowbridge IS, Thomas ML. CD45: An emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 35.Justement LB, Campbell KS, Chien NC, Cambier JC. Regulation of B cell antigen receptor signal transduction and phosphorylation by CD45. Science. 1991;252(5014):1839–1842. doi: 10.1126/science.1648262. [DOI] [PubMed] [Google Scholar]
- 36.Hesslein DGT, et al. Differential requirements for CD45 in NK-cell function reveal distinct roles for Syk-family kinases. Blood. 2011;117(11):3087–3095. doi: 10.1182/blood-2010-06-292219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes N. CD45: All is not yet crystal clear. Immunology. 2006;117(2):145–155. doi: 10.1111/j.1365-2567.2005.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hesslein DG, Takaki R, Hermiston ML, Weiss A, Lanier LL. Dysregulation of signaling pathways in CD45-deficient NK cells leads to differentially regulated cytotoxicity and cytokine production. Proc Natl Acad Sci USA. 2006;103(18):7012–7017. doi: 10.1073/pnas.0601851103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irles C, et al. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003;4(2):189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 40.Gabaev I, et al. The human cytomegalovirus UL11 protein interacts with the receptor tyrosine phosphatase CD45, resulting in functional paralysis of T cells. PLoS Pathog. 2011;7(12):e1002432. doi: 10.1371/journal.ppat.1002432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newman W, Fast LD, Rose LM. Blockade of NK cell lysis is a property of monoclonal antibodies that bind to distinct regions of T-200. J Immunol. 1983;131(4):1742–1747. [PubMed] [Google Scholar]
- 42.Lilley BN, Ploegh HL, Tirabassi RS. Human cytomegalovirus open reading frame TRL11/IRL11 encodes an immunoglobulin G Fc-binding protein. J Virol. 2001;75(22):11218–11221. doi: 10.1128/JVI.75.22.11218-11221.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atalay R, et al. Identification and expression of human cytomegalovirus transcription units coding for two distinct Fcgamma receptor homologs. J Virol. 2002;76(17):8596–8608. doi: 10.1128/JVI.76.17.8596-8608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ballingall KT, et al. The CD45 locus in cattle: Allelic polymorphism and evidence for exceptional positive natural selection. Immunogenetics. 2001;52(3-4):276–283. doi: 10.1007/s002510000276. [DOI] [PubMed] [Google Scholar]
- 45.Filip LC, Mundy NI. Rapid evolution by positive Darwinian selection in the extracellular domain of the abundant lymphocyte protein CD45 in primates. Mol Biol Evol. 2004;21(8):1504–1511. doi: 10.1093/molbev/msh111. [DOI] [PubMed] [Google Scholar]
- 46.Yang Z, et al. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279(5353):1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- 47.Sester M, Burgert H-G. Conserved cysteine residues within the E3/19K protein of adenovirus type 2 are essential for binding to major histocompatibility complex antigens. J Virol. 1994;68(9):5423–5432. doi: 10.1128/jvi.68.9.5423-5432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Körner H, Fritzsche U, Burgert H-G. Tumor necrosis factor alpha stimulates expression of adenovirus early region 3 proteins: Implications for viral persistence. Proc Natl Acad Sci USA. 1992;89(24):11857–11861. doi: 10.1073/pnas.89.24.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilgendorf A, et al. Two distinct transport motifs in the adenovirus E3/10.4-14.5 proteins act in concert to down-modulate apoptosis receptors and the epidermal growth factor receptor. J Biol Chem. 2003;278(51):51872–51884. doi: 10.1074/jbc.M310038200. [DOI] [PubMed] [Google Scholar]
- 50.Watzl C, Long EO. Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J Exp Med. 2003;197(1):77–85. doi: 10.1084/jem.20020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mesecke S, Urlaub D, Busch H, Eils R, Watzl C. Integration of activating and inhibitory receptor signaling by regulated phosphorylation of Vav1 in immune cells. Sci Signal. 2011;4(175):ra36. doi: 10.1126/scisignal.2001325. [DOI] [PubMed] [Google Scholar]
- 52.Sester M, et al. Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus. J Virol. 2002;76(8):3748–3755. doi: 10.1128/JVI.76.8.3748-3755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.