Significance
This study addresses one of the most fundamental issues in sleep rhythm generation. The theory that low-threshold burst firing mediated by T-type calcium channels in thalamocortical neurons is the key component for sleep spindles, has been accepted as dogma and appears throughout the literature. In this study, however, in vivo and in vitro evidence shows that sleep spindles are generated normally in the absence of T-type channels and burst firing in thalamocortical neurons. Furthermore, our data indicate a potentially important role of tonic firing in this rhythm generation. This study advances the knowledge of sleep and vigilance control to another level of understanding.
Abstract
T-type Ca2+ channels in thalamocortical (TC) neurons have long been considered to play a critical role in the genesis of sleep spindles, one of several TC oscillations. A classical model for TC oscillations states that reciprocal interaction between synaptically connected GABAergic thalamic reticular nucleus (TRN) neurons and glutamatergic TC neurons generates oscillations through T-type channel-mediated low-threshold burst firings of neurons in the two nuclei. These oscillations are then transmitted from TC neurons to cortical neurons, contributing to the network of TC oscillations. Unexpectedly, however, we found that both WT and KO mice for CaV3.1, the gene for T-type Ca2+ channels in TC neurons, exhibit typical waxing-and-waning sleep spindle waves at a similar occurrence and with similar amplitudes and episode durations during non-rapid eye movement sleep. Single-unit recording in parallel with electroencephalography in vivo confirmed a complete lack of burst firing in the mutant TC neurons. Of particular interest, the tonic spike frequency in TC neurons was significantly increased during spindle periods compared with nonspindle periods in both genotypes. In contrast, no significant change in burst firing frequency between spindle and nonspindle periods was noted in the WT mice. Furthermore, spindle-like oscillations were readily generated within intrathalamic circuits composed solely of TRN and TC neurons in vitro in both the KO mutant and WT mice. Our findings call into question the essential role of low-threshold burst firings in TC neurons and suggest that tonic firing is important for the generation and propagation of spindle oscillations in the TC circuit.
Sleep spindles are one type of several rhythmic brain waves detected by electroencephalography (EEG) during normal non-rapid eye movement (NREM) sleep. A spindle consists of characteristic waxing-and-waning field potentials grouped into 7- to 14-Hz oscillations that last for 1–3 s and recur once every 5–10 s in the thalamus and the cortex (1–3). Spindles are also visible under anesthesia, particularly with barbiturates but also with ketamine-xylazine combinations (4, 5). These oscillations are generated in the thalamus as a result of synaptic interactions between inhibitory [i.e., thalamic reticular nucleus (TRN)] neurons and excitatory thalamocortical (TC) neurons, and are propagated to the cortex. Corticothalamic projections back to the thalamus complete the cortico-thalamo-cortical loop.
In vivo data suggest that TRN neurons are spindle pacemakers, because spindles can be generated in deafferented TRN neurons (6) but disappear in TC regions after disconnection from TRN neurons (7). However, in vitro data suggest that an intact TC-TRN network is a necessity, because spindles are abolished after disconnection of TC and TRN neurons (8).
Two distinct firing patterns, tonic and burst, are displayed by both TRN and TC neurons. Burst firing is mediated by low-threshold T-type Ca2+ channels (9). Of the three subtypes of T-type channels, CaV3.1 is expressed exclusively in TC regions, whereas CaV3.2 and CaV3.3 are abundant in TRN regions (10). T-type channels in TC neurons have been proposed to be a critical component in the generation of physiological and pathological TC oscillations, such as sleep rhythms (1, 11, 12) and the spike-wave discharges (SWDs) of absence seizures (11, 13, 14). One generally accepted hypothesis proposes that inhibitory inputs from TRN neurons de-inactivate T-type Ca2+ channels in TC neurons, leading to induction of burst firings in TC neurons, which in return excite reciprocally connected TRN neurons. These thalamic oscillations are then transmitted from TC neurons to cortical neurons. This model proposes that T-type channel-mediated burst firing in TC neurons underlies sleep spindles and other sleep rhythms within TC circuits (1, 11).
There have long been doubts regarding the extent to which TC T-type Ca2+ channels contribute to the heterogeneity of TC oscillations during NREM sleep, which consists of multiple EEG components including slow waves (<1 Hz), delta waves (1–4 Hz), and sleep spindles (7–14 Hz). T-type channels have received particular attention in the genesis of spindles and delta waves, both of which are thought to originate from thalamic neurons (8), although cortically generated delta waves also have been found in cats with thalamic lesions (15). A role for TC T-type channels in sleep has been demonstrated in two studies, one using mice with a global deletion (16) and the other using mice with a thalamus-restricted deletion (17) of CaV3.1 T-type channels. Both mice exhibited reduced delta waves with intact slow waves (16, 17). Fragmented sleep was observed in both mice, indicating that this sleep phenotype in the global CaV3.1−/− mice is related to a defect in TC neurons. The effect of the mutation on spindle rhythms was unclear, however (16).
In the present study, we examined the role of low-threshold burst firing in sleep spindles expressed in TC neurons using mice lacking CaV3.1 T-type Ca2+ channels. We observed intact sleep spindles in CaV3.1−/− mice during NREM sleep. Our findings suggest that the classical view of the roles of T-type channels and burst firing in TC neurons with respect to the generation of spindle oscillations may need to be revised.
Results
Sleep Spindles Are Not Altered in Cav3.1−/− Mice During Natural NREM Sleep.
First, in an attempt to elucidate the role of low-threshold burst firing mediated by TC T-type channels in generating sleep spindles, we examined EEGs recorded during a state of natural sleep in mice lacking the CaV3.1 T-type channels that are highly expressed in TC neurons. EEG/electromyography (EMG) recordings were acquired with a telemetry system over a continuous period of 48 h in mice housed in their home cages. These data were analyzed to assign the sleep categories of awake, NREM, and rapid eye movement (REM) states based on standard criteria for the analysis of rodent sleep (18).
The sleep spindle episodes in both CaV3.1+/+ and CaV3.1−/− mice exhibited a characteristic waxing-and-waning pattern and typical recurring sequences (Fig. 1A) that met the stereotypical criteria for sleep spindles (1, 8, 19). There were no significant differences in the number of spindle episodes, mean duration of episodes, peak-to-peak amplitudes, or peak frequencies of spindle oscillations between the CaV3.1+/+ and CaV3.1−/− mice (Fig. 1B). These findings appear to indicate that sleep spindles are not altered in CaV3.1−/− mice.
Fig. 1.
Sleep spindles during NREM sleep in CaV3.1+/+ (WT) and CaV3.1−/− (KO) mice. (A) Sample traces show the raw (upper trace) and filtered (lower trace) EEG signals recorded during NREM sleep. Bandpass-filtered (6–15 Hz) EEG signals clearly show spindle events (arrowheads) in both genotypes. (B) There were no differences in the mean length of each spindle episode, number of episodes, mean peak-to-peak amplitude, and peak frequency between CaV3.1+/+ and CaV3.1−/− mice.
Decreased Duration of Barbiturate-Induced Spindle Episodes in Cav3.1−/− Mice.
We next examined the characteristics of spindle oscillations induced by barbiturate injection. In agreement with the literature (20), we found that barbiturate-induced spindles were characterized by large-amplitude oscillations of increased duration compared with sleep spindles (Fig. 2A). The mean duration of barbiturate-induced spindle episodes was almost doubled compared with the spindle episode duration during NREM sleep in the CaV3.1+/+ mice, but not in the CaV3.1−/− mice. Compared with the CaV3.1+/+ mice, the CaV3.1−/− mice exhibited shorter barbiturate-induced spindle episodes (*P < 0.05; Fig. 2B), as well as smaller peak-to-peak amplitudes (*P < 0.05), whereas there was no difference in the peak frequency of spindles between the two genotypes (Fig. 2B). Of note, the spindle episodes in the CaV3.1−/− mice were composed of shorter episodes compared with those seen in the WT mice (Fig. 2C).
Fig. 2.
The duration of spindle episodes induced by barbiturate injection was diminished in CaV3.1−/− mice compared with CaV3.1+/+ mice. (A) Sample traces illustrate EEG signals with a systemic injection of barbiturate (20 mg/kg i.p.). The upper trace is a raw EEG trace, and the lower trace shows bandpass-filtered (6–15 Hz) EEG signals. Arrowheads denote spindle events. (B) CaV3.1−/− mice had a shorter mean spindle episode duration and smaller peak-to-peak spindle amplitude compared with WT mice, but similar number of episodes and peak frequency. (C) Barbiturate-induced spindle sequences were categorized according to the duration of each event. Spindle events in CaV3.1−/− mice were of short duration.
Taken together, these results suggest that low-threshold spikes (LTSs) mediated by CaV3.1 T-type Ca2+ channels in TC neurons are not essential for spindle generation, but may play a role in increasing the duration of spindles episodes induced by barbiturates.
Spike Discharges in TC Neurons During in Vivo Spindle Oscillations.
Our findings regarding natural sleep spindles and barbiturate-induced spindles in CaV3.1−/− mice prompted us to investigate the properties of TC neuron spike discharges during spindle episodes in the absence of low-threshold burst firing. To do this, we performed in vivo extracellular single-unit recordings in TC neurons while simultaneously monitoring the cortical EEG under barbiturate anesthesia.
Both tonic and burst spikes were observed in CaV3.1+/+ TC cells, whereas only tonic spikes were detected in CaV3.1−/− TC cells (Fig. 3 A–C), confirming previous in vitro data (13). Bursts were identified according to the conventional criteria (21), with a burst defined as a cluster of two or more spikes with interspike intervals of ≤4 ms, in which the first spike in the burst has a preceding interspike interval of >100 ms (Fig. S1). Spikes recorded over 15 consecutive spindle sequences (SP1–SP15) were aligned with the initiation time of each cortical EEG spindle event. The perievent histograms showed a large number of TC spikes co-occurring within cortical spindle events (Fig. 3B), as reported previously (22, 23). Of note, we found the spike frequency was significantly higher during spindle periods compared with nonspindle periods in both WT mice (1.71 ± 0.23 Hz vs. 0.73 ± 0.16 Hz) and KO mice (1.94 ± 0.43 Hz vs. 0.18 ± 0.04 Hz) (Fig. 3C, a). Of particular importance, there was no difference in the total spiking rate (across tonic and burst activity) within spindle events between two genotype TC neurons (P = 0.42; Fig. 3C, a) with event correlation (Fig. S2), whereas the nonspindle spike frequency was significantly lower in CaV3.1−/− neurons compared with CaV3.1+/+ neurons (Fig. 3C, a).
Fig. 3.
TC neurons discharge tonic spikes abundantly during spindle events in both CaV3.1+/+ and CaV3.1−/− mice in vivo. (A) Sample traces showing cortical EEG signals over the ipsilateral frontal lobe (upper trace) and extracellular single-unit recordings from TC regions (lower trace) in CaV3.1+/+ and CaV3.1−/− mice under barbiturate anesthesia. Burst and tonic spikes are represented by vertical red and blue lines, respectively. (B) Perievent histograms depicting the number of spikes in a 200-ms bin for 15 cortical spindle episodes (SP1–SP15). Red, blue, and turquiose bars represent the spiking frequency of burst, tonic, and mixed spikes, respectively. The mixed spikes describe the events in which both tonic and burst firing were detected within a 250-ms bin. The duration of each spindle episode is shown as a horizontal gray line. (C, a) Total spike frequency during spindle and nonspindle events between WT (solid bar) and CaV3.1−/− (open bar with diagonal stripes) mice. Burst spikes are in red, and tonic spikes are in blue. (C, b) Tonic and burst firing frequencies during spindle and nonspindle periods in WT and KO mutant mice. Values are mean ± SEM. *P < 0.05; **P < 0.005.
Further analysis of the firing mode of TC neurons revealed a significantly higher tonic spike frequency in both WT and mutant mice during spindle periods than during nonspindle periods (Fig. 3C, b). During spindle episodes, the tonic spike frequency was significantly higher in mutant KO mice compared with WT mice, perhaps explaining why the total spiking rate of mutant TC neurons within cortical spindle events was as high as that in WT neurons, even though the mutants are devoid of burst spikes. In contrast, there was no significant difference in burst firing between spindle and nonspindle periods (Fig. 3C, b), either in the frequency of burst spikes (0.58 ± 0.15 Hz vs. 0.35 ± 0.09 Hz) or in the occurrence of burst events (0.28 ± 0.09 Hz vs. 0.16 ± 0.06 Hz; P = 0.27). In other words, the majority of CaV3.1+/+ TC cells discharged burst spikes without preference for spindle episodes; just 2 of 13 fired bursts preferentially during the spindle period.
Also of interest is our finding of no difference in the overall spike frequency of TC neurons during spindle events between the two genotypes. Of note, WT TC neurons exhibited a higher tonic spike frequency than burst spike frequency during spindle events. More importantly, the tonic spike frequency in WT mice was significantly (∼3.5-fold) higher during spindle periods than during nonspindle periods, whereas the burst spike frequency did not differ in the two periods. The tonic spike frequency observed in CaV3.1−/− TC cells coincided even more strongly with spindles (∼10-fold higher). In addition, spike discharges recorded in both CaV3.1+/+ and CaV3.1−/− TRN neurons were more frequent during spindle episodes (Fig. S3), in parallel with the findings in TC neurons.
Spindle-Like Oscillations Elicited in Intrathalamic Circuits in Cav3.1−/− Mice.
Our finding that robust generation of cortical spindles is accompanied by increased TC spike discharges in CaV3.1−/− mice prompted us to further investigate whether spindle oscillations can be generated within an intrathalamic circuit composed only of TRN and TC neurons in the presence or absence of LTSs in TC neurons. For this purpose, we assessed thalamic network activity in TC slices with and without attached cortex (24). Previous studies have demonstrated that oscillations within the range observed for sleep spindles in vivo (22) can be evoked in thalamic slices by electrical stimulation of the internal capsule (IC) (25). These are thus referred to as “spindle-like oscillations” (SLOs) (26). We adopted this paradigm using the multielectrode array system as described in SI Materials and Methods.
Thalamic oscillations were evoked by a single 20- to 100-μV, 60- to 80-μs electrical stimulation in the IC in TC slices. Clusters of spikes composed of multiple units with various amplitudes were detected simultaneously in TRN and TC nuclei (Fig. 4A). SLOs were readily generated in these thalamic slices from both CaV3.1+/+ and CaV3.1−/− mice in the presence and absence of cortex (Fig. 4A). There were no significant differences in the durations of evoked oscillatory activities between CaV3.1+/+ and CaV3.1−/− thalamic slices with cortex (7 slices from 7 WT mice vs. 8 slices from 7 mutant mice) or without cortex (13 slices from 8 WT mice vs. 12 slice from 9 mutant mice) (Fig. 4B), although the total TC spike numbers in CaV3.1−/− thalamic slices were lower than those in CaV3.1+/+ slices in the absence of cortex (P = 0.047; Fig. 4C).
Fig. 4.
SLOs were elicited in the intrathalamic network in both CaV3.1+/+ and CaV3.1−/− thalamic slices in either the presence or absence of the cortex. (A) Sample traces of simultaneous multiunit recordings of SLOs from TRN (upper trace) and TC (lower trace) nuclei in CaV3.1+/+ (Left) and CaV3.1−/− (Right) thalamic slices demonstrate oscillatory activity in both nuclei. Network oscillations were evoked by single electrical shocks to the IC. Magnified traces show that multiple units with various amplitudes were detected in both nuclei in the thalamus. (B) Duration of SLOs in TRN and TC nuclei in CaV3.1+/+ and CaV3.1−/− thalamic slices in the presence or absence of cortex in the slice. (C) Total spike activity in TRN during the oscillatory activity evoked by IC stimulation in the presence or absence of cortex in the slice.
The foregoing results provide direct evidence contrary to the classical view that postinhibitory low-threshold burst firing mediated by T-type Ca2+ channels in TC neurons is essential for the generation of oscillations within the TRN-TC circuit. They suggest that instead, sleep spindles observed in CaV3.1−/− mice may be generated within the TRN-TC circuit in the absence of TC bursts.
Excitability of CaV3.1−/− TC Neurons in Vitro.
Our next step was to examine whether generation of spindle oscillations within the CaV3.1−/− intrathalamic circuit might be related to changes in the intrinsic excitability of TC neurons rather than to a loss of low-threshold burst firing by deletion of CaV3.1 T-type channels. To do this, we used the whole-cell patch-clamp technique to measure the excitability of TC neurons. We found no differences between CaV3.1+/+ (n = 12) and CaV3.1−/− (n = 14) TC neurons in membrane capacitance (141.2 ± 15.6 pF vs. 137.4 ±10.5 pF), resting membrane potential (−59.11 ± 5.34 mV vs. −57.54 ± 6.31 mV), or membrane resistance (219.5 ± 48.2 MΩ vs. 175.4 ± 38.2 MΩ).
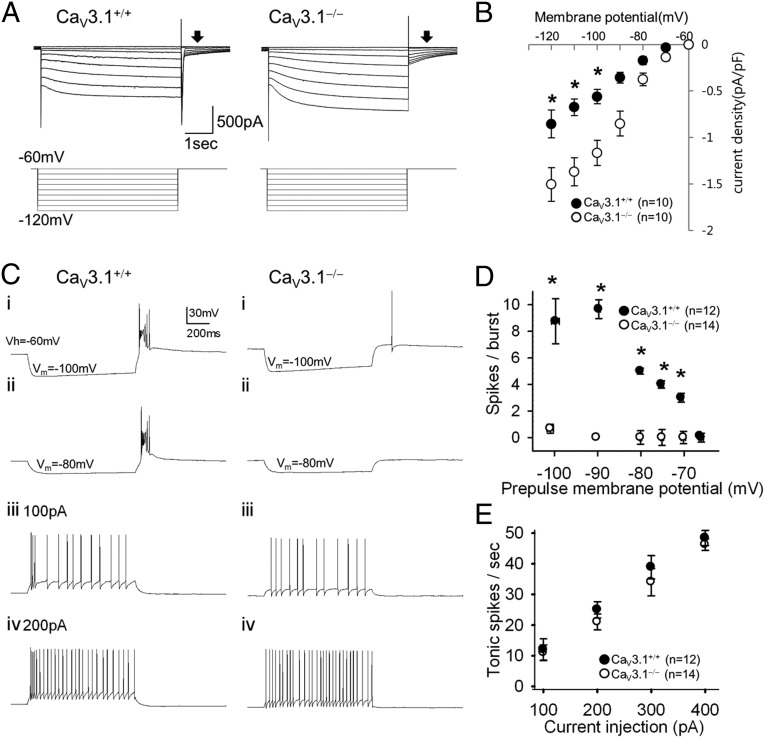
We then measured hyperpolarization-activated cyclic nucleotide-gated channel-mediated current (Ih) in TC neurons at the tail current at 0.7 s after a test voltage pulse (Fig. 5A) to minimize the capacitative contribution (27) or non–Ih-active current (28). We observed an increase in the current density of Ih in CaV3.1−/− TC neurons compared with CaV3.1+/+ TC neurons only when membrane potential was hyperpolarized to ≥−100 mV (Fig. 5 A and B). We then measured the firing pattern of TC neurons. Low-threshold burst firing was ablated in CaV3.1−/− TC neurons, whereas vigorous burst firing was induced in CaV3.1+/+ TC neurons, with a membrane potential rebound to −60 mV after hyperpolarization to −70 to −120 mV (Fig. 5 C and D). Some CaV3.1−/− TC neurons (6 of 10) produced a single spike when the membrane potential was returned to −60 mV after hyperpolarization to ≥−100 mV (Fig. 5 C and D), possibly related to the enhanced Ih current (Fig. 5 A and B). However, the enhanced Ih current did not affect either the tonic or burst firing rate elicited by depolarizing current injections (Fig. 5 C–E), and did not alter any other intrinsic membrane properties of CaV3.1−/− TC neurons. Thus, we conclude that deletion of CaV3.1 T-type channel did not significantly alter the intrinsic excitability of TC neurons. The firing pattern of TRN neurons was not changed either (Fig. S4).
Fig. 5.
Firing properties of CaV3.1−/− TC neurons in vitro. (A) Hyperpolarization-activated cyclic nucleotide-gated channel current (Ih) responses (upper traces) to voltage steps (lower traces) recorded from CaV3.1+/+ (Left) and CaV3.1−/− (Right) TC neurons were measured at the tail current (arrowheads). The membrane potential was held at −60 mV, stepped to a hyperpolarizing potential between −120 and −70 mV to elicit the current, and then returned to −60 mV. (B) The current density of Ih was larger in CaV3.1−/− TC cells compared with CaV3.1+/+ TC cells when membrane potential was hyperpolarized to ≥−100 mV. (C) Representative traces from whole-cell patch recordings show the firing pattern of CaV3.1+/+ and CaV3.1−/− TC neurons responding to hyperpolarizing or depolarizing current inputs. (D) The number of spikes per burst was counted in WT TC neurons (closed circles). No bursts were induced in mutants (open circles). (E) Number of tonic spikes per s induced by various depolarizing currents.
Discussion
We have demonstrated here that sleep spindles and intrathalamic SLOs are generated in the absence of CaV3.1 T-type Ca2+ channels in TC neurons. This finding calls into question the validity of the long-standing hypothesis that LTSs mediated by T-type channels in TC neurons are essential for spindle oscillations.
CaV3.1 T-Type Channels in Sleep Rhythms.
Sleep spindles have been proposed to arise from the intrathalamic circuit composed of TRN and TC neurons (8, 29). Postinhibitory LTSs in TC neurons has been considered essential for the generation of intrathalamic oscillations and their propagation to the cortex (1). According to this hypothesis, spindle oscillations should be diminished, if not abolished, in cortical EEGs of CaV3.1−/− mice lacking LTSs in TC neurons.
In contrast to this expectation, we clearly observed sleep spindle waves in cortical EEG recordings from both CaV3.1+/+ and CaV3.1−/− mice during NREM sleep. We chose a method suitable for analyzing spindle waves in mice (19) used in previous studies (1–3). In a previous study on the sleep phenotype of CaV3.1−/− mice, we filtered EEG data with a high-pass cutoff at 0.1 Hz to include and analyze all sleep rhythms. This did not exclude the possibility that a significant reduction in delta power in CaV3.1−/− mice might exaggerate the difference in the EEG power density of the spindle component (16). In the present study, we used a 6- to 15-Hz bandpass filter to clearly reveal spindle oscillations and exclude delta and slow waves (Figs. 1A and 2A and Fig. S5), and found no difference in the number of sleep spindle episodes between CaV3.1+/+ and CaV3.1−/− mice.
Neither the duration nor the amplitude of the spindle episodes was reduced in CaV3.1−/− mice during natural NREM sleep, raising questions about the essential role of TC neuron T-type channels in the generation of sleep spindles. However, the mean episode duration and amplitude of barbiturate-induced spindles were substantially reduced in CaV3.1−/− mice, in accordance with our previous findings of a reduced EEG power density at delta and spindle frequencies in CaV3.1−/− mice under high-dose urethane (2 mg/kg), another spindle-inducing anesthesia (16).
These results suggest that T-type Ca2+ channels and the resulting LTSs in TC neurons are not required for the generation of spindle oscillations in TC circuits, but may play a role in enhancing the strength of spindles, as observed in barbiturate-induced spindles. This view is quite different from the classical theory that the generation of sleep spindles requires low-threshold burst firing of TC neurons.
A recent study in mice lacking the CaV3.3 T-type channels abundantly expressed in TRN found a selective reduction in spindle power before the onset of REM sleep (30). The same study also found that delta power was unaffected by the absence of CaV3.3 channels (30), whereas CaV3.1−/− mice demonstrated a significantly reduced power density of the delta range (1–4 Hz) during NREM sleep (16). These findings suggest that CaV3.3 T-type Ca2+ channels in TRN neurons contribute to spindles, whereas channels encoded by CaV3.1 genes in TC neurons contribute to the delta waves during NREM sleep.
Firing Patterns During Spindle Oscillations in TC Neurons.
We examined an intriguing question regarding the firing pattern of TC neurons that lack T-type channels in response to inhibitory inputs from the TRN neurons during spindle episodes. Only tonic spikes were observed in TC nuclei in CaV3.1−/− mice, as expected (Fig. 3). Tonic spikes were more abundant than burst spikes during spindles in CaV3.1+/+ TC neurons, whereas no difference in tonic and burst spike frequency was seen during nonspindle periods.
With regard to the critical role of tonic firing in spindles, our most compelling observation may be that in WT TC neurons, the tonic spike frequency increases significantly during cortical spindle events compared with nonspindle periods. This pattern is clearly different from that seen for burst spike frequency in WT TC neurons, which is almost equal during spindle and nonspindle periods. Of interest, the overall spike frequency during spindle episodes in mutant TC neurons with no burst spikes did not differ from that in WT TC neurons, because the degree of increase in tonic spike frequency during spindle periods was much larger in mutant TC neurons. Nevertheless, the duration of barbiturate-induced spindles was shorter in the mutant mice compared with the WT mice (Fig. 2) even though the total spike frequency was similar in the two mice during barbiturate-induced spindles (Fig. 3), implying that TC burst firing may play a role in increasing the duration of barbiturate-induced spindle oscillations. The total spike frequency during nonspindle periods was lower in mutant mice than in the WT mice, a finding that remains to be explained.
The explicit induction of oscillations within an isolated CaV3.1−/− intrathalamic circuit convinced us that spindle oscillations may be generated within the TRN-TC network as suggested previously (31), but in the absence of LTSs in TC neurons. We also confirmed that deletion of CaV3.1 T-type channels had no effect on the intrinsic firing properties of TC neurons (Fig. 5). Taken together, these findings suggest that spindle oscillations can be generated and propagated via any type of TC spike activity as long as TC neurons discharge spikes at an appropriate frequency. This conclusion differs from previous observations on the critical role of TC T-type channels in sleep delta waves (16) and paroxysmal SWDs (13, 32, 33), and leads us to suggest that the various TC oscillations may be generated through diverse mechanisms.
CaV3.1 T-Type Channels in Spindles vs. SWDs.
It has been proposed that TC oscillations occurring during sleep spindles and SWDs of absence seizures share common elements and mechanisms (11). Clinical observations of the temporal coincidence between sleep spindles and SWDs and experimental findings showing that penicillin, a weak GABAAR antagonist, induces the transformation of sleep spindles into SWDs gave rise to the view that SWDs may be a perverted form of sleep spindles (34).
Previous studies have found that CaV3.1−/− mice are resistant to GABAB receptor agonist-induced SWDs (13), and that deletion of CaV3.1 completely suppresses or markedly reduces SWDs in genetic mouse models of absence seizures (35), implying that CaV3.1 T-type Ca2+ channels play a crucial role in the genesis of SWDs. In contrast, the increased T-type Ca2+ currents in TC neurons occur alongside the generation of SWDs and absence seizure phenotypes in transgenic or mutant mice (32, 33). Our present findings support the hypothesis that CaV3.1 T-type Ca2+ channels are not an essential component in the generation of sleep spindles. Overall, these results suggest that paroxysmal TC oscillations during SWDs require the postinhibitory low-threshold burst firing mediated by T-type channels in TC neurons, whereas physiological TC oscillations during sleep spindles do not.
In conclusion, our findings suggest that T-type Ca2+ channels in TC neurons might not be essential for the generation and propagation of spindle oscillations. This study provides valuable insights into the realm of T-type channels as a possible target for differentiation of various TC oscillations and implies that the TC oscillations generated under pathological conditions may be controlled by modulating T-type channels in TC neurons without affecting natural physiological sleep spindles. Our findings raise further questions, including what drives TC neurons to fire during spindles and what determines the spindle frequency. Future studies should be aimed at investigating the detailed mechanism through which each type of TC oscillation is generated.
Materials and Methods
Treatment of animals and all experiments were conducted in accordance with the ethical guidelines of the Institutional Animal Care and Use Committee of Yonsei University, Institute of Basic Science and the Korea Institute of Science and Technology.
The mice and experiments for this study are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank G. Ha for help with the PCR analysis. This work was supported by funding from the Institute for Basic Science (Grant HQ1301), the Translational Research Center for Protein Function Control (Grant NRF-2011-0012170), the Basic Science Research Program (Grant NRF-2011-0014699), and the Pioneer Research Center Program (Grant 2012-0001087), all of which are funded by the Korean Ministry of Science, Information and Communication Technology, and Future Planning.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320572110/-/DCSupplemental.
References
- 1.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 2.Meeren HK, Veening JG, Möderscheim TA, Coenen AM, van Luijtelaar G. Thalamic lesions in a genetic rat model of absence epilepsy: Dissociation between spike-wave discharges and sleep spindles. Exp Neurol. 2009;217(1):25–37. doi: 10.1016/j.expneurol.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Schofield CM, Huguenard JR. GABA affinity shapes IPSCs in thalamic nuclei. J Neurosci. 2007;27(30):7954–7962. doi: 10.1523/JNEUROSCI.0377-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: A study of dynamic corticothalamic relationships. J Neurosci. 1995;15(1 Pt 2):604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contreras D, Destexhe A, Steriade M. Spindle oscillations during cortical spreading depression in naturally sleeping cats. Neuroscience. 1997;77(4):933–936. doi: 10.1016/s0306-4522(96)00573-8. [DOI] [PubMed] [Google Scholar]
- 6.Steriade M, Domich L, Oakson G, Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57(1):260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 7.Steriade M, Deschênes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 8.von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261(5119):361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- 9.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 10.Talley EM, et al. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19(6):1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: Is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62(5):612–632. doi: 10.1016/j.neuron.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheong E, Shin HS. T-type Ca2+ channels in normal and abnormal brain functions. Physiol Rev. 2013;93(3):961–992. doi: 10.1152/physrev.00010.2012. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, et al. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31(1):35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 14.Cheong E, Shin HS. T-type Ca²⁺ channels in absence epilepsy. Biochim Biophys Acta. 2013;1828(7):1560–1571. doi: 10.1016/j.bbamem.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M, Nuñez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13(8):3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Kim D, Shin HS. Lack of delta waves and sleep disturbances during non-rapid eye movement sleep in mice lacking alpha1G-subunit of T-type calcium channels. Proc Natl Acad Sci USA. 2004;101(52):18195–18199. doi: 10.1073/pnas.0408089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson MP, et al. Thalamic Cav3.1 T-type Ca2+ channel plays a crucial role in stabilizing sleep. Proc Natl Acad Sci USA. 2005;102(5):1743–1748. doi: 10.1073/pnas.0409644102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984;228(2):268–274. [PubMed] [Google Scholar]
- 19.Wang XJ, Liu Y, Sanchez-Vives MV, McCormick DA. Adaptation and temporal decorrelation by single neurons in the primary visual cortex. J Neurophysiol. 2003;89(6):3279–3293. doi: 10.1152/jn.00242.2003. [DOI] [PubMed] [Google Scholar]
- 20.Fuentealba P, Timofeev I, Bazhenov M, Sejnowski TJ, Steriade M. Membrane bistability in thalamic reticular neurons during spindle oscillations. J Neurophysiol. 2005;93(1):294–304. doi: 10.1152/jn.00552.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman SM. Dual response modes in lateral geniculate neurons: Mechanisms and functions. Vis Neurosci. 1996;13(2):205–213. doi: 10.1017/s0952523800007446. [DOI] [PubMed] [Google Scholar]
- 22.Contreras D, Steriade M. Spindle oscillation in cats: The role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490(Pt 1):159–179. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95(6):3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 24.Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41(2-3):365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- 25.Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283(5401):541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsen RB, Ulrich D, Huguenard JR. GABA(B) and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. J Neurophysiol. 2001;86(3):1365–1375. doi: 10.1152/jn.2001.86.3.1365. [DOI] [PubMed] [Google Scholar]
- 27.Paz JT, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16(1):64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim U, Bal T, McCormick DA. Spindle waves are propagating synchronized oscillations in the ferret LGNd in vitro. J Neurophysiol. 1995;74(3):1301–1323. doi: 10.1152/jn.1995.74.3.1301. [DOI] [PubMed] [Google Scholar]
- 30.Astori S, et al. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci USA. 2011;108(33):13823–13828. doi: 10.1073/pnas.1105115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30(7):350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Ernst WL, Zhang Y, Yoo JW, Ernst SJ, Noebels JL. Genetic enhancement of thalamocortical network activity by elevating alpha 1g-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci. 2009;29(6):1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheong E, et al. Deletion of phospholipase C beta4 in thalamocortical relay nucleus leads to absence seizures. Proc Natl Acad Sci USA. 2009;106(51):21912–21917. doi: 10.1073/pnas.0912204106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leresche N, Lambert RC, Errington AC, Crunelli V. From sleep spindles of natural sleep to spike and wave discharges of typical absence seizures: Is the hypothesis still valid? Pflugers Arch. 2012;463(1):201–212. doi: 10.1007/s00424-011-1009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song I, et al. Role of the alpha1G T-type calcium channel in spontaneous absence seizures in mutant mice. J Neurosci. 2004;24(22):5249–5257. doi: 10.1523/JNEUROSCI.5546-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.