Abstract
Plasmodium falciparum, the causative agent of malaria, completely remodels the infected human erythrocyte to acquire nutrients and to evade the immune system. For this process, the parasite exports more than 10% of all its proteins into the host cell cytosol, including the major virulence factor PfEMP1 (P. falciparum erythrocyte surface protein 1). This unusual protein trafficking system involves long-known parasite-derived membranous structures in the host cell cytosol, called Maurer’s clefts. However, the genesis, role, and function of Maurer’s clefts remain elusive. Similarly unclear is how proteins are sorted and how they are transported to and from these structures. Recent years have seen a large increase of knowledge but, as yet, no functional model has been established. In this perspective we review the most important findings and conclude with potential possibilities to shed light into the enigma of Maurer’s clefts. Understanding the mechanism and function of these structures, as well as their involvement in protein export in P. falciparum, might lead to innovative control strategies and might give us a handle with which to help to eliminate this deadly parasite.
Since Charles Louis Alphonse Laveran discovered the malaria parasite in 1881 (1) in Algeria, while examining the blood of a patient who had died from marsh fever, research has been conducted on these deadly parasites. Laveran received the Nobel Prize in medicine for his discovery in 1907, which causally explained that malaria symptoms are caused by protozoan parasites, eventually described as Plasmodium species of the phylum Apicomplexa. Among the five species infecting humans, Plasmodium falciparum causes the most lethal forms of the disease, but zoonotic Plasmodium knowlesi infections can also be lethal.
Plasmodium falciparum and Its Exceptional Host Cell
During the complex life cycle, all morbidity is linked only to the intraerythrocytic cycles. Sporozoites injected by an infected Anopheline mosquito are carried to the liver, invade hepatocytes, and multiply asexually. Subsequently, merozoites are released to invade erythrocytes to start multiple rounds of the vicious 48-h life cycle in which another schizogony takes place until rupture of the host cell and release of new merozoites. The intracellular habitat and the lack of a major histocompatibility complex in the erythrocyte provide an ideal hideaway in which the parasite can ensconce itself. This hideaway in turn presents the parasite with exceptional difficulties because the terminally differentiated and metabolically highly reduced erythrocyte provides no machinery for protein or lipid synthesis and transport. In fact, the host cell provides very little to the parasite except a major source of nutrient, hemoglobin, which also is limited. Plasmodium digests hemoglobin, which lacks the amino acid isoleucine, and provides a very limited source of cysteine, glutamate, methionine, proline, and tyrosine (2–4). Thus, just to survive, the parasite remarkably must remodel its host cell.
Remodeling to Make the Home Habitable
In the early hours after erythrocyte invasion the parasite, embedded in a parasitophorous vacuole, induces dramatic host cell modifications already noticed first by Marchiafava and Celli (5), and then in more detail by Maurer (6) and Schüffner (7), the latter with the Tertian-parasite (most probably Plasmodium vivax). These extensive reconstructions are facilitated by the export of at least 10% of all proteins of P. falciparum (8). Now, over 130 y after the description of Marchifava and Celli (5), a function can be assigned for only a few proteins exported to selective sites in the erythrocyte cytosol or membrane. Proteins transported beyond the parasite’s confines are translocated across the parasite plasma membrane, the parasitophorous vacuolar membrane (PVM), and in certain cases inserted into the erythrocyte membrane. Protein insertion and cytoskeleton interaction seems to lead to increased permeability of the erythrocyte plasma membrane, facilitating nutrient uptake as has recently been shown with the Plasmodium surface anion channel (PSAC), consisting of members of the cytoadherence linked antigen (CLAG) protein family (9, 10). As a consequence, erythrocyte rigidity increases and deformability decreases dramatically (11, 12), and the previously very flexible and heavily deformable erythrocyte can no longer penetrate slits much smaller than the actual size of the cell as required for splenic passage (13).
Consequently, a few hours after the cell is infected, electron-dense protrusions appear on the surface of the host cell forming the anchor for the erythrocyte surface protein 1 (PfEMP1). This large protein is considered to be the major or sole virulence factor in tropica malaria and mediates cytoadherence and sequestration of late-stage–infected erythrocytes in deep tissues, avoiding passage through the spleen (14–16). Hence, PfEMP1 plays a key role in the pathology of falciparum malaria and displays different binding phenotypes, most probably corresponding to tissue-specific sequestration in different organs. In turn, exposure on the erythrocyte surface triggers antibody-dependent immune responses in which semi-immunity of exposed individuals is rooted. In a typical “arms race” the parasite circumvents elimination by the immune system through antigenic variation, exclusively expressing one of many PfEMP1 variants at any specific time point (17). Thus, again, export and trafficking of proteins into the host cell cytosol are key for parasite survival, chronic infections, and pathology of malaria.
In this light, the appearance of Golgi-like membranous structures in the cytosol of the host cell, already described as “stippling” by Maurer—the Maurer’s clefts—become critically important. These structures play a crucial role in the refurbishment of the host cell, and most proteins destined to the surface, like PfEMP1, transiently localize to these structures. In the past, an increasing number of Maurer’s cleft resident proteins have been identified; still, we know little of the function of Maurer’s clefts nor of their creation or origin, and least about their dynamics and fate during the intraerythrocytic cycle. In the following, we discuss the current knowledge on the enigma described first by Maurer, in the context of their importance in parasite survival and the dependence of successful remodeling the host cell. Finally, we believe that these modification processes could provide potential targets for new malaria intervention strategies.
Maurer’s Clefts Morphology
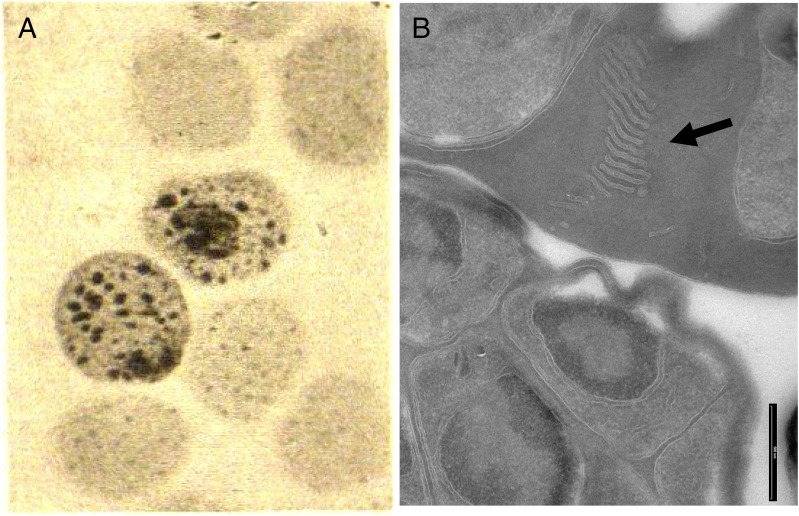
Already in 1902, Maurer described spots beyond the parasite’s confines in the red blood cells inhabited by older ring stages of P. falciparum (6) when stained with alkaline methylene blue. Maurer observed various numbers of spots from very small up to 1 µm. These spots appear during growth of the parasite and were considered sufficient for the diagnosis of malaria perniciosa, today malaria tropica. His explanations of these spots differed from what we know today. Maurer proposed them to be a “change or loss in substance” (e.g., hemoglobin) on the surface of the erythrocyte as a consequence of parasite attacks, which it undertakes to adhere onto the cell and acquire nutrients. In short, the spots were injuries of the host. In those days it was believed that the parasite lived on the erythrocyte and only older stages were intracellular (Fig. 1). Today we know much more about the shape, appearance, and structure of these clefts but still know little of their origin or their precise function. Moreover, we know virtually nothing about similar structures described by Schüffner in P. vivax (7). It is startling that after more than 100 years of research our knowledge is so sparse regarding organelles or structures so essential for parasite survival.
Fig. 1.
(A) Image from Georg Maurer’s publication (6). (Magnification: 1000×.) (B) Today’s view of Maurer’s clefts by electron microscopy (P. falciparum D10 strain) (image courtesy of F. Brand). (Scale bar: 500 nm.)
What do we know? Trager et al. (18), using electron microscopy, described Maurer’s clefts as narrow clefts scattered through the cytoplasm of the infected cell bounded on each side by a double membrane. Today it is accepted that clefts are only bordered by a single membrane and that these double membranes, when seen by transmission electron microscopy (TEM), represent whorls of the PVM, referred to as the tubulovesicular network (19–24). Maurer’s clefts are membrane-limited vacuoles or sack-like structures in the cytosol of the erythrocyte, formed early after invasion by the parasite, visible as blue dots in Giemsa-stained blood smears. The clefts appear as flattened or circular structures with an electron-dense coat and lumen, the latter appearing electron-lucent after fixation (25–27), and are believed to originate from the PVM (28–30). There are various descriptions of morphology, size, number, and position in the host cell (21, 26, 31–35) but the simplest representation of a Maurer’s cleft is a single, disk-shaped cistern of about 500-nm width. New observations from early stages indicate initial globular structures that only in later stages acquire a flattened feature (34, 35). Forms have also been described as an extended network bridging the distance from the PVM to the erythrocyte membrane (21, 31, 32, 36). There is also strain diversity observed in laboratory strains, such as in 3D7, which produces mostly single slender, slit-like lamella, whereas multiple lamellae were observed resembling stacks of Golgi in D10, Dd2, HB3, NF54, and FCQ-27 (Fig. 1) (21, 32, 37). Whether this finding also relates to the number of surface molecules and pathology in field isolates remains to be tested. Maurer’s clefts look highly convoluted and interconnected in whole-cell imaging (21) and connections are observed between individual Maurer’s clefts, between Maurer’s clefts and the PVM, as well as between Maurer’s clefts and the erythrocyte membrane (21, 31, 32). Maurer’s cleft resident proteins, however, are restricted to Maurer’s cleft bodies only, and are not distributed uniformly among these interconnected structures (21). Fluorescence microscopy of erythrocytes infected with transfectant parasites expressing GFP-chimera of resident proteins shows discrete puncta (38–45), which cannot be replenished by lateral diffusion (41).
Maurer’s Cleft as Sorting Stations
With the identification of the protein export motif Plasmodium export element (PEXEL) (46, 47), the list of parasite-derived proteins that are exported beyond the boundaries of the parasite has expanded considerably (Table S1). An increasing number of these proteins localize permanently to Maurer’s clefts, whereas proteins destined for the erythrocyte membrane transiently associate with Maurer’s clefts. Only a few proteins are known to be trafficked to structures in the host cell without passing through the Maurer’s cleft hub. These proteins include MAHRP2 at tether-like structures (48), heat shock protein 40 (HSP40) and HSP70-x in or at J-dots (49, 50), and some soluble proteins in the cytosol, such as ring exported protein 3 (REX3) (40). Although most resident proteins are encoded by single-copy genes, such as membrane associated histidine rich protein 1 (MAHRP1) (41) or knob associated histidine rich protein (KAHRP) (38, 51), Maurer’s clefts gain their importance as sorting stations of a number of exported protein families. The foremost important exported protein family is PfEMP1, which is found on the surface of the infected cell. This virulence factor is transported via the Maurer’s clefts at which it transiently localizes. Other proteins destined to the erythrocyte membrane [such as RIFINs (39, 52–57), STEVOR (58–62), PfMC-2TM (60, 63, 64), FIKK kinase (65–67), and probably members of the CLAG family] also pass through the Maurer’s clefts on their way to the erythrocyte membrane, again highlighting the tremendous significance of these structures.
Recently, two gene families have been predicted to be exported (68) and to partially reside at Maurer’s clefts. The Plasmodium helical interspersed subtelomeric (PHIST) protein family, comprising 71 proteins that carry a characteristic conserved PHIST domain, includes the ring-infected erythrocyte surface antigen (RESA). These PHISTs cluster into three groups based on their length, distribution, and domains, and at least one member was shown to localize to Maurer’s clefts (MAL7P1.172) (11), and others to localize to the erythrocyte membrane [PFI1780w (68) and PFE1605w]. The latter two members seem to directly interact with the ATS domain of PfEMP1 via their PHIST domain (69). Although abundant proteins, no further function has been ascribed, but even less understood are the 17 annotated HYP families. Recently, a Maurer’s clefts localization has been suggested for members of the HYP4 (PfEPF3, P. falciparum exported protein family) and HYP5 (PfEPF3) families (70). Mbengue et al. also showed that the PfEPF1 family lacking an export motif localizes to the clefts (70). Members of the CLAG family have also been implicated to be transported through Maurer’s clefts, but there is a controversy about their final destination and origin (71). Why are so many proteins destined to the Maurer’s clefts? What is the function of so many players?
Are Maurer’s Clefts Independent, Continuous, or Anchored Entities? Where Do They Come From?
Using reconstructed regions from serial continuous sections, Wickert et al. proposed that all membranous structures form a continuous membrane system originating from one or more sites of the PVM (31, 32). This finding was supported by confocal microscopy using lipid dyes in which dots in the erythrocyte cytoplasm were observed connected by fine threads originating at the parasitophorous vacuole (19, 72–74). In contrast, GFP-tagged Maurer’s clefts resident proteins were found only in a punctuate manner at Maurer’s clefts, indicating distinct, independent structures. In the case of a continuous network, fluorescent signal would have been observed throughout the network (38–45), but in photo-bleaching experiments no lateral diffusion of Maurer’s clefts resident proteins or lipids (41, 44, 75, 76) was observed. In contrast, the erythrocyte membrane showed a fast recovery after photo-bleaching when BODIPY-ceramide was used (44). Hence, we must conclude that there is no diffusion between Maurer’s clefts or between the clefts and the erythrocyte membrane or the PVM; there is no lipid continuum between these compartments. It is also known that the tubulovesicular network contains exclusive markers not found at Maurer’s clefts (44, 77, 78). Using structured illumination microscopy, Hanssen et al. visualized the complex exomembrane system of the entire infected cell and also could not find an extended membrane network stretching from the PVM to the surface of the infected cell in trophozoites; additionally, only very mature stages showed highly intertwined but not interconnected networks (36).
Although fluorescence microscopy offers ease of use and a variety of fluorochromes, its resolution is limited to ∼200 nm; recent advances in 3D structured illumination microscopy (SIM) permit analyses at around 100 nm (79). However, most structures in the infected erythrocyte cytosol are around or smaller than this. Higher resolution must be obtained by electron microscopy. Because electrons are strongly absorbed, only thin sections of 300–400 nm can be observed. Hence, techniques to generate composite whole-cell images have been developed. Nevertheless, the harsh fixation conditions used for electron microscopy can introduce artifacts (80) and the extensive sample preparation needed for electron tomography similarly might disturb the natural distribution of structures, such as vesicles, and thus can produce artifacts (36).
From where do Maurer’s clefts originate? The genesis of Maurer’s clefts has only vaguely been described. Live fluorescence microscopy of GFP-tagged Maurer’s cleft proteins indicated budding from the PVM (41). Maurer’s clefts membranes are equally susceptible to saponin, as is the PVM (40), but are less susceptible to Streptolysin O compared with the PVM, which argues against a continuous network (40). It is also unclear if Maurer’s clefts resident proteins are loaded into the clefts because they are formed at the PVM, or if there is continuous transport of cargo to already existing Maurer’s clefts. Recent live-imaging studies demonstrated that some proteins arrived at Maurer’s clefts after these had been formed (45, 81).
Maurer’s clefts are formed very early and REX1 can be detected already at 2–4 h postinvasion (hpi) in characteristic punctuate staining. Other Maurer’s clefts resident proteins, skeleton binding protein 1 (SBP1) and MAHRP1, appear somewhat later at 4–6 hpi (45, 81), and at 6 hpi all three proteins are detected in most of the Maurer’s clefts (45). All clefts seem to be formed at the early ring stage because no new clefts were observed after invasion (81) and the number of clefts remained constant as early as 8 hpi (45), supporting the hypothesis of protein transport to preexisting clefts. Other resident proteins, such as REX2, or transient proteins, such as a RIFIN protein, expressed under the chloroquine resistance transporter (crt) promoter, were found later in clefts in which REX1 and SBP1 were already present (81). The major virulence factor PfEMP1, displayed at the erythrocyte membrane ∼20 hpi, is initially found at the parasite surface at 8–11 hpi and subsequently at Maurer’s clefts, though not until 16 hpi (45). Taken together, these data indicate a constant flow of cargo from the parasite to the Maurer’s clefts, the means by which, however, remains elusive.
Although Maurer’s clefts are formed very early after invasion, they occur as highly mobile structures (41, 45, 81). Live imaging over the 48-h life cycle using REX2-GFP–expressing parasites revealed a movement of Maurer’s clefts over three phases. Clefts move rapidly in young stages and become fixed before the parasite develops into the trophozoites stage at around 22 hpi (45, 81). This arrest is not a limitation of space because clefts stop moving before the parasite grows rapidly, indicating the completion of host cell modification (81). In the third phase, the spatial arrangement of clefts collapses shortly before merozoites egress, suggesting an active process of host cell modification disassembly. Contemporaneously with the arrest in cleft mobility, PfEMP1 appears on the erythrocyte surface (81).
The sudden cessation of Maurer’s cleft movement requires an active fixation or anchoring process. For a long time, it has been known that Maurer’s clefts are attached to the erythrocyte membrane (82–84) and it was postulated that proteinaceous tethers possibly connect the Maurer’s clefts to the erythrocyte membrane (24, 83). During recent years, evidence of stalk-like extensions connecting Maurer’s clefts to the erythrocyte membrane or sometimes to the PVM has been gathered through TEM and SIM (21, 36, 48, 85). These tethers are tubular, cylindrical structures with an electron-dense lumen of about 200–300 nm in length and a diameter of around 30 nm. Using antibodies, only SBP1 as known Maurer’s cleft protein could be detected to some extent, but to date only MAHRP2 was found to localize specifically to these tether structures (48). MAHRP2 is a conserved PEXEL-negative protein with no ortholog in any other Plasmodium species, and is closely associated with Maurer’s clefts markers SBP1 or MAHRP1 (48). Tether-like structures are highly mobile when MAHRP2-GFP transfectants are observed by live imaging (45, 48) and appear 4 hpi in close proximity to Maurer’s clefts (45). McMillan et al. described that initially only a subset of Maurer’s clefts were associated with MAHRP2-labeled structures, whereas 8 hpi most clefts are labeled. The number of these mobile structures increases 16–22 hpi before Maurer’s cleft complexes attach to the erythrocyte membrane (45). The temporal and physical behavior, as well as close proximity of tethers and Maurer’s clefts, suggests an involvement of tethers in the immobilization event. Adversely, Kilian et al. proposed a different model, in which the morphological change of Maurer’s clefts from an initial globular to the well-known disk-shaped structure of late stages negatively affects the ability to move by Brownian motion (35). Cyrklaff et al. observed an extended network of long, sometimes branched actin-like filaments that connected the Maurer’s clefts with the knobs but did not detect tether-like structures by cryo-electron microscopy (34). The authors suggested a highly dynamic turnover of actin filaments along which vesicles might be transported from Maurer’s clefts to the erythrocyte membrane (34). The authors further postulate that actin filaments are needed to keep the morphology of Maurer’s clefts (34, 35). When parasites were treated with the actin depolymerizing agent cytochalasin D, the filaments were destroyed and Maurer’s clefts were aberrantly depicted. Cytochalasin D treatment was not sufficient to completely prevent arrest of Maurer’s cleft movement, indicating that actin filaments do not play a crucial role in this process (35). It remains to be determined whether the immobilization of Maurer’s clefts really is an active process mediated by MAHRP2 tethering or if it is a passive process based on structural changes of the clefts.
Proteins Reach Maurer’s Clefts via a Complex Transport Pathway
Although much evidence has been compiled that Maurer’s clefts are important hubs for protein distribution and trafficking, and essential for parasite survival, we only now begin to understand how protein trafficking beyond the PVM works. All proteins exported to the host cell cytoplasm must cross the parasite plasma membrane and the PVM. Proteins localizing to Maurer’s clefts or the erythrocyte membrane must even cross a third membrane, a complexity in protein transport not comparable to any other organism, and a unique situation for which no molecular concepts exist that could be used as conceptual basis. In addition, the host cell gives no hand to any protein translocation or transport.
A large number of proteins exported to the host cytosol possess an amino acid motif close to the N terminus, called PEXEL (46) or vacuolar transport signal (47), with a hydrophobic domain about 20 amino acids upstream of the motif that mediates co- or posttranslational insertion into the endoplasmic reticulum (ER) for soluble and transmembrane proteins. The identification of this motif allowed the prediction of the P. falciparum exportome with 463 proteins, including protein families (8) carrying this motif. That amount represents nearly 10% of all genes present in P. falciparum (46, 47, 68, 86). Upon export the PEXEL motif is processed in the ER by plasmepsin V (87, 88), with a subsequent N-terminal acetylation (89, 90), and possibly involving binding of phosphatidylyinositol-3-phosphate [PI(3)P] to the PEXEL (91). Export into the parasitophorous vacuole is via vesicular traffic, but how proteins destined for the host cytosol are sorted beyond the plasma membrane remains speculative (89, 92, 93). Whether proteins are chaperoned or transported in vesicles to discrete regions in the PVM where translocation takes place remains to be shown. Specific sites in the PVM where translocation takes place have been seen as a necklace of beads, with several GFP-tagged exported proteins (38, 41, 42, 45). De Koning-Ward et al. identified this Plasmodium translocation complex (PTEX) comprising an ATPase (HSP101), a predicted pore forming protein (EXP2), and the potential regulators TRX2, PTEX150, and PTEX88 (94). It was already shown in 1996 that the translocation across the PVM was ATP-dependent (95) and that proteins must unfold (96). The presence of a P. falciparum translocon for exported proteins (PTEX) has been observed at discrete foci in the PVM (97), but its evolutionary origin is unclear and might explain why PEXEL-containing proteins are unique to Plasmodium.
Not only PEXEL motif-carrying proteins were found to be exported; there is a growing body of evidence for non-PEXEL–carrying proteins to be exported to the host cytosol, which includes proteins that do not even contain a recognizable N-terminal ER-targeting signal peptide (92, 93, 98–101). This group of proteins includes some well-characterized proteins known to be located to the Maurer’s clefts, including Maurer’s clefts resident proteins MAHRP1, SBP1, REX1, and REX2 (40, 43, 83, 102, 103), as well as the tether protein MAHRP2 (48). Heiber et al. recently based their prediction on rather general criteria and demonstrated that, most likely, many more PEXEL-negative proteins exist with various hydrophobic domains (104). It has been suggested that all these proteins enter the classic secretory pathway (41–43, 48, 105), and lately several research groups tried to identify sequence requirements for export, yet no common motif or perceptible sequence has been identified. A transmembrane domain or a signal peptide seems to be essential, most likely for entering the ER and the secretory pathway (41, 43, 48, 106). Unfolding appears to be equally required for PEXEL-positive proteins, suggesting similar mode of translocation across the PVM (104, 107). Interestingly, processed N termini of PEXEL-positive proteins promote the export of a reporter gene, like unprocessed N termini of PEXEL-negative proteins (107). An open question is whether these proteins are guided through the same or totally distinct translocon.
A major piece in this puzzle of transport is the appearance of the virulence factor PfEMP1 on the surface of the host cell, which coincides with the immobilization of Maurer’s clefts. It has now been speculated that PfEMP1 is transported in vesicles to the surface once Maurer’s clefts have attached to the erythrocyte membrane. Indeed, Cyrklaff et al. (34) observed vesicles apparently moving along the actin filaments from the Maurer’s clefts to the erythrocyte surface. However, adding to the enigma of Maurer’s clefts it is unclear how these proteins reach the clefts. Previously, a vesicle-based transport was assumed when components, such as PfSec31, PfSar1p, and PfSec23c were found to be exported to Maurer’s clefts (108–110), which could not be confirmed by transfection (111). Therefore, it was hypothesized that proteins are loaded into nascent clefts (40, 41, 112, 113) before they bud off. However, recently it was clearly shown that proteins arrive at already existing Maurer’s clefts, and thus cannot be transported by diffusion (45, 81). Additionaly, there is no known vesicular transport to the Maurer’s clefts because none of the vesicles observed could be labeled with antibodies against any Maurer’s clefts protein (21, 36). Whether these proteins travel via a nontypical vesicular transport or as soluble chaperoned protein complexes (76, 81, 114) remains to be shown, and recently described J-dots carrying parasite-derived HSP40 and HSP70 proteins might play a role in this process (49, 50).
To complicate matters, a number of vesicular structures in the host cell cytosol have been described by electron microscopy (21, 23, 24, 31, 34, 36, 37, 45, 115–118) and, furthermore, in electron tomograms vesicles smaller than conventional transport vesicles lacking an obvious coat have also been observed budding from Maurer’s clefts (21) and in close proximity to the erythrocyte membrane (31). Trelka et al. showed strings of vesicles (116), and those associated with Maurer’s clefts had a diameter of about 25 nm (21, 24, 31, 36), but a few larger vesicles of about 80 nm in diameter with an electron-dense coat around a lumen of approximately 25 nm were also found in trophozoite-infected erythrocytes and were called electron-dense vesicles (36, 45, 116). These vesicles share morphological features with the knob complex, but are both present in knob-carrying and knobless infected erythrocytes (36). They can be heavily labeled with antibodies against PfEMP1 and seem to fuse occasionally with the erythrocyte membrane (36, 45). Whether these vesicles resemble the recently shown exosomes (see below) remains to be shown (119, 120).
Very recently, cell–cell communication through exosome-like vesicles promoting sexual differentiation and also involving Maurer’s clefts has been described for the first time (119, 120). It was shown that parasites were able to pass transfected plasmid DNA onto other parasite cells, irrespective of cell–cell contact. Inhibition of actin polymerization and depolymerization suggested that these functions are necessary. Vesicles of ∼70-nm diameter were observed to be released into the culture supernatant, a size analogous to mammalian exosomes or of the electron-dense vesicles observed underneath the erythrocyte membrane. A Maurer’s clefts protein previously shown to be essential for PfEMP1 surface translocation (MAL7P1.172/PfPTP2, PfEMP1 trafficking protein) is required for proper cell–cell communication. PfPTP2 belongs to the family of exported proteins comprising a PHIST domain, and it might have an essential function in budding and release of the exosome-like vesicles. PfPTP2 localized to membranous structures budding from Maurer’s clefts shown by immuno-electron microscopy, suggesting that exosome-like vesicles derive from Maurer’s clefts rather than from the erythrocyte membrane. Mantel et al. analyzed the proteome of microvesicles and found Maurer’s clefts resident proteins to be enriched (120). The authors show that these vesicles are released before egress, which coincides with the disappearance of Maurer’s clefts (81). In contrast, Regev-Rudzki et al. showed that optimal plasmid transfer occurred between ring-stage parasites (119); it remains to be shown whether these microvesicles are the same, and even more so what their biological function would be. Mantel et al. observed internalization of microvesicles that stimulated conversion to sexual parasites and speculated that released microvesicles could regulate gametocyte production on a population level (120).
Gene-Deletion Studies Indicate Functions of Maurer’s Clefts
Why does the parasite invest such an enormous amount of protein synthesis and newly evolved translocation machinery just to export some membranous structures and less than a handful surface proteins? The function of the Maurer’s clefts remains to a certain degree in the dark. Gene-deletion technology made it more possible to study the function of specific genes. First, knock out experiments eliminating the Maurer’s clefts proteins MAHRP1 or SBP1 surprisingly revealed that PfEMP1 is not exported to the surface of the infected erythrocyte anymore (41, 121, 122). This finding was the first proof that Maurer’s clefts have a function as an intermediate compartment in transport or sorting of proteins, at least for those destined for the erythrocyte membrane. Erythrocytes infected with parasites lacking MAHRP1 showed widened and fragmented clefts by electron microscopy that indicated a stabilizing role for MAHRP1 (123). Deletions of REX1 and truncation mutants showed a stacked Maurer’s clefts phenotype similar to parasites of the D10 strain, which has a naturally occurring deletion of parts of chromosome 9, including all of the REX-encoding genes (44). In that case, PfEMP1 was retained at Maurer’s clefts (124). When proteins were deleted that are part of the knob complex in the erythrocyte membrane, such as PfEMP3, a reduced cytoadherence phenotype was observed with a reduction of PfEMP1 (125). In the case of KAHRP knockout, PfEMP1 was correctly trafficked to the surface but showed a significantly reduced cytoadherence under flow conditions, probably because of the lack of cytoskeleton anchoring (126).
After the identification of the PEXEL motif, Maier et al. conducted a large-scale gene-knockout approach of PEXEL-carrying proteins (11). The authors attempted to knock out ∼80 genes and the phenotypical read-out was cytoadherence, membrane rigidity, and the presence of knobs. Four knockout clones resulted in no or very reduced levels of PfEMP1 on the erythrocyte surface (PFB0106c, MAL7P1.172, PF13_0076, and PF14_0758), reduced surface expression of PfEMP1 was shown for two clones (MAL7P1.171, PF10_0025), and in one clone (MAL7P1.172) reduced levels of PfEMP1 were observed. An increase in host cell membrane rigidity was seen with four clones (PFB0920w, PF10_0159, PF13_0073, PF14_0758), whereas eight clones showed a reduced rigidity (PFA0110w, MAL7P1.171, PF10_0025, PFD0225w, PFD1160w, PFE0065w, MAL8P1.154, PF14_0018). Furthermore, the PFD1170c knockout clone lacked knobs because KAHRP was only transported to Maurer’s clefts, leading to reduced cytoadherence. Knockout clone PF10_0381 showed only rudimentary knobs, which were significantly smaller and less protrusive. This study reflects the difficulties in determining direct functions of any exported protein because of no or slightly modified phenotypes only (Table 1). This finding is again exemplified in knockout experiments of a member of the recently described Maurer’s cleft protein family PfEPF1, which resulted in a 50–60% reduction of reinvasion (70). It appeared that only a small number of merozoites could be released and that most were trapped within the disintegrating erythrocyte membrane. The authors speculated that this effect was because of modifications of the mechanical properties of the erythrocyte membrane (70), once again failing to describe conclusively the functional interaction of the protein.
Table 1.
Phenotypical read out possibilities for transgenic parasites
| Monitored cell characteristics | Measurements |
| Parasite growth rate | Cycle length, number of merozoites, parasite counts |
| Parasite shape and structure | Light microscopy, EM images |
| Cytoadherence, endothelial binding | Static assays, number of bound cells to CSA or CD26 or other receptors, under flow assays, rosetting capacity |
| Presence of knobs | EM images, gelatin floatation |
| Protein export and localization | Fluorescence microscopy, colocalizations, Western blots |
| Structure and mobility of Maurer’s clefts | EM images, fluorescent live-cell time-lapse imaging |
| Solute and nutrient uptake | Percoll gradient, metabolic labeling, radioactivity uptake, osmotic fragility measurement, patch-clamp experiments |
| Membrane rigidity | Shear stress measures, micropipette aspiration measures |
| Gametocytogenesis | Ability to produce gametocytes, numbers of gametocytes (infectivity of gametocytes) |
| Exosome production | Plasmid transfer |
| Transcriptome analysis | Microarrays/RNA seq at various time points |
| Proteome analysis | Whole proteome at various time points, proteome of cellular fractions |
How Can We Shed Light into the Darkness of the Maurer’s Clefts?
During the last decade much knowledge has been accumulated on proteins and structures exported to the erythrocyte cytosol during the intraerythrocytic stage of P. falciparum (Fig. 2). However, we still know very little on the function of individual proteins or of structures like Maurer’s clefts or vesicles. The inability to knock out essential genes prevents this route of studying, and conditional knockout strategies are yet not working routinely, except for the FK506 binding protein (FKBP)-mediated protein-destabilizing system (127). Even when successful, knockout or knockdown parasites have been generated, often no obvious phenotype of the genetically modified parasite can be observed. To overcome this, we need to close this gap and identify broader read-out measures to monitor the effect of perturbations of this export system. Currently, it is standard to test the altered distribution of PfEMP1 and accessory molecules, cytoadherence properties, membrane rigidity, and nutrient up-take (Table 1). Some studies looked at growth rates and others on the capacity to form gametocytes (Table 1). In many cases, no discernible alteration could be observed, suggesting either a surprisingly large redundancy of proteins or other yet to be discovered functions. Admittedly, with no animal model at hand, all alterations tested occur only in culture and requirements in culture may well be lower than in the natural human host.
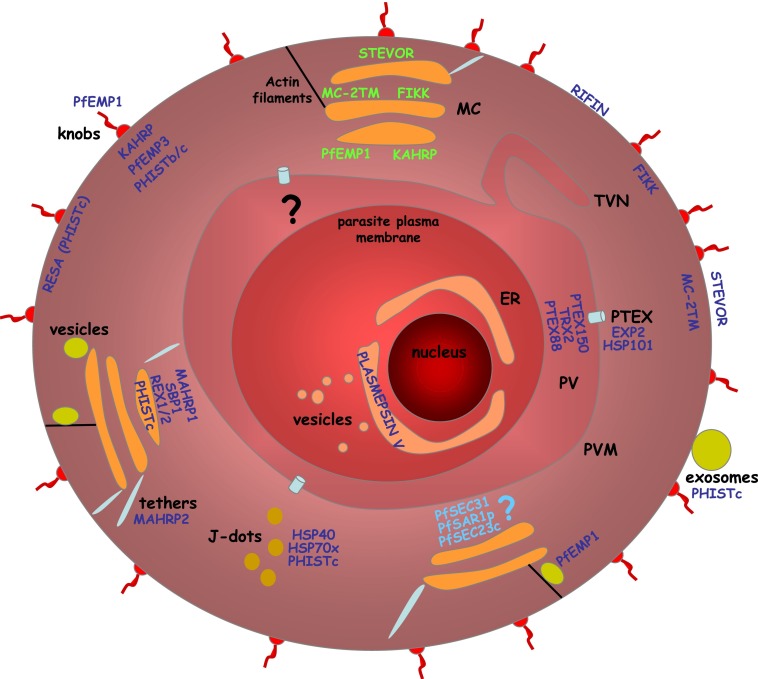
Fig. 2.
Schematic representation of a P. falciparum-infected erythrocyte. Parasite structures and proteins involved in export. ER, endoplasmatic reticulum; MC, Maurer’s clefts; PV, parasitophorous vacuole; PVM, parasitophorous vacuolar membrane; TVN, tubular-vesicular network. Protein names in dark blue indicate resident proteins of that organelle, names in green indicate transient location, and names in light blue indicate proteins, the of which location has not been confirmed.
What is needed to understand the function of this exportome? We and others have started to routinely compare the transcriptome of parasites upon knocking down genes with the transcriptome of unaltered isogenic parasites to observe other modifications of this system. However, there is an urgent need to use systems biological approaches and to look into quantitative proteomics upon genetic modifications. To do so, we need to master much better fractionation techniques and need to use other approaches to solubilize membrane-embedded or -associated proteins. Once these technical problems have been solved, it should be possible to compare various proteomes of parasites having any of these exported genes knocked out or down. With network analyses, bioinformatic approaches, and refined imaging technologies, it should then be possible to establish the interaction network of these exported proteins and thus understand their function in the host cell.
After all, we are interested in understanding these functions because it might be the hidden Achilles’ heel. If it were possible to interfere with this export system, the parasite might not be able to acquire nutrients, might not be able to evade the immune system through antigenic variation and sequestration, and probably would be eliminated in a human infection instantly. Therefore, it is somewhat surprising that very little has been invested in research to find specific inhibitors for this exported network.
Besides these important medical implications, the study of the Maurer’s clefts addresses a fundamental question in Apicomplexan biology, which is the interplay of parasite and host. Have parasites evolved a new protein distribution system? Do the Maurer’s clefts represent secondary Golgi apparatuses? Is there a new way of vesicular transport? And, is there even a new cell–cell communication system via vesicular export? For certain, parasites would not invest so heavily into this machinery if there were not a tremendous need and advantage.
Finally, there are other Plasmodia, and as yet exported and Maurer’s cleft proteins of P. falciparum have not been systematically compared with Schüffner’s dots and exported proteins in P. vivax. There are a number of exported proteins in P. falciparum that have homologous proteins in P. vivax, but there are also a many proteins for which no homologous protein exist. Again, a systematic comparative approach could shed some light on the function of these proteins.
As Rejewski (128) cracked the Enigma code in 1939, we should be able to use our biological reasoning to crack the secrets of this deadly parasite and to relieve the human population of the risk of malaria.
Supplementary Material
Acknowledgments
We thank Françoise Brand (Swiss Tropical and Public Health Institute) for providing the electron micrograph. This study was funded in part by the Swiss National Science Foundation (Grant 31003A_132709).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309247110/-/DCSupplemental.
References
- 1.Laveran A. Nature Parasitaire des Accidents de l'Impaludisme, Description d'un Nouveau Parasite Trouvé dans le Sung des Malades Atteints de Fièvre Palustre. Paris: J.-B. Baillière; 1881. [Google Scholar]
- 2.Divo AA, Geary TG, Davis NL, Jensen JB. Nutritional requirements of Plasmodium falciparum in culture. I. Exogenously supplied dialyzable components necessary for continuous growth. J Protozool. 1985;32(1):59–64. doi: 10.1111/j.1550-7408.1985.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 3.Hill RJ, Konigsberg W, Guidotti G, Craig LC. The structure of human hemoglobin. I. The separation of the alpha and beta chains and their amino acid composition. J Biol Chem. 1962;237:1549–1554. [PubMed] [Google Scholar]
- 4.Guidotti G, Hill RJ, Konigsberg W. The structure of human hemoglobin. II. The separation and amino acid composition of the tryptic peptides from the alpha and beta chains. J Biol Chem. 1962;237:2184–2195. [PubMed] [Google Scholar]
- 5.Marchiafava E, Celli A. Die Veränderung der rothen Blutscheiben bei Malaria-Kranken. Fortschr Med. 1883;1(18):573–575. [Google Scholar]
- 6.Maurer G. Die Malaria perniciosa. Centralblatt für Bakteriologie. Parasitenkunde und Infektionskrankheiten. 1902;32:695–719. [Google Scholar]
- 7.Schüffner WAP. Beitrag zur Kenntniss der Malaria. Deutsch Arch Klin Med. 1899;64:428–449. [Google Scholar]
- 8.Boddey JA, et al. Role of plasmepsin V in export of diverse protein families from the Plasmodium falciparum exportome. Traffic. 2013;14(5):532–550. doi: 10.1111/tra.12053. [DOI] [PubMed] [Google Scholar]
- 9.Nguitragool W, et al. Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell. 2011;145(5):665–677. doi: 10.1016/j.cell.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillai AD, et al. Solute restriction reveals an essential role for clag3-associated channels in malaria parasite nutrient acquisition. Mol Pharmacol. 2012;82(6):1104–1114. doi: 10.1124/mol.112.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier AG, et al. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008;134(1):48–61. doi: 10.1016/j.cell.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke BM, Mohandas N, Coppel RL. The malaria-infected red blood cell: Structural and functional changes. Adv Parasitol. 2001;50:1–86. doi: 10.1016/S0065-308X(01)50029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohandas N, Gallagher PG. Red cell membrane: Past, present, and future. Blood. 2008;112(10):3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leech JH, Barnwell JW, Miller LH, Howard RJ. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984;159(6):1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su XZ, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82(1):89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 16.Kyes S, Horrocks P, Newbold C. Antigenic variation at the infected red cell surface in malaria. Annu Rev Microbiol. 2001;55:673–707. doi: 10.1146/annurev.micro.55.1.673. [DOI] [PubMed] [Google Scholar]
- 17.Voss TS, et al. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439(7079):1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- 18.Trager W, Rudzinska MA, Bradbury PC. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- 19.Haldar K, Samuel BU, Mohandas N, Harrison T, Hiller NL. Transport mechanisms in Plasmodium-infected erythrocytes: Lipid rafts and a tubovesicular network. Int J Parasitol. 2001;31(12):1393–1401. doi: 10.1016/s0020-7519(01)00251-x. [DOI] [PubMed] [Google Scholar]
- 20.Haldar K. Intracellular trafficking in Plasmodium-infected erythrocytes. Curr Opin Microbiol. 1998;1(4):466–471. doi: 10.1016/s1369-5274(98)80067-2. [DOI] [PubMed] [Google Scholar]
- 21.Hanssen E, et al. Electron tomography of the Maurer’s cleft organelles of Plasmodium falciparum-infected erythrocytes reveals novel structural features. Mol Microbiol. 2008;67(4):703–718. doi: 10.1111/j.1365-2958.2007.06063.x. [DOI] [PubMed] [Google Scholar]
- 22.Aikawa M. Parasitological review. Plasmodium: The fine structure of malarial parasites. Exp Parasitol. 1971;30(2):284–320. doi: 10.1016/0014-4894(71)90094-4. [DOI] [PubMed] [Google Scholar]
- 23.Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol Today. 2000;16(10):427–433. doi: 10.1016/s0169-4758(00)01755-5. [DOI] [PubMed] [Google Scholar]
- 24.Kriek N, et al. Characterization of the pathway for transport of the cytoadherence-mediating protein, PfEMP1, to the host cell surface in malaria parasite-infected erythrocytes. Mol Microbiol. 2003;50(4):1215–1227. doi: 10.1046/j.1365-2958.2003.03784.x. [DOI] [PubMed] [Google Scholar]
- 25.Langreth SG, Jensen JB, Reese RT, Trager W. Fine structure of human malaria in vitro. J Protozool. 1978;25(4):443–452. doi: 10.1111/j.1550-7408.1978.tb04167.x. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson CT, Aikawa M. Ultrastructure of malaria-infected erythrocytes. Blood Cells. 1990;16(2-3):351–368. [PubMed] [Google Scholar]
- 27.Henrich P, Kilian N, Lanzer M, Cyrklaff M. 3-D analysis of the Plasmodium falciparum Maurer’s clefts using different electron tomographic approaches. Biotechnol J. 2009;4(6):888–894. doi: 10.1002/biot.200900058. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson CT, et al. Ultrastructure of the erythrocytic stages of Plasmodium malariae. J Protozool. 1987;34(3):267–274. doi: 10.1111/j.1550-7408.1987.tb03173.x. [DOI] [PubMed] [Google Scholar]
- 29.Aikawa M, Miller LH, Rabbege J. Caveola—Vesicle complexes in the plasmalemma of erythrocytes infected by Plasmodium vivax and P. cynomolgi. Unique structures related to Schüffner’s dots. Am J Pathol. 1975;79(2):285–300. [PMC free article] [PubMed] [Google Scholar]
- 30.Kara UA, Stenzel DJ, Ingram LT, Kidson C. The parasitophorous vacuole membrane of Plasmodium falciparum: Demonstration of vesicle formation using an immunoprobe. Eur J Cell Biol. 1988;46(1):9–17. [PubMed] [Google Scholar]
- 31.Wickert H, et al. Evidence for trafficking of PfEMP1 to the surface of P. falciparum-infected erythrocytes via a complex membrane network. Eur J Cell Biol. 2003;82(6):271–284. doi: 10.1078/0171-9335-00319. [DOI] [PubMed] [Google Scholar]
- 32.Wickert H, Göttler W, Krohne G, Lanzer M. Maurer’s cleft organization in the cytoplasm of Plasmodium falciparum-infected erythrocytes: New insights from three-dimensional reconstruction of serial ultrathin sections. Eur J Cell Biol. 2004;83(10):567–582. doi: 10.1078/0171-9335-00432. [DOI] [PubMed] [Google Scholar]
- 33.Lanzer M, Wickert H, Krohne G, Vincensini L, Braun Breton C. Maurer’s clefts: A novel multi-functional organelle in the cytoplasm of Plasmodium falciparum-infected erythrocytes. Int J Parasitol. 2006;36(1):23–36. doi: 10.1016/j.ijpara.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Cyrklaff M, et al. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science. 2011;334(6060):1283–1286. doi: 10.1126/science.1213775. [DOI] [PubMed] [Google Scholar]
- 35.Kilian N, et al. Haemoglobin S and C affect the motion of Maurer’s clefts in Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2013;15(7):1111–1126. doi: 10.1111/cmi.12102. [DOI] [PubMed] [Google Scholar]
- 36.Hanssen E, et al. Whole cell imaging reveals novel modular features of the exomembrane system of the malaria parasite, Plasmodium falciparum. Int J Parasitol. 2010;40(1):123–134. doi: 10.1016/j.ijpara.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Wickert H, Krohne G. The complex morphology of Maurer’s clefts: From discovery to three-dimensional reconstructions. Trends Parasitol. 2007;23(10):502–509. doi: 10.1016/j.pt.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Wickham ME, et al. Trafficking and assembly of the cytoadherence complex in Plasmodium falciparum-infected human erythrocytes. EMBO J. 2001;20(20):5636–5649. doi: 10.1093/emboj/20.20.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khattab A, Klinkert MQ. Maurer’s clefts-restricted localization, orientation and export of a Plasmodium falciparum RIFIN. Traffic. 2006;7(12):1654–1665. doi: 10.1111/j.1600-0854.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- 40.Spielmann T, et al. A cluster of ring stage-specific genes linked to a locus implicated in cytoadherence in Plasmodium falciparum codes for PEXEL-negative and PEXEL-positive proteins exported into the host cell. Mol Biol Cell. 2006;17(8):3613–3624. doi: 10.1091/mbc.E06-04-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spycher C, et al. Genesis of and trafficking to the Maurer’s clefts of Plasmodium falciparum-infected erythrocytes. Mol Cell Biol. 2006;26(11):4074–4085. doi: 10.1128/MCB.00095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saridaki T, Sanchez CP, Pfahler J, Lanzer M. A conditional export system provides new insights into protein export in Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2008;10(12):2483–2495. doi: 10.1111/j.1462-5822.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- 43.Haase S, et al. Sequence requirements for the export of the Plasmodium falciparum Maurer’s clefts protein REX2. Mol Microbiol. 2009;71(4):1003–1017. doi: 10.1111/j.1365-2958.2008.06582.x. [DOI] [PubMed] [Google Scholar]
- 44.Hanssen E, et al. Targeted mutagenesis of the ring-exported protein-1 of Plasmodium falciparum disrupts the architecture of Maurer’s cleft organelles. Mol Microbiol. 2008;69(4):938–953. doi: 10.1111/j.1365-2958.2008.06329.x. [DOI] [PubMed] [Google Scholar]
- 45.McMillan PJ, et al. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell Microbiol. 2013;15(8):1401–1418. doi: 10.1111/cmi.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306(5703):1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 47.Hiller NL, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306(5703):1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 48.Pachlatko E, et al. MAHRP2, an exported protein of Plasmodium falciparum, is an essential component of Maurer’s cleft tethers. Mol Microbiol. 2010;77(5):1136–1152. doi: 10.1111/j.1365-2958.2010.07278.x. [DOI] [PubMed] [Google Scholar]
- 49.Külzer S, et al. Parasite-encoded Hsp40 proteins define novel mobile structures in the cytosol of the P. falciparum-infected erythrocyte. Cell Microbiol. 2010;12(10):1398–1420. doi: 10.1111/j.1462-5822.2010.01477.x. [DOI] [PubMed] [Google Scholar]
- 50.Külzer S, et al. Plasmodium falciparum-encoded exported hsp70/hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell Microbiol. 2012;14(11):1784–1795. doi: 10.1111/j.1462-5822.2012.01840.x. [DOI] [PubMed] [Google Scholar]
- 51.Taylor DW, et al. Localization of Plasmodium falciparum histidine-rich protein 1 in the erythrocyte skeleton under knobs. Mol Biochem Parasitol. 1987;25(2):165–174. doi: 10.1016/0166-6851(87)90005-3. [DOI] [PubMed] [Google Scholar]
- 52.Kyes SA, Rowe JA, Kriek N, Newbold CI. Rifins: A second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc Natl Acad Sci USA. 1999;96(16):9333–9338. doi: 10.1073/pnas.96.16.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petter M, et al. Variant proteins of the Plasmodium falciparum RIFIN family show distinct subcellular localization and developmental expression patterns. Mol Biochem Parasitol. 2007;156(1):51–61. doi: 10.1016/j.molbiopara.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Petter M, Bonow I, Klinkert MQ. Diverse expression patterns of subgroups of the rif multigene family during Plasmodium falciparum gametocytogenesis. PLoS ONE. 2008;3(11):e3779. doi: 10.1371/journal.pone.0003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haeggström M, et al. Common trafficking pathway for variant antigens destined for the surface of the Plasmodium falciparum-infected erythrocyte. Mol Biochem Parasitol. 2004;133(1):1–14. doi: 10.1016/j.molbiopara.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Haeggström M, Von Euler A, Kironde F, Fernandez V, Wahlgren M. Characterization of Maurer’s clefts in Plasmodium falciparum-infected erythrocytes. Am J Trop Med Hyg. 2007;76(1):27–32. [PubMed] [Google Scholar]
- 57.Joannin N, Abhiman S, Sonnhammer EL, Wahlgren M. Sub-grouping and sub-functionalization of the RIFIN multi-copy protein family. BMC Genomics. 2008;9:19. doi: 10.1186/1471-2164-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaviratne M, Khan SM, Jarra W, Preiser PR. Small variant STEVOR antigen is uniquely located within Maurer’s clefts in Plasmodium falciparum-infected red blood cells. Eukaryot Cell. 2002;1(6):926–935. doi: 10.1128/EC.1.6.926-935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Przyborski JM, et al. Trafficking of STEVOR to the Maurer’s clefts in Plasmodium falciparum-infected erythrocytes. EMBO J. 2005;24(13):2306–2317. doi: 10.1038/sj.emboj.7600720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lavazec C, Sanyal S, Templeton TJ. Hypervariability within the Rifin, Stevor and Pfmc-2TM superfamilies in Plasmodium falciparum. Nucleic Acids Res. 2006;34(22):6696–6707. doi: 10.1093/nar/gkl942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blythe JE, et al. Plasmodium falciparum STEVOR proteins are highly expressed in patient isolates and located in the surface membranes of infected red blood cells and the apical tips of merozoites. Infect Immun. 2008;76(7):3329–3336. doi: 10.1128/IAI.01460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niang M, Yan Yam X, Preiser PR. The Plasmodium falciparum STEVOR multigene family mediates antigenic variation of the infected erythrocyte. PLoS Pathog. 2009;5(2):e1000307. doi: 10.1371/journal.ppat.1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sam-Yellowe TY, et al. A Plasmodium gene family encoding Maurer’s cleft membrane proteins: structural properties and expression profiling. Genome Res. 2004;14(6):1052–1059. doi: 10.1101/gr.2126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsarukyanova I, Drazba JA, Fujioka H, Yadav SP, Sam-Yellowe TY. Proteins of the Plasmodium falciparum two transmembrane Maurer’s cleft protein family, PfMC-2TM, and the 130 kDa Maurer’s cleft protein define different domains of the infected erythrocyte intramembranous network. Parasitol Res. 2009;104(4):875–891. doi: 10.1007/s00436-008-1270-3. [DOI] [PubMed] [Google Scholar]
- 65.Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: The kinome of a divergent eukaryote. BMC Genomics. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider AG, Mercereau-Puijalon O. A new Apicomplexa-specific protein kinase family: Multiple members in Plasmodium falciparum, all with an export signature. BMC Genomics. 2005;6:30. doi: 10.1186/1471-2164-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nunes MC, Goldring JP, Doerig C, Scherf A. A novel protein kinase family in Plasmodium falciparum is differentially transcribed and secreted to various cellular compartments of the host cell. Mol Microbiol. 2007;63(2):391–403. doi: 10.1111/j.1365-2958.2006.05521.x. [DOI] [PubMed] [Google Scholar]
- 68.Sargeant TJ, et al. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 2006;7(2):R12. doi: 10.1186/gb-2006-7-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mayer C, Slater L, Erat MC, Konrat R, Vakonakis I. Structural analysis of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) intracellular domain reveals a conserved interaction epitope. J Biol Chem. 2012;287(10):7182–7189. doi: 10.1074/jbc.M111.330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mbengue A, Audiger N, Vialla E, Dubremetz JF, Braun-Breton C. Novel Plasmodium falciparum Maurer’s clefts protein families implicated in the release of infectious merozoites. Mol Microbiol. 2013;88(2):425–442. doi: 10.1111/mmi.12193. [DOI] [PubMed] [Google Scholar]
- 71.Ekland EH, Akabas MH, Fidock DA. Taking charge: Feeding malaria via anion channels. Cell. 2011;145(5):645–647. doi: 10.1016/j.cell.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 72.Behari R, Haldar K. Plasmodium falciparum: Protein localization along a novel, lipid-rich tubovesicular membrane network in infected erythrocytes. Exp Parasitol. 1994;79(3):250–259. doi: 10.1006/expr.1994.1088. [DOI] [PubMed] [Google Scholar]
- 73.Elmendorf HG, Haldar K. Plasmodium falciparum exports the Golgi marker sphingomyelin synthase into a tubovesicular network in the cytoplasm of mature erythrocytes. J Cell Biol. 1994;124(4):449–462. doi: 10.1083/jcb.124.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haldar K, et al. Protein and lipid trafficking induced in erythrocytes infected by malaria parasites. Cell Microbiol. 2002;4(7):383–395. doi: 10.1046/j.1462-5822.2002.00204.x. [DOI] [PubMed] [Google Scholar]
- 75.Knuepfer E, Rug M, Klonis N, Tilley L, Cowman AF. Trafficking determinants for PfEMP3 export and assembly under the Plasmodium falciparum-infected red blood cell membrane. Mol Microbiol. 2005;58(4):1039–1053. doi: 10.1111/j.1365-2958.2005.04895.x. [DOI] [PubMed] [Google Scholar]
- 76.Knuepfer E, Rug M, Klonis N, Tilley L, Cowman AF. Trafficking of the major virulence factor to the surface of transfected P. falciparum-infected erythrocytes. Blood. 2005;105(10):4078–4087. doi: 10.1182/blood-2004-12-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haldar K, Hiller NL, van Ooij C, Bhattacharjee S. Plasmodium parasite proteins and the infected erythrocyte. Trends Parasitol. 2005;21(9):402–403. doi: 10.1016/j.pt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Tamez PA, et al. An erythrocyte vesicle protein exported by the malaria parasite promotes tubovesicular lipid import from the host cell surface. PLoS Pathog. 2008;4(8):e1000118. doi: 10.1371/journal.ppat.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shao L, Kner P, Rego EH, Gustafsson MG. Super-resolution 3D microscopy of live whole cells using structured illumination. Nat Methods. 2011;8(12):1044–1046. doi: 10.1038/nmeth.1734. [DOI] [PubMed] [Google Scholar]
- 80.McIntosh R, Nicastro D, Mastronarde D. New views of cells in 3D: An introduction to electron tomography. Trends Cell Biol. 2005;15(1):43–51. doi: 10.1016/j.tcb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Grüring C, et al. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat Commun. 2011;2:165. doi: 10.1038/ncomms1169. [DOI] [PubMed] [Google Scholar]
- 82.Martinez SL, Clavijo CA, Winograd E. Identification of peripheral membrane proteins associated with the tubo-vesicular network of Plasmodium falciparum infected erythrocytes. Mol Biochem Parasitol. 1998;91(2):273–280. doi: 10.1016/s0166-6851(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 83.Blisnick T, et al. Pfsbp1, a Maurer’s cleft Plasmodium falciparum protein, is associated with the erythrocyte skeleton. Mol Biochem Parasitol. 2000;111(1):107–121. doi: 10.1016/s0166-6851(00)00301-7. [DOI] [PubMed] [Google Scholar]
- 84.Vincensini L, et al. Proteomic analysis identifies novel proteins of the Maurer’s clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Mol Cell Proteomics. 2005;4(4):582–593. doi: 10.1074/mcp.M400176-MCP200. [DOI] [PubMed] [Google Scholar]
- 85.Tilley L, Hanssen E. A 3D view of the host cell compartment in P. falciparum-infected erythrocytes. Transfus Clin Biol. 2008;15(1–2):72–81. doi: 10.1016/j.tracli.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 86.van Ooij C, et al. The malaria secretome: From algorithms to essential function in blood stage infection. PLoS Pathog. 2008;4(6):e1000084. doi: 10.1371/journal.ppat.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boddey JA, et al. An aspartyl protease directs malaria effector proteins to the host cell. Nature. 2010;463(7281):627–631. doi: 10.1038/nature08728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russo I, et al. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature. 2010;463(7281):632–636. doi: 10.1038/nature08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boddey JA, Moritz RL, Simpson RJ, Cowman AF. Role of the Plasmodium export element in trafficking parasite proteins to the infected erythrocyte. Traffic. 2009;10(3):285–299. doi: 10.1111/j.1600-0854.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang HH, et al. N-terminal processing of proteins exported by malaria parasites. Mol Biochem Parasitol. 2008;160(2):107–115. doi: 10.1016/j.molbiopara.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhattacharjee S, Stahelin RV, Speicher KD, Speicher DW, Haldar K. Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell. Cell. 2012;148(1–2):201–212. doi: 10.1016/j.cell.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crabb BS, de Koning-Ward TF, Gilson PR. Protein export in Plasmodium parasites: From the endoplasmic reticulum to the vacuolar export machine. Int J Parasitol. 2010;40(5):509–513. doi: 10.1016/j.ijpara.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 93.Haase S, de Koning-Ward TF. New insights into protein export in malaria parasites. Cell Microbiol. 2010;12(5):580–587. doi: 10.1111/j.1462-5822.2010.01455.x. [DOI] [PubMed] [Google Scholar]
- 94.de Koning-Ward TF, et al. A newly discovered protein export machine in malaria parasites. Nature. 2009;459(7249):945–949. doi: 10.1038/nature08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ansorge I, Benting J, Bhakdi S, Lingelbach K. Protein sorting in Plasmodium falciparum-infected red blood cells permeabilized with the pore-forming protein streptolysin O. Biochem J. 1996;315(Pt 1):307–314. doi: 10.1042/bj3150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gehde N, et al. Protein unfolding is an essential requirement for transport across the parasitophorous vacuolar membrane of Plasmodium falciparum. Mol Microbiol. 2009;71(3):613–628. doi: 10.1111/j.1365-2958.2008.06552.x. [DOI] [PubMed] [Google Scholar]
- 97.Riglar DT, et al. Spatial association with PTEX complexes defines regions for effector export into Plasmodium falciparum-infected erythrocytes. Nat Commun. 2013;4:1415. doi: 10.1038/ncomms2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lingelbach K, Przyborski JM. The long and winding road: Protein trafficking mechanisms in the Plasmodium falciparum infected erythrocyte. Mol Biochem Parasitol. 2006;147(1):1–8. doi: 10.1016/j.molbiopara.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 99.Spielmann T, Gilberger TW. Protein export in malaria parasites: Do multiple export motifs add up to multiple export pathways? Trends Parasitol. 2010;26(1):6–10. doi: 10.1016/j.pt.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 100.Deponte M, et al. Wherever I may roam: Protein and membrane trafficking in P. falciparum-infected red blood cells. Mol Biochem Parasitol. 2012;186(2):95–116. doi: 10.1016/j.molbiopara.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 101.Marti M, Spielmann T. Protein export in malaria parasites: Many membranes to cross. Curr Opin Microbiol. 2013;16(4):445–451. doi: 10.1016/j.mib.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hawthorne PL, et al. A novel Plasmodium falciparum ring stage protein, REX, is located in Maurer’s clefts. Mol Biochem Parasitol. 2004;136(2):181–189. doi: 10.1016/j.molbiopara.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 103.Spycher C, et al. MAHRP-1, a novel Plasmodium falciparum histidine-rich protein, binds ferriprotoporphyrin IX and localizes to the Maurer’s clefts. J Biol Chem. 2003;278(37):35373–35383. doi: 10.1074/jbc.M305851200. [DOI] [PubMed] [Google Scholar]
- 104.Heiber A, et al. Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog. 2013;9(8):e1003546. doi: 10.1371/journal.ppat.1003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dixon MW, et al. Targeting of the ring exported protein 1 to the Maurer’s clefts is mediated by a two-phase process. Traffic. 2008;9(8):1316–1326. doi: 10.1111/j.1600-0854.2008.00768.x. [DOI] [PubMed] [Google Scholar]
- 106.Saridaki T, Fröhlich KS, Braun-Breton C, Lanzer M. Export of PfSBP1 to the Plasmodium falciparum Maurer’s clefts. Traffic. 2009;10(2):137–152. doi: 10.1111/j.1600-0854.2008.00860.x. [DOI] [PubMed] [Google Scholar]
- 107.Grüring C, et al. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe. 2012;12(5):717–729. doi: 10.1016/j.chom.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 108.Adisa A, Albano FR, Reeder J, Foley M, Tilley L. Evidence for a role for a Plasmodium falciparum homologue of Sec31p in the export of proteins to the surface of malaria parasite-infected erythrocytes. J Cell Sci. 2001;114(Pt 18):3377–3386. doi: 10.1242/jcs.114.18.3377. [DOI] [PubMed] [Google Scholar]
- 109.Albano FR, et al. A homologue of Sar1p localises to a novel trafficking pathway in malaria-infected erythrocytes. Eur J Cell Biol. 1999;78(7):453–462. doi: 10.1016/S0171-9335(99)80072-7. [DOI] [PubMed] [Google Scholar]
- 110.Wickert H, Rohrbach P, Scherer SJ, Krohne G, Lanzer M. A putative Sec23 homologue of Plasmodium falciparum is located in Maurer’s clefts. Mol Biochem Parasitol. 2003;129(2):209–213. doi: 10.1016/s0166-6851(03)00117-8. [DOI] [PubMed] [Google Scholar]
- 111.Adisa A, et al. Re-assessing the locations of components of the classical vesicle-mediated trafficking machinery in transfected Plasmodium falciparum. Int J Parasitol. 2007;37(10):1127–1141. doi: 10.1016/j.ijpara.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 112.Tilley L, Sougrat R, Lithgow T, Hanssen E. The twists and turns of Maurer’s cleft trafficking in P. falciparum-infected erythrocytes. Traffic. 2008;9(2):187–197. doi: 10.1111/j.1600-0854.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 113.Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol. 2009;7(5):341–354. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- 114.Papakrivos J, Newbold CI, Lingelbach K. A potential novel mechanism for the insertion of a membrane protein revealed by a biochemical analysis of the Plasmodium falciparum cytoadherence molecule PfEMP-1. Mol Microbiol. 2005;55(4):1272–1284. doi: 10.1111/j.1365-2958.2004.04468.x. [DOI] [PubMed] [Google Scholar]
- 115.Atkinson CT, et al. Ultrastructural localization of erythrocyte cytoskeletal and integral membrane proteins in Plasmodium falciparum-infected erythrocytes. Eur J Cell Biol. 1988;45(2):192–199. [PubMed] [Google Scholar]
- 116.Trelka DP, Schneider TG, Reeder JC, Taraschi TF. Evidence for vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes. Mol Biochem Parasitol. 2000;106(1):131–145. doi: 10.1016/s0166-6851(99)00207-8. [DOI] [PubMed] [Google Scholar]
- 117.Taraschi TF, Trelka D, Martinez S, Schneider T, O’Donnell ME. Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes. Int J Parasitol. 2001;31(12):1381–1391. doi: 10.1016/s0020-7519(01)00256-9. [DOI] [PubMed] [Google Scholar]
- 118.Taraschi TF, et al. Generation of an erythrocyte vesicle transport system by Plasmodium falciparum malaria parasites. Blood. 2003;102(9):3420–3426. doi: 10.1182/blood-2003-05-1448. [DOI] [PubMed] [Google Scholar]
- 119.Regev-Rudzki N, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013;153(5):1120–1133. doi: 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 120.Mantel PY, et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. 2013;13(5):521–534. doi: 10.1016/j.chom.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maier AG, et al. Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood. 2007;109(3):1289–1297. doi: 10.1182/blood-2006-08-043364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cooke BM, et al. A Maurer’s cleft-associated protein is essential for expression of the major malaria virulence antigen on the surface of infected red blood cells. J Cell Biol. 2006;172(6):899–908. doi: 10.1083/jcb.200509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spycher C, et al. The Maurer’s cleft protein MAHRP1 is essential for trafficking of PfEMP1 to the surface of Plasmodium falciparum-infected erythrocytes. Mol Microbiol. 2008;68(5):1300–1314. doi: 10.1111/j.1365-2958.2008.06235.x. [DOI] [PubMed] [Google Scholar]
- 124.Dixon MW, et al. Genetic ablation of a Maurer’s cleft protein prevents assembly of the Plasmodium falciparum virulence complex. Mol Microbiol. 2011;81(4):982–993. doi: 10.1111/j.1365-2958.2011.07740.x. [DOI] [PubMed] [Google Scholar]
- 125.Waterkeyn JG, et al. Targeted mutagenesis of Plasmodium falciparum erythrocyte membrane protein 3 (PfEMP3) disrupts cytoadherence of malaria-infected red blood cells. EMBO J. 2000;19(12):2813–2823. doi: 10.1093/emboj/19.12.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Crabb BS, et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89(2):287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 127.Armstrong CM, Goldberg DE. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat Methods. 2007;4(12):1007–1009. doi: 10.1038/nmeth1132. [DOI] [PubMed] [Google Scholar]
- 128.Rejewski M. How Polish mathematicians deciphered the Enigma. Ann Hist Comput. 1981;3(3):213–234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.