Significance
Sleep is a homeostatically regulated process. Slow wave sleep is characterized by slow waves detectable from the cerebral cortex by EEG. When homeostatic sleep “drive” is manipulated by varying durations of sleep deprivation, the intensity of EEG slow waves proportionally increases. The neural circuitry underlying this homeostatic response is little understood. In this study we describe a systematic relationship between homeostatic sleep drive and activation of cortical neurons that express neuronal nitric oxide synthase (nNOS). We also find that transgenic mice lacking nNOS have a greatly diminished response to sleep deprivation. We conclude that cortical nNOS neurons and nNOS enzymatic activity are likely involved in the homeostatic regulation of slow wave sleep.
Keywords: sleep homeostasis, cerebral cortex, interneurons, Fos, Nos1
Abstract
Although the neural circuitry underlying homeostatic sleep regulation is little understood, cortical neurons immunoreactive for neuronal nitric oxide synthase (nNOS) and the neurokinin-1 receptor (NK1) have been proposed to be involved in this physiological process. By systematically manipulating the durations of sleep deprivation and subsequent recovery sleep, we show that activation of cortical nNOS/NK1 neurons is directly related to non-rapid eye movement (NREM) sleep time, NREM bout duration, and EEG δ power during NREM sleep, an index of preexisting homeostatic sleep drive. Conversely, nNOS knockout mice show reduced NREM sleep time, shorter NREM bouts, and decreased power in the low δ range during NREM sleep, despite constitutively elevated sleep drive. Cortical NK1 neurons are still activated in response to sleep deprivation in these mice but, in the absence of nNOS, they are unable to up-regulate NREM δ power appropriately. These findings support the hypothesis that cortical nNOS/NK1 neurons translate homeostatic sleep drive into up-regulation of NREM δ power through an NO-dependent mechanism.
The electrical activity of the cerebral cortex has been used to distinguish sleep vs. wakefulness since the earliest EEG studies of sleep (1). Several neural circuits have been implicated in the synchronization and desynchronization of cortical activity that distinguish non-rapid eye movement sleep (NREM) from wakefulness and rapid eye movement sleep (REM). Input from the basal forebrain (BF), likely from both cholinergic and noncholinergic neurons, is critical for the desynchronized EEG characteristic of wakefulness and REM (2, 3). Synchronization of the EEG during NREM depends on thalamic as well as intrinsic cortical oscillators (4).
The firing rate of cortical neurons has generally been reported to be reduced during NREM relative to wakefulness and REM (5, 6). A few studies have reported cortical neurons with the opposite pattern. For example, 4 of 177 neurons in the monkey orbitofrontal cortex increased their firing rates during NREM (7). In the cat parietal cortex, 25% of neurons discharged in phase with NREM slow waves during up states but ceased firing during quiet wakefulness (8).
Using Fos immunohistochemistry as a marker of cellular activity, we described a population of cortical GABAergic interneurons that are activated during sleep in three species (9, 10). These neurons express neuronal nitric oxide synthase (nNOS) and thus likely release nitric oxide (NO) as well as GABA (11). The percentage of activated cortical nNOS neurons was proportional to NREM δ energy (NRDE), the product of NREM time and NREM EEG δ power. Because NREM time and δ power increase in response to prolonged wakefulness through a regulated process referred to as sleep homeostasis, NRDE is an electrophysiological marker of homeostatic sleep “drive.” Consequently, activation of cortical nNOS neurons during sleep seems to be related to the sleep need that accrues during wakefulness.
Cortical nNOS neurons receive cholinergic (12), monoaminergic (13), and peptidergic (14, 15) inputs. Sleep-active nNOS cells correspond to type I nNOS neurons, which are larger and less numerous than type II cells, express the neurokinin-1 receptor (NK1; 14, 15), and are likely projection neurons (16, 17). On the basis of these observations, we have proposed that cortical nNOS/NK1 neurons are fundamentally related to the homeostatic regulation of sleep and play a critical role in coordinating slow waves within the cortex, possibly through release of NO (18).
To further test this hypothesis, we subjected rats to varying durations of sleep deprivation (SD) and recovery sleep opportunities (RS) and evaluated which sleep parameters were most closely correlated with cortical nNOS/NK1 neuron activation. We then asked whether those sleep parameters were altered in nNOS KO mice. Consistent with a role for cortical nNOS/NK1 neurons in sleep homeostasis, we find that these neurons are most active during consolidated NREM with increased δ power, that the absence of nNOS results in attenuation of NREM consolidation and NREM δ power, and that nNOS KO mice are unable to respond appropriately to challenges to the sleep homeostatic system.
Results
Fos Expression in Cortical nNOS Neurons Is Associated with Spontaneous Sleep and Wakefulness.
Because Fos expression in cortical nNOS neurons declines during spontaneous sleep and wakefulness across the day in mouse and hamster (10), we determined whether this decline also occurred in the rat. Fig. S1 presents results from four groups of rats killed at 2-h intervals across the day (protocol 1). Fig. S1 A and B illustrate typical results in which rats killed early in the day show a higher proportion of Fos-positive cortical nNOS neurons (%Fos/nNOS) than rats killed late in the day. Fig. S1C presents summary data showing that the %Fos/nNOS declined from Zeitgeber Time (ZT)4 to ZT10, i.e., from 4 to 10 h after lights on. Kruskal-Wallis one-way ANOVA revealed significant variation among groups [H(3) = 10.49, P = 0.015] and the Bonferroni-Dunn post hoc test established that the ZT6 and ZT10 groups differed from each other (Q = 2.91, P < 0.05).
The Proportion of Cortical nNOS Neurons That Express Fos Depends on the Magnitude of Homeostatic Sleep Drive and Recovery Sleep Duration.
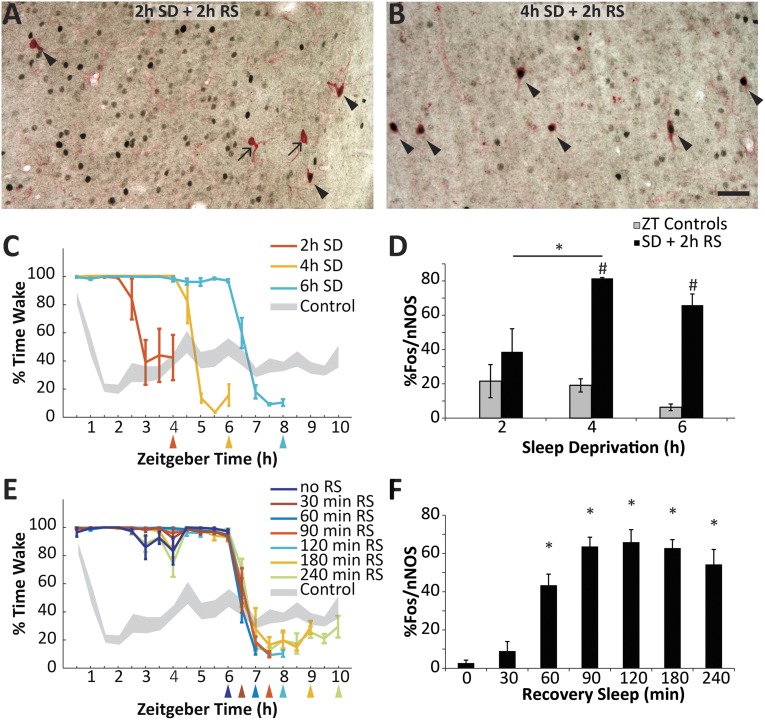
Because we established that Fos expression in cortical nNOS neurons can be increased by SD (10), we determined the minimum SD duration necessary to increase the %Fos/nNOS when RS was fixed at 2 h (protocol 2). Fig. 1 A and B shows representative immunohistochemical results. Although relatively few double-labeled cells were evident after 2 h SD followed by 2 h RS (Fig. 1A), the proportion of double-labeled neurons was greatly increased by 4 h SD followed by 2 h RS (Fig. 1B). Fig. 1C presents the time course of wakefulness observed in three groups of rats that were subjected to SD beginning at light onset for 2–6 h followed by 2 h RS. Fig. 1D presents summary results indicating that the %Fos/nNOS increased significantly above the respective time-matched control groups after both 4 h (t = 13.73, n = 5; P < 0.001) and 6 h of SD (t = 7.16, n = 8; P < 0.001). One-way ANOVA revealed an effect of SD duration (F2,11 = 4.35; P = 0.04), and the Holm-Sidak post hoc test confirmed a significant increase from 2 h to 4 h of SD (t = 2.78; P = 0.035).
Fig. 1.
The proportion of cortical nNOS neurons expressing Fos is dependent on homeostatic sleep drive and recovery sleep duration. (A) Representative results from the cortex of a rat after 2 h SD plus 2 h RS. Arrows indicate single-labeled nNOS neurons; arrowheads indicate double-labeled Fos+/nNOS cells. (B) Representative results from the cortex of a rat after 4 h SD plus 2 h RS. (C) The percent time awake in rats sleep deprived beginning at ZT0 for either 2 h, 4 h, or 6 h followed by a 2 h RS opportunity (protocol 2). Colored lines denote the experimental groups; gray shading is the pooled control (non-SD) rats (±SEM from mean). Colored triangles below the x axis indicate the time of killing for each group. Because control rats were also killed at the times indicated by the triangles in C and E, the number of rats in the pooled control group decreases from 25 at ZT4 to 8 at ZT10. (D) Proportion of double-labeled nNOS neurons in the three experimental groups depicted in C (black); gray bars are the control values at ZT4, ZT6, and ZT8 replotted from Fig. S1. *P < 0.05 for Holm-Sidak post hoc test after ANOVA; #P < 0.05 for t test against time-matched control group. (E) Percent time awake in rats after 6 h SD followed by RS opportunities ranging from 0 to 240 min. Gray shading represents the pooled control as in C. The 6 h SD + 2 h RS group is also plotted in C. (F) Proportion of double-labeled cortical nNOS neurons in the seven groups depicted in E. *P < 0.05 for Holm-Sidak post hoc test against RS = 0 min after ANOVA. (Scale bar, 50 µm.)
Conversely, in protocol 3, the duration of SD was fixed at 6 h (Fig. 1E), and the time course of Fos expression was determined as the RS duration was increased. Fig. 1F demonstrates that the %Fos/nNOS is dependent on the RS duration. Almost no double-labeled cells were found without RS, and 30 min RS was insufficient to produce a significant increase. However, ANOVA revealed a significant variation among the groups (F6,33 = 19.62; P < 0.001), and the Holm-Sidak post hoc test confirmed a significant increase in %Fos/nNOS for all groups with RS >30 min relative to the RS = 0 min group. The proportion of double-labeled cells reached a plateau after 90 min.
Activation of Cortical nNOS Neurons Is Related to NREM Sleep and Most Strongly Correlated with NREM δ Energy.
The results presented in Fig. 1 established that the %Fos/nNOS depends on both the magnitude of homeostatic sleep drive and RS duration. RS is characterized by increased levels of NREM, REM, and EEG power in the δ range (0.5–4.5 Hz). To determine which of these sleep parameters was most closely linked to activation of cortical nNOS neurons, we correlated the magnitude of these parameters with %Fos/nNOS during the time preceding killing. Because the %Fos/nNOS reached a plateau after 90 min of RS (Fig. 1F), we focused our analyses on the 90 min before perfusion. The dataset comprised 75 rats from 15 experimental groups: the 13 distinct groups from protocols 1–3 that are depicted in Fig. 1 and Fig. S1, an additional group from protocol 1 killed at ZT9 after spontaneous sleep and wakefulness, and a group that underwent 2 h SD followed by 4 h RS.
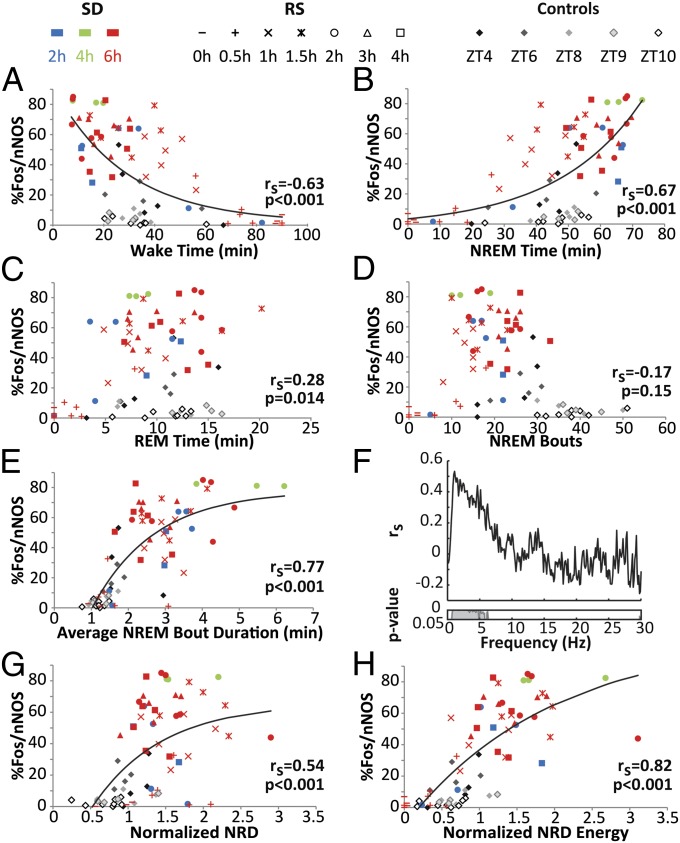
Fig. 2 presents correlations between the %Fos/nNOS and several physiological parameters. Table S1 presents the amount of each state and other sleep parameters of the 15 groups during the 90 min before killing. The %Fos/nNOS was inversely correlated with the duration of wakefulness (Fig. 2A) and directly correlated with the duration of NREM (Fig. 2B) but only weakly correlated with REM (Fig. 2C). Because the strongest correlation was with NREM, we explored this further and found that, although there was no significant correlation with the number of NREM bouts (Fig. 2D), there was a strong correlation with NREM bout duration (Fig. 2E).
Fig. 2.
The proportion of cortical nNOS neurons expressing Fos is related to NREM and most strongly correlated with NREM δ energy. (A–E) Correlations between the %Fos/nNOS and (A) wakefulness, (B) NREM, (C) REM, (D) number of NREM bouts, and (E) average NREM bout duration during the 90 min before killing for the 75 rats across the 15 groups. Spearman’s correlation coefficients and corresponding P values are at bottom right in each panel. (F) Spearman’s correlation coefficients calculated between the %Fos/nNOS and 0.122-Hz frequency bins of the EEG power spectra during NREM sleep are plotted from 0 to 30 Hz. For each rat, the NREM power spectrum during the 90 min before killing was normalized by division by the NREM power spectrum during baseline (ZT5–7). Inset below shows P values for each 0.122-Hz bin. (G) Correlation between %Fos/nNOS and normalized NREM δ power. (H) Correlation between %Fos/nNOS and normalized NREM δ energy. Exponential growth or exponential growth to maximum functions were fitted for correlations where rs > 0.3.
To determine whether activation of cortical nNOS neurons was related to particular EEG frequencies during NREM, we correlated the %Fos/nNOS with the normalized spectral power in the NREM EEG in 0.122-Hz bins. Fig. 2F indicates that only frequencies between 0.5 and 6.0 Hz were significantly correlated with %Fos/nNOS, which includes the δ range. Given the results in Fig. 2 A–F, we calculated the correlations of %Fos/nNOS with normalized NREM δ power (0.5–4.5 Hz; NRD) and with NRDE, the mathematical product of normalized NRD × NREM time. NRDE had a higher correlation with %Fos/nNOS than normalized NRD (Fig. 2 G and H).
nNOS Null Mutant Mice Have Impaired Regulation of EEG δ Power.
Given the above results, we hypothesized that nNOS enzymatic activity was involved in sleep homeostasis and investigated sleep/wake in nNOS KO mice. The results obtained in baseline recordings using abdominally placed telemeters were discordant from those previously published in the same strain (19). Consequently, we replicated our study of nNOS KO and WT mice using tethered EEG and electromyographic (EMG) recordings, which more closely approximated the previously published procedures and which we have also used previously in both rats (20) and mice (21). Because the results from these two types of recordings in our laboratory were largely coincident (compare Fig. S2 vs. Fig. 3 and Fig. S3), we report here the results obtained from telemetry recordings of 8 male nNOS KO mice and 12 male WT mice.
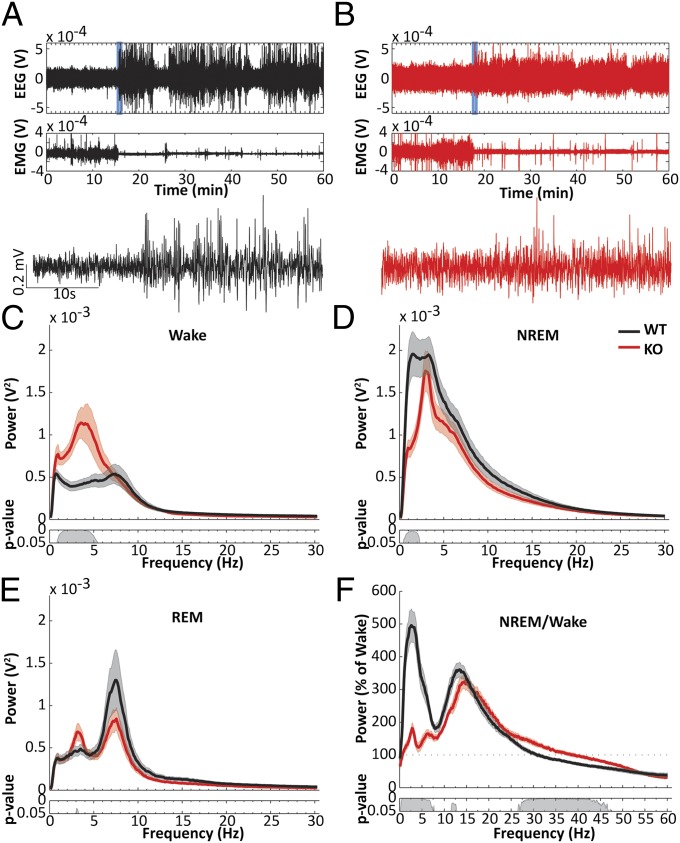
Fig. 3.
EEG activity is abnormal during both waking and sleep in nNOS KO mice. (A and B) Example EEG and EMG recordings from a WT (A; black) and an nNOS KO mouse (B; red). (Lower) EEG during a transition from wakefulness to NREM in the periods depicted by the blue boxes in the EEG trace above. (C) Mean wake EEG spectrum across a 24-h period from WT (black) and nNOS KO mice (red). (Inset) P values for post hoc t tests between the two genotypes after permutation ANOVA. (D) Mean NREM EEG spectra. (E) Mean REM EEG spectra. (F) NREM spectra expressed as percent of wake spectra. Note that the increase during NREM in the low (<5 Hz) frequencies is strongly attenuated in nNOS KO mice, whereas the increase during NREM from 7.5 Hz to 25 Hz is comparable between the two strains.
As illustrated in Fig. 3 A and B, the distinction between wakefulness and sleep was much less clear in the EEG of nNOS KO than WT mice. During wakefulness, nNOS KO mice showed significantly greater spectral power in the δ range between 1 and 5.5 Hz (Fig. 3C). Conversely, during NREM, nNOS KO mice showed significantly less spectral power in the low δ range between 0.5 and 2.5 Hz (Fig. 3D). In all states, nNOS KO mice seemed to have a narrow peak of spectral power between 3 and 4 Hz (Fig. 3 C–E). When EEG spectral power during NREM was expressed as a percentage of the corresponding wake spectra, the characteristic increase in δ power during NREM was virtually absent, and the reduction in low γ frequencies (26–47 Hz) was attenuated in nNOS KO mice (Fig. 3F). In contrast, the increase in the β range (12–25 Hz) was largely unaffected by absence of nNOS, although the peak was shifted to a slightly higher frequency.
nNOS KO Mice Have Less NREM Sleep and Shorter NREM Bouts than WT Mice.
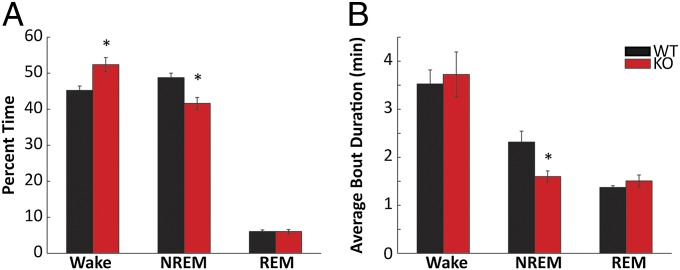
When examined across the 24-h period, nNOS KO mice showed more wakefulness (P = 0.004) and less NREM (P = 0.003; Fig. 4A) than WT mice. Although the number of NREM bouts did not change, the average NREM bout duration was one-third shorter in nNOS KO mice than in WT mice (P = 0.03; Fig. 4B), suggesting that the mechanism underlying the reduction in NREM time is the inability of nNOS KO mice to sustain longer NREM bouts. These effects were independent of time of day (Fig. S3 A and C). Mixed-model ANOVA revealed main effects for genotype for both NREM time (F1,239 = 12.23, P = 0.003) and NREM bout duration (F1,239 = 6.46, P = 0.02) but no interactions between genotype and time (2-h bins) for either parameter.
Fig. 4.
nNOS KO mice have less NREM and shorter NREM bouts than WT mice. (A) Percentage of time spent in wake, NREM, and REM across the 24 h period in WT (black) and nNOS KO (red) mice. (B) Average bout durations of each of the three states across the 24-h period. *P < 0.05 for t test against WT.
Although the average wake bout duration did not differ significantly between the two strains, nNOS KO mice spent less time in long bouts of wakefulness (>32 min) than WT mice and more time in shorter wake bouts (16–32 min; Fig. S3B). The reduced NREM sleep and shorter NREM bout duration in nNOS KO mice was reflected in an overall shift to NREM bouts of shorter duration compared with WT mice, with more time being spent in bouts <1 min in the KOs (Fig. S3D).
The Homeostatic Response to Sleep Loss Is Greatly Diminished in nNOS KO Mice.
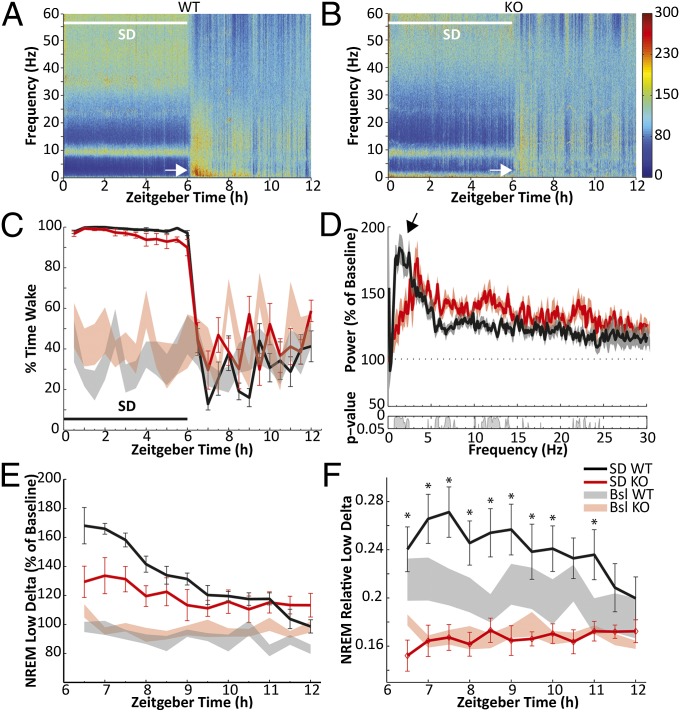
In the absence of nNOS, the homeostatic response to SD was greatly reduced. Fig. 5 A and B presents heat maps of the EEG frequency spectra recorded during 6 h SD and subsequent 6 h RS in WT and nNOS KO mice. These heat maps are expressed as a percentage of the corresponding 24-h baseline spectra which, to account for the genotypic differences in amounts of NREM sleep, were calculated as the mean of the average wake and NREM spectra recorded on the baseline day. Whereas an increase in spectral power in the δ range (0.5–4.5 Hz) of the EEG is clearly evident for at least 90 min after termination of SD in WT mice (white arrow in Fig. 5A), little or no response was evident in nNOS KO mice (white arrow in Fig. 5B). Fig. 5C demonstrates the efficacy of our procedures during the 6-h SD period. Fig. 5D presents the NREM EEG spectrum (0–30 Hz) for the first 90 min of RS for both genotypes as a percentage of the NREM spectrum during the time-matched period on the baseline day. Both genotypes increased power across the entire spectrum in response to 6 h of SD. However, whereas WT mice showed the greatest increase in the low δ range (0.5–2.5 Hz, arrow), the increase in this range was selectively attenuated in nNOS KO mice. The time course of low δ during 6 h RS shows this difference in greater detail (Fig. 5E). When NREM low δ during RS was normalized by the baseline values and compared between genotypes by mixed-model two-way permutation ANOVA, a main effect for time (F11,197 = 17.65, P < 0.001) and for the time × genotype interaction (F11,197 = 4.89, P < 0.001) was revealed. Although the Holm-Sidak post hoc test did not identify the source of the interaction effect, the largest differences between genotypes were in the first 2 h of RS (Fig. 5E). Last, when expressed as a proportion of spectral power of all frequencies (0–60 Hz), the increase in low δ evident in WT mice during the recovery period was completely absent in nNOS KO mice (Fig. 5F). Thus, despite an overall increase in spectral power in response to 6 h of SD (Fig. 5 D and E), in the absence of nNOS, spectral power was not shifted toward the low δ range (Fig. 5F) as would be expected after SD.
Fig. 5.
The homeostatic response to sleep loss is diminished in nNOS KO mice. (A) Heat map presenting average normalized EEG spectra from 12 WT mice during 6 h SD (white bar) and subsequent 6 h RS. White arrow indicates intense δ activity during the first hours of RS. (B) Heat map from eight nNOS KO mice plotted as in A. White arrow indicates diminished response in the δ range during RS. (C) Wakefulness during SD (black bar) and RS corresponding to A and B for WT (black) and KO (red). Shaded areas indicate wakefulness during the baseline day. (D) NREM spectral power during the first 90 min of RS (ZT6–7.5) relative to NREM spectra during the same time period of the baseline day in WT and KO mice. Arrow highlights greater increase in the low δ (0.5–2.5 Hz) range in WT. No differences were found above 30 Hz. (Inset) P values for post hoc t tests between the two genotypes after permutation ANOVA. (E) Spectral power in the low δ (0.5–2.5 Hz) range during RS relative to the average baseline value in WT and KO. The increase is blunted in KO mice. Shaded areas indicate NREM low δ power on the baseline day. (F) Spectral power in the low δ range (0.5–2.5 Hz) as the proportion of the entire NREM spectra during RS in WT and KO. Shaded areas indicate the relative NREM δ power on the baseline day. In KO mice, there is essentially no response to SD. *P < 0.05 within-subject Holm-Sidak post hoc test against baseline after two-way repeated-measures ANOVA with factors “SD condition” and “time bin.”
NREM bout duration is also considered an indicator of the homeostatic response to SD. As in baseline conditions, NREM bout duration in response to 6 h SD was shorter in nNOS KO than WT mice (Fig. S4D), although the proportional increase relative to baseline was comparable between the genotypes (Fig. S4E).
nNOS KO Mice Are Sleepier Than WT Mice.
The Multiple Sleep Latency Test (MSLT), a tool used in both clinical and research settings to quantify sleepiness, has been adapted for use in rodents (22). During the 20-min nap opportunities in the murine MSLT, nNOS KO mice had more NREM (P = 0.005; Fig. S5 A and C) and REM (P = 0.019; Fig. S5 B and D) sleep as well as a shorter latency to NREM (P = 0.014; Fig. S5C) than WT mice. Thus, despite having a reduced homeostatic response to SD (Fig. 5), nNOS KO mice were objectively sleepier than WT mice. Experimenter observations during the period of SD reported that some mice were more difficult to keep awake during SD than others. Once the blind was revealed, retrospective analyses established that these mice were nNOS KO mice.
Cortical nNOS/NK1 Cells Are Activated by Sleep Deprivation in the Absence of nNOS.
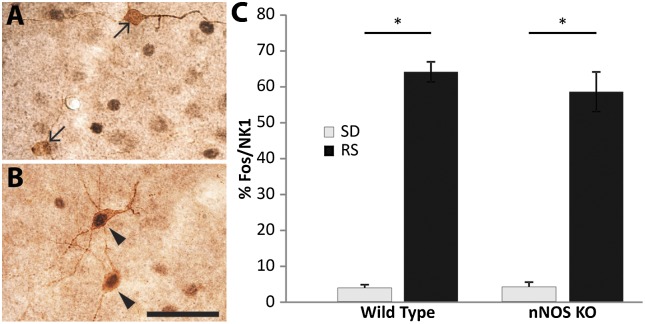
Immunohistochemical staining confirmed the absence of nNOS cells in the cerebral cortex of mice established to be nNOS KO by PCR genotyping (Fig. S6). Although only ∼70% of cortical NK1 neurons in rats coexpress nNOS (14, 15), overlap of these two markers in the mouse cortex is virtually complete (14). Because nNOS KO mice are deficient in the homeostatic sleep response (Fig. 5), we investigated whether the presence of nNOS was necessary for activation of these cells. When nNOS KO mice were deprived of sleep for 6 h and then allowed 2 h RS, the %Fos/NK1 was indistinguishable between nNOS KO and WT mice (Fig. 6). Thus, nNOS KO mice can detect homeostatic sleep drive but, in the absence of nNOS, are unable to recruit an appropriate homeostatic response.
Fig. 6.
Cortical NK1 neurons are activated during RS in the absence of nNOS. (A) Cortical NK1 neurons (brown) do not express Fos (black) in nNOS KO mice killed at the end of 6 h SD. (B) In contrast, cortical NK1 neurons express Fos after 6 h SD followed by a 2 h RS opportunity. (C) The %Fos/NK1 neurons is significantly increased after 2 h RS in both WT and KO mice, even though the homeostatic response differs between these two strains as demonstrated in Fig. 5. Arrows, single-labeled NK1 neurons; arrowheads, double-labeled Fos/NK1 cells. (Scale bar, 50 µM.)
Discussion
By systematically manipulating the durations of SD and RS, we found that activation of cortical nNOS/NK1 neurons was most highly correlated with NREM consolidation and NREM δ energy. In the absence of nNOS, mice cannot sustain long bouts of NREM sleep, and both cortical slow wave activity and homeostatic sleep regulation are disrupted, yet cortical NK1 neurons are still activated by increased sleep pressure. These results are consistent with our hypothesis that cortical nNOS/NK1 neurons are part of the neural substrate underlying sleep homeostasis (18). We suggest here that cortical nNOS/NK1 neurons coordinate the homeostatic sleep response by integrating homeostatic sleep drive and linking it to EEG slow wave activity, likely through release of NO.
As shown previously in mice and hamsters (10), the %Fos/nNOS in rats undergoing spontaneous sleep and wakefulness decreased across the light (i.e., inactive) period when homeostatic sleep drive is expected to dissipate. When sleep pressure was increased by SD, up to 80% of cortical nNOS neurons were activated. Although 2 h of SD did not produce a significant increase, SD of 4 h or longer maximally activated these cells. When SD duration was kept constant and RS duration was varied, %Fos/nNOS significantly increased after 60 min and reached a plateau after 90 min. A similar time course of Fos induction has been found in many studies, for example in rat visual cortex after ongoing photic stimulation (23). Consequently, we can infer that activation of cortical nNOS neurons is initiated in conjunction with RS. Conversely, decay of Fos could be expected to occur by 60–90 min after stimulus cessation (23). The fact that %Fos/nNOS remained elevated even after 240 min of RS implies that activation of these neurons was ongoing, although the slight downward trend may indicate a slow decrease.
We found that time awake during the 90 min before killing was a negative predictor of cortical nNOS neuron activation, indicating that these cells are inactive during wake as observed previously (10). Conversely, time spent in NREM was highly predictive of %Fos/nNOS, indicating that these neurons are highly active during NREM. The weak correlation of %Fos/nNOS with REM time is likely a secondary consequence of the fact that the groups with lowest %Fos/nNOS—rats kept awake without RS—were acutely deprived of both NREM and REM.
Because NREM bout number was not correlated with %Fos/nNOS, it is unlikely that these neurons are activated during wake/NREM transitions (i.e., during the process of falling asleep) rather than during NREM itself. In contrast, a high correlation was found between %Fos/nNOS and NREM bout duration. The %Fos/nNOS was also highly correlated with average NRD, but NRDE had the highest predictive value of all measures tested. Because Fos expression is an integrative signal of neuronal activation and NRDE is an integrative measure of NRD over time, this result indicates that cortical nNOS neurons are most active during NREM sleep with elevated NRD. Intriguingly, nNOS KO mice had defects in these same parameters: NREM time and mean bout duration were reduced relative to WT mice, and nNOS KO mice exhibited a deficit in the low NREM δ power. Both of these deficits likely contributed to the inability to respond appropriately to a homeostatic sleep challenge.
It is unclear why our results differed from an earlier report on the same strain of nNOS KO mice (19). The location of EEG electrodes was comparable in both studies. It is possible that drift in the genetic background has occurred and changed expression of the phenotype. However, our results are in agreement with pharmacological blockade of nNOS (24) and knockout of cGMP-dependent protein kinase type I, a downstream effector of NO signaling (25), indicating that the phenotype we observed is caused by the lack of nNOS. Although it could be argued that nNOS KO mice simply need less sleep, the MSLT results suggest that nNOS KO mice are under elevated sleep pressure, likely a consequence of their NREM consolidation deficit and diminished ability to generate slow wave activity. The increased δ power during wake may be a result of elevated sleep pressure in nNOS KO mice. However, it cannot be excluded that the wake EEG abnormalities in nNOS KO mice result from the absence of nNOS in a wake-promoting system, rather than being an indirect consequence of impaired NREM regulation.
The deficits described here in nNOS KO mice suggest that NO is necessary to maintain sustained NREM bouts and for the synchronization of firing among cortical neurons that underlies EEG activity in the low δ range (4). NO release might facilitate δ waves by reducing neuronal excitability or synaptic transmission, thereby increasing the propensity for neuronal down-states (26).
A limitation of the present study is that the nNOS KO mice we studied had constitutive, rather than cortex-specific, elimination of nNOS. nNOS is expressed in several subcortical areas involved in sleep/wake control, including the BF, dorsal raphe nuclei, and laterodorsal tegmental nuclei. On the other hand, we have recently shown that type I cortical nNOS neurons are the only nNOS population in the brain that is sleep-active (9), with the possible exception of some striatal neurons (10). Thus, we think it is likely that the deficits described here are due largely to the absence of nNOS from the cortex.
An extensive literature indicates that buildup of adenosine in the BF during wakefulness, possibly originating from astrocytes (27), is critical for the homeostatic sleep response, and that BF cholinergic neurons may be required (28–30). NO production in the BF has been proposed to be essential for RS (31), with inducible NOS (iNOS) being implicated specifically in NREM recovery (32, 33). Because BF cholinergic neurons are known to project to cortical nNOS neurons (12), a neural circuit involving adenosine, BF cholinergic neurons, iNOS, cortical nNOS/NK1 neurons, NO, and likely other yet-to-be-identified elements may underlie global homeostatic sleep regulation. Use-dependent accumulation of sleep-regulatory substances, such as adenosine and cytokines, during wakefulness is thought to underlie local regulation of NREM δ activity (34). Because type I sleep-active nNOS neurons are distributed throughout all cortical areas, these cells might integrate signals of increased sleep need and respond with locally increased NO release during subsequent sleep.
The synaptic homeostasis hypothesis (35) posits that average synaptic strength is reduced during sleep to restore the ability to acquire new information during waking by synaptic potentiation. NREM slow waves are proposed to contribute to this process because they resemble the 1-Hz stimulation used to experimentally induce long-term depression. Interestingly, induction of synaptic depression in the cortex by 1-Hz stimulation requires NO and is blocked by an nNOS-specific inhibitor (36). Thus, cortical nNOS neurons might contribute to synaptic downscaling by facilitating slow waves and providing NO as part of the required neuromodulatory environment (37).
As discussed above, nNOS KO mice have defects in the same parameters that are highly correlated with cortical nNOS/NK1 neuron activation: NREM time, NREM bout duration, and NREM δ power. However, even in the absence of nNOS, cortical NK1 neurons are activated in response to elevated sleep pressure. Consequently, we propose that cortical nNOS/NK1 neurons are not only responsive to homeostatic sleep drive but integrate this input and transform it to an output signal in the form of NO which, in turn, plays a critical role in the production of cortical slow wave activity.
Materials and Methods
Detailed methodology is provided in SI Materials and Methods.
Animals.
All experimental procedures involving animals were approved by SRI International’s Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health (NIH) guidelines. For the functional anatomical studies, 75 male Sprague-Dawley rats were distributed among the 15 experimental groups described in SI Materials and Methods, Experimental Protocols. To determine the sleep/wake phenotype of nNOS KO mice, 13 male B6;129S4-Nos1tm1 Plh nNOS KO and 17 B6;129SF2/J WT mice (Jackson Laboratories) were separated into two groups for either telemetric or tethered EEG recordings.
EEG/EMG Recording and Analyses.
Rats were recorded by telemetry (10, 38, 39); mice were recorded either by telemetry (40) or by tethered (10, 21) procedures. State determinations and data analysis were conducted as described in these publications.
Immunohistochemistry and Cell Counts.
Rat brain sections were stained for Fos and nNOS as described previously (9, 10). Single-labeled nNOS and double-labeled Fos/nNOS cells were counted in two hemisections of the cerebral cortex between 2.8 and 3.8 mm posterior to bregma. Mouse brain sections (40 µm) were stained for Fos, incubated in rabbit anti-NK1 antibody (1:1,000; gift from Ryuichi Shigemoto, IST Austria, Klosterneuburg) and visualized with a peroxidase system (Nova Red, SK-4805, Vector Laboratories). Single-labeled NK1 and double-labeled Fos/NK1 cells were counted in two hemisections of the cerebral cortex, one at +0.9 to +1.1 mm and one at −1.2 to −1.7 mm from bregma.
Supplementary Material
Acknowledgments
We thank Kristy Silveira, Alan Wilk, and Deepti Warrier for technical assistance, and Prof. Ryuichi Shigemoto for the NK1 antisera. Research was supported by NIH Grant R01 HL59658, US Army Medical Research Acquisition Activity Grant DR080789P1, Deutsche Forschungsgemeinschaft Fellowship DI 1718/1-1, and SRI International internal research funds.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 19982.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314762110/-/DCSupplemental.
References
- 1.Loomis AL, Harvey EN, Hobart G. Potential rhythms of the cerebral cortex during sleep. Science. 1935;81(2111):597–598. doi: 10.1126/science.81.2111.597. [DOI] [PubMed] [Google Scholar]
- 2.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: A dialogue between three cardinal oscillators. Nat Neurosci. 2010;13(1):9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel JM. The neurobiology of sleep. Semin Neurol. 2009;29(4):277–296. doi: 10.1055/s-0029-1237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szymusiak R. Hypothalamic versus neocortical control of sleep. Curr Opin Pulm Med. 2010;16(6):530–535. doi: 10.1097/MCP.0b013e32833eec92. [DOI] [PubMed] [Google Scholar]
- 7.Rolls ET, Inoue K, Browning A. Activity of primate subgenual cingulate cortex neurons is related to sleep. J Neurophysiol. 2003;90(1):134–142. doi: 10.1152/jn.00770.2002. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph M, Pospischil M, Timofeev I, Destexhe A. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci. 2007;27(20):5280–5290. doi: 10.1523/JNEUROSCI.4652-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasumarthi RK, Gerashchenko D, Kilduff TS. Further characterization of sleep-active neuronal nitric oxide synthase neurons in the mouse brain. Neuroscience. 2010;169(1):149–157. doi: 10.1016/j.neuroscience.2010.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerashchenko D, et al. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci USA. 2008;105(29):10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota Y, et al. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011;21(8):1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- 12.Vaucher E, Linville D, Hamel E. Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neuroscience. 1997;79(3):827–836. doi: 10.1016/s0306-4522(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 13.Cauli B, et al. Cortical GABA interneurons in neurovascular coupling: Relays for subcortical vasoactive pathways. J Neurosci. 2004;24(41):8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dittrich L, et al. Cortical nNOS neurons co-express the NK1 receptor and are depolarized by Substance P in multiple mammalian species. Front Neural Circuits. 2012;6:31. doi: 10.3389/fncir.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vruwink M, Schmidt HH, Weinberg RJ, Burette A. Substance P and nitric oxide signaling in cerebral cortex: Anatomical evidence for reciprocal signaling between two classes of interneurons. J Comp Neurol. 2001;441(4):288–301. doi: 10.1002/cne.1413. [DOI] [PubMed] [Google Scholar]
- 16.Tomioka R, et al. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21(6):1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- 17.Higo S, Akashi K, Sakimura K, Tamamaki N. Subtypes of GABAergic neurons project axons in the neocortex. Front Neuroanat. 2009;3:25. doi: 10.3389/neuro.05.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilduff TS, Cauli B, Gerashchenko D. Activation of cortical interneurons during sleep: An anatomical link to homeostatic sleep regulation? Trends Neurosci. 2011;34(1):10–19. doi: 10.1016/j.tins.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Majde JA, Krueger JM. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 2003;973(2):214–222. doi: 10.1016/s0006-8993(03)02484-3. [DOI] [PubMed] [Google Scholar]
- 20.Morairty SR, Wisor J, Silveira K, Sinko W, Kilduff TS. The wake-promoting effects of hypocretin-1 are attenuated in old rats. Neurobiol Aging. 2011;32(8):1514–1527. doi: 10.1016/j.neurobiolaging.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher SP, et al. Longitudinal analysis of the EEG and sleep phenotype in the R6/2 mouse model of Huntington’s disease. Brain. 2013;136(Pt 7):2159–2172. doi: 10.1093/brain/awt132. [DOI] [PubMed] [Google Scholar]
- 22.Veasey SC, Yeou-Jey H, Thayer P, Fenik P. Murine Multiple Sleep Latency Test: Phenotyping sleep propensity in mice. Sleep. 2004;27(3):388–393. doi: 10.1093/sleep/27.3.388. [DOI] [PubMed] [Google Scholar]
- 23.Zangenehpour S, Chaudhuri A. Differential induction and decay curves of c-fos and zif268 revealed through dual activity maps. Brain Res Mol Brain Res. 2002;109(1-2):221–225. doi: 10.1016/s0169-328x(02)00556-9. [DOI] [PubMed] [Google Scholar]
- 24.Cavas M, Navarro JF. Effects of selective neuronal nitric oxide synthase inhibition on sleep and wakefulness in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(1):56–67. doi: 10.1016/j.pnpbp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Langmesser S, et al. cGMP-dependent protein kinase type I is implicated in the regulation of the timing and quality of sleep and wakefulness. PLoS ONE. 2009;4(1):e4238. doi: 10.1371/journal.pone.0004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vyazovskiy VV, Harris KD. Sleep and the single neuron: The role of global slow oscillations in individual cell rest. Nat Rev Neurosci. 2013;14(6):443–451. doi: 10.1038/nrn3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halassa MM, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61(2):213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porkka-Heiskanen T. Sleep homeostasis. Curr Opin Neurobiol. 2013;23(5):799–805. doi: 10.1016/j.conb.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Porkka-Heiskanen T, et al. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276(5316):1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalinchuk AV, McCarley RW, Stenberg D, Porkka-Heiskanen T, Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: Lessons from 192 IgG-saporin lesions. Neuroscience. 2008;157(1):238–253. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinchuk AV, Lu Y, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Nitric oxide production in the basal forebrain is required for recovery sleep. J Neurochem. 2006;99(2):483–498. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- 32.Kalinchuk AV, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. Eur J Neurosci. 2006;24(5):1443–1456. doi: 10.1111/j.1460-9568.2006.05019.x. [DOI] [PubMed] [Google Scholar]
- 33.Kalinchuk AV, McCarley RW, Porkka-Heiskanen T, Basheer R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J Neurosci. 2010;30(40):13254–13264. doi: 10.1523/JNEUROSCI.0014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krueger JM, et al. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9(12):910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Le Roux N, Amar M, Moreau AW, Fossier P. Roles of nitric oxide in the homeostatic control of the excitation-inhibition balance in rat visual cortical networks. Neuroscience. 2009;163(3):942–951. doi: 10.1016/j.neuroscience.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Wisor JP, Gerashchenko D, Kilduff TS. Sleep-active neuronal nitric oxide synthase-positive cells of the cerebral cortex: A local regulator of sleep? Curr Top Med Chem. 2011;11(19):2483–2489. doi: 10.2174/156802611797470367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morairty SR, Hedley L, Flores J, Martin R, Kilduff TS. Selective 5HT2A and 5HT6 receptor antagonists promote sleep in rats. Sleep. 2008;31(1):34–44. doi: 10.1093/sleep/31.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morairty SR, et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS ONE. 2012;7(7):e39131. doi: 10.1371/journal.pone.0039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Black SW, et al. Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy. Sleep. 2013;36(3):325–336. doi: 10.5665/sleep.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.