Significance
Sexual monogamy is potentially costly for males, and few mammalian species along with humans exhibit it. The hypothalamic peptide oxytocin (OXT) has been implicated in mediating pair bonds in various species, but as yet, we know little about neurobiological factors that might act to promote fidelity, especially in men. Here we provide evidence for a mechanism by which OXT may contribute to romantic bonds in men by enhancing their partner's attractiveness and reward value compared with other women.
Keywords: emotion, functional imaging, love, monogamy
Abstract
The biological mechanisms underlying long-term partner bonds in humans are unclear. The evolutionarily conserved neuropeptide oxytocin (OXT) is associated with the formation of partner bonds in some species via interactions with brain dopamine reward systems. However, whether it plays a similar role in humans has as yet not been established. Here, we report the results of a discovery and a replication study, each involving a double-blind, placebo-controlled, within-subject, pharmaco-functional MRI experiment with 20 heterosexual pair-bonded male volunteers. In both experiments, intranasal OXT treatment (24 IU) made subjects perceive their female partner's face as more attractive compared with unfamiliar women but had no effect on the attractiveness of other familiar women. This enhanced positive partner bias was paralleled by an increased response to partner stimuli compared with unfamiliar women in brain reward regions including the ventral tegmental area and the nucleus accumbens (NAcc). In the left NAcc, OXT even augmented the neural response to the partner compared with a familiar woman, indicating that this finding is partner-bond specific rather than due to familiarity. Taken together, our results suggest that OXT could contribute to romantic bonds in men by enhancing their partner's attractiveness and reward value compared with other women.
Love and enduring romantic bonds can bring the elation of profound joy and pleasure but also, when broken, the deepest sorrow and despair. Although love is the source of a large variety of emotions and feelings and celebrated in all human cultures by countless works of art, as yet surprisingly little is known about the neurobiological underpinnings of long-term pair bonds (i.e., enduring attachments between sexual partners) in humans.
Indeed, very few other mammalian species [∼3–5% (1)] exhibit pair bonds, with the most investigated species being the prairie vole (Microtus ochrogaster) (2). In prairie voles, partner bonds are formed after the neuropeptides oxytocin (OXT) or arginine vasopressin (AVP) are released in the brain during mating. Their effects on bonding are mediated via interactions with dopamine (DA) release, particularly in the ventral tegmental area (VTA) and nucleus accumbens (NAcc). Furthermore, in different species of voles, a monogamous, as opposed to polygamous, pattern of behavior is associated with a higher density of dopamine D2 receptors (D2R) in the medial prefrontal cortex (mPFC) (3) and of OXT receptors (OXTR) in mPFC, NAcc, and caudate putamen (4, 5). In prairie voles, D2R activation in the NAcc facilitates a partner preference and bond formation in the absence of mating in both sexes (6), whereas the direct injection of OXT into the NAcc (7) does so in females and of AVP into the lateral septum of males (8). Conversely, the infusion of an AVP antagonist into the ventral pallidum of male prairie voles prevents partner preference formation (9). However, nonspecific intracerebroventricular administration of OXT can induce a partner preference and bonding in both males and females (10).
These findings in voles are paralleled in humans by functional MRI (fMRI) studies showing that viewing the face of a romantic partner while recalling experiences with them activates reward-associated regions such as the VTA, NAcc, caudate, and lentiform nuclei (11–14). Genetic variability in OXT influences DA signaling in brain reward centers (15), and there are also D2R-OXTR heteromers with facilitatory receptor–receptor interactions in the striatum (16). Moreover, numerous studies have reported prosocial effects of OXT including increased social affiliation and attachment (17–19). Plasma OXT levels can be elevated by several attachment-related experiences, including partner hugs (20), social support (21), massages (22), or orgasms (23), and consequently, OXT levels are higher in new lovers than in unattached singles (24). Nevertheless, it is currently unclear to what extent peripheral measurements reflect central OXT concentrations (25). An OXTR polymorphism has also been associated with pair-bonding traits in females (26). Intranasal administration of OXT increases ratings of facial attractiveness (27), positive communication behavior during couple conflicts (28), and may facilitate the fidelity of men in a monogamous relationship by making them keep a greater distance from other women (29). We therefore predicted that OXT would strengthen romantic bonds in men by increasing the perceived attractiveness and arousal evoked by face pictures of their female partner and concomitant neural activation in the VTA, NAcc, caudate, and lentiform nuclei.
To address this fascinating hypothesis, we conducted a discovery (DSC) and a replication (RPL) study, each involving a counter balanced, double-blind, within-subject design experiment. In both studies, we used fMRI to repeatedly scan the brains of 20 heterosexual pair-bonded men (mean age ± SD and relationship length, DSC study: 25.1 ± 3.3 y and 28.8 ± 15.4 mo; RPL study: 26.6 ± 3.8 y and 36.4 ± 25.3 mo) after they received either intranasal OXT (24 IU) or placebo (PLC) treatment. During fMRI scanning of the DSC study, they were exposed to gray-scaled face photographs of their loved one, of an unfamiliar woman, or of a nonface control stimulus, a house. The photographs of other women were carefully matched for attractiveness, arousal, and picture quality with those of the partner in each case by using ratings obtained from a sample of 10 independent judges. Each picture of the three categories was presented 24 times (six blocks with four picture presentations). In the RPL study, we omitted the nonsocial control condition of the fMRI paradigm and instead used photographs of highly familiar, but nonrelated, women to control for possible nonspecific effects of OXT on familiarity. To avoid ceiling effects and to limit cognitive processing, we asked our subjects to passively view the pictures. After each fMRI scan, subjects completed the Passionate Love Scale (PLS) questionnaire (30) and rated the perceived attractiveness and arousal evoked by the faces using a visual analog scale ranging from 0 (minimum) to 100 (maximum). Further, love styles (e.g., Eros, a committed romantic relationship) (31) were measured before the administration of the nasal spray.
Results
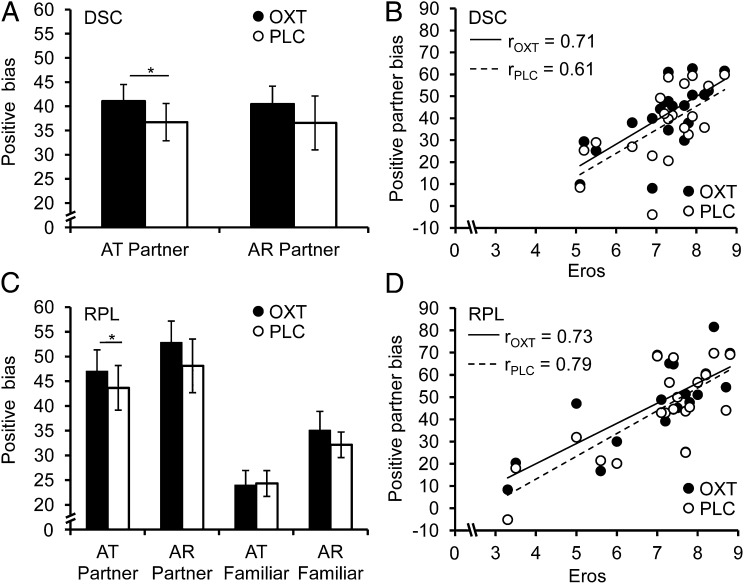
All subjects reported being passionately in love (Table S1). A repeated-measures ANOVA with bond (DSC: partner vs. unfamiliar; RPL: partner, unfamiliar and familiar) and treatment (OXT vs. PLC) as within-subject variables and attractiveness ratings as a dependent variable yielded a main effect of bond (DSC: F(1,19) = 120.96, P < 0.01, ƞ2 = 0.88; RPL: F(2,18) = 61.61, P < 0.01, ƞ2 = 0.87) and an interaction effect of bond and treatment (DSC: F(1,19) = 7.00, P = 0.02, ƞ2 = 0.27; RPL: F(2,18) = 2.82, P = 0.086, ƞ2 = 0.24). For arousal ratings, we detected only a main effect of bond (DSC: F(1,19) = 74.96, P < 0.01, ƞ2 = 0.80; RPL: F(2,18) = 84.47, P < 0.01, ƞ2 = 0.90) and a main effect of treatment in the DSC study (F(1,19) = 7.47, P = 0.01, ƞ2 = 0.28), but no interaction effects (all P > 0.14). The main effect of bond became evident as a positive partner bias, i.e., the participants gave their partners significantly higher attractiveness and arousal ratings than other either unfamiliar or familiar women (attractiveness: DSCOXT: t(19) = 12.11, P < 0.01, d = 4.09; DSCPLC: t(19) = 9.52, P < 0.01, d = 3.68; RPLOXT: t(19) = 10.92, P < 0.01, d = 3.90; RPLPLC: t(19) = 9.68, P < 0.01, d = 3.53 and arousal: DSCOXT: t(19) = 11.03, P < 0.01, d = 3.20; DSCPLC: t(19) = 6.57, P < 0.01, d = 2.06; RPLOXT: t(19) = 12.21, P < 0.01, d = 3.51; RPLPLC: t(19) = 8.87, P < 0.01, d = 2.60). Interestingly, post hoc t tests in the RPL study also revealed a positive bias for attractiveness and arousal ratings of the highly familiar woman (attractiveness: RPLOXT: t(19) = 8.12, P < 0.01, d = 1.87; RPLPLC: t(19) = 9.30, P < 0.01, d = 2.26, arousal: RPLOXT: t(19) = 8.12, P < 0.01, d = 2.14; RPLPLC: t(19) = 9.30, P < 0.01, d = 2.13), but these effects were significantly smaller than the partner bias (attractiveness: RPLOXT: t(19) = 5.63, P < 0.01, d = 1.39; RPLPLC: t(19) = 5.79, P < 0.01, d = 1.17; arousal: RPLOXT: t(19) = 4.44, P < 0.01, d = 0.97; RPLPLC: t(19) = 3.19, P < 0.01, d = 0.84). The arousal-based treatment effect in the DSC study is related to higher arousal ratings under OXT, irrespective of the bond. However, this effect could not be replicated in the RPL study. Importantly, as indicated by the interaction effect of treatment and bond, OXT enhanced the positive partner bias in attractiveness ratings in both the DSC (t(19) = 2.65, P = 0.02, d = 0.27; Fig. 1A) and RPL studies (t(19) = 2.32, P = 0.03, d = 0.17; Fig. 1C). OXT neither altered PLS scores nor the positive bias for familiar as opposed to unfamiliar women (all P > 0.05). Moreover, a correlation analysis between the positive partner bias and love styles revealed that only a positive association with Eros was significant in the DSC and RPL studies, which survived Bonferroni correction for multiple testing (DSC: rPLC = 0.61, P < 0.01; rOXT = 0.71, P < 0.01; Fig. 1B; RPL: rPLC = 0.79, P < 0.01; rOXT = 0.73, P < 0.01; Fig. 1D), providing further evidence that this bias indeed constitutes a fundamental element of a romantic relationship.
Fig. 1.
OXT effects on partner judgments. Both in the DSC (A) and RPL (C) study, the intranasal administration of OXT significantly increased the positive partner bias for attractiveness. This bias was positively correlated with Eros (a romantic love style) in the DSC (B) and RPL (D) study. Error bars indicate SEM. AR, arousal; AT, attractiveness; DSC, discovery; OXT, oxytocin; PLC, placebo; RPL, replication; *P < 0.05.
On the neural level, a whole-brain analysis in both the DSC and RPL study revealed an increased blood oxygen level-dependent (BOLD) signal in the middle occipital gyrus (DSC: MNI coordinates 30, −85, 4, t(19) = 6.85, family wise error corrected: PFWE < 0.01; RPL: MNI coordinates −39, −61, −8, t(19) = 5.74, PFWE < 0.01) and anterior cingulate cortex (DSC: MNI coordinates −6, 41, −5, t(19) = 4.46, PFWE < 0.01; RPL: MNI coordinates −3, 50, 7, t(19) = 5.74, PFWE < 0.01) when the partner's face was presented compared with an unfamiliar woman (Tables S2 and S3). We did not observe any comparable effects for the contrast [Partner > Familiar] and there were also no nonspecific main treatment effects across conditions (all P > 0.05).
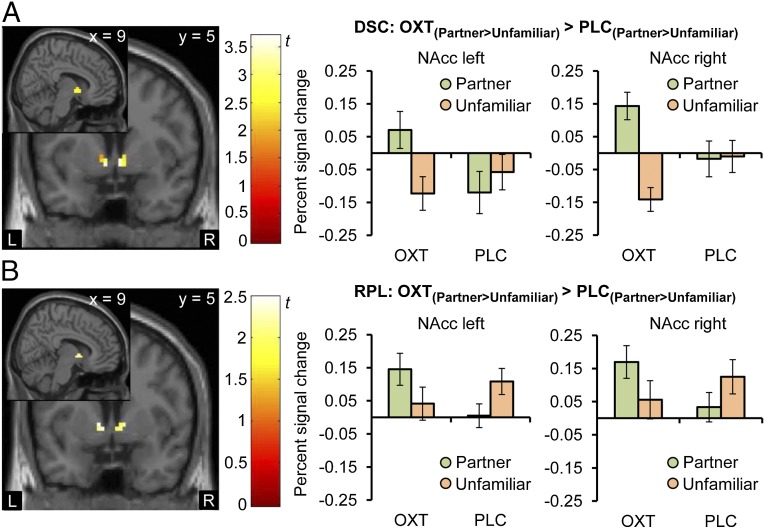
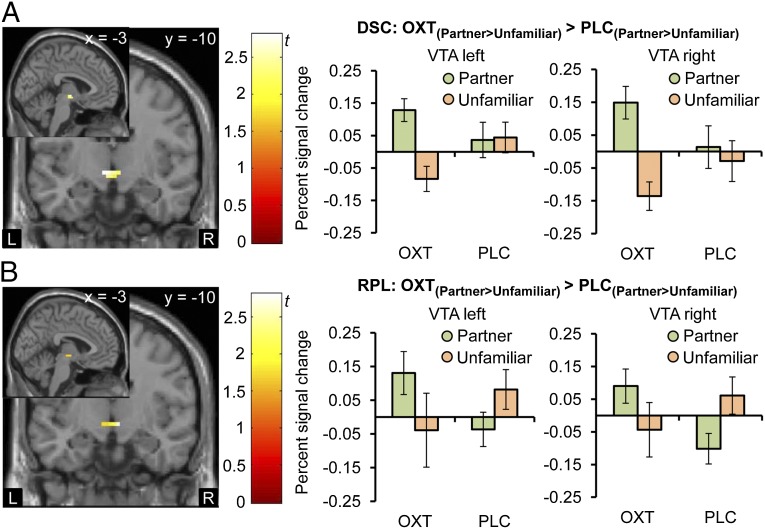
To examine the specific OXT effect on the processing of the partner's face, we computed the contrast [PartnerOXT > UnfamiliarOXT] > [PartnerPLC > UnfamiliarPLC] in both studies and additionally [PartnerOXT > FamiliarOXT] > [PartnerPLC > FamiliarPLC] in the RPL study. When we restricted our analysis to the VTA, NAcc, caudate, and lentiform nucleus as hypothesis-driven predefined regions of interest (ROIs), we found enhanced responsiveness for the contrast [PartnerOXT > UnfamiliarOXT] > [PartnerPLC > UnfamiliarPLC] bilaterally in the NAcc (Fig. 2A) and VTA (Fig. 3A) (PFWE < 0.05; DSC study). This finding was replicated bilaterally in the NAcc (Fig. 2B) and unilaterally in the right VTA (Fig. 3B; RPL study). Inspection of the extracted individual percent signal changes confirmed that this effect was due to a differential response as a result of OXT treatment. With houses as control stimuli in the DSC study ([PartnerOXT > HouseOXT] > [PartnerPLC > HousePLC]), we found similar OXT effects as with unfamiliar women for the NAcc (DSC left peak MNI coordinates −6, 5, −5; t(114) = 2.69, PFWE = 0.03; right peak MNI coordinates 6, 2, −5; t(114) = 2.05, PFWE = 0.098). For the VTA, no significant effect was detected, indicating that not only an increased response to the partner but also a decreased response to alternative women is necessary for OXT effects to become evident. In fact, a direct comparison of the neural response to the unfamiliar woman under OXT and PLC ([UnfamiliarPLC > UnfamiliarOXT]) showed a trend for a reduced activation in the left VTA after OXT administration (DSC peak MNI coordinates −6, −10, −5; t(19) = 2.58, PFWE = 0.051; Fig. S1).
Fig. 2.
OXT effects on NAcc responses. Both in the DSC (A) and RPL (B) study, the intranasal administration of OXT increased NAcc response to the female partner's face compared with a matched, but unfamiliar woman (OXT(Partner > Unfamiliar) > PLC(Partner > Unfamiliar); DSC: left peak MNI coordinates −6, 5, −5; t(114) = 3.22, PFWE < 0.01; right peak MNI coordinates 6, 2, −2; t(114) = 3.70, PFWE < 0.01; RPL: left peak MNI coordinates −9, 5, 1; t(152) = 2.40, PFWE = 0.052; right peak MNI coordinates 12, 2, 1; t(152) = 2.48, PFWE = 0.04; display threshold P < 0.05 uncorrected). Percent signal change in the bilateral NAcc showed the greatest response to the mate after OXT administration. Error bars indicate SEM. DSC, discovery; L, left hemisphere; NAcc, nucleus accumbens; OXT, oxytocin; PLC, placebo; R, right hemisphere; RPC, replication.
Fig. 3.
OXT effects on VTA responses. Both in the DSC (A) and RPL (B) study, the intranasal administration of OXT increased neural responses in the VTA to the face of the female partner compared with a matched, but unfamiliar woman (OXT(Partner > Unfamiliar) > PLC(Partner > Unfamiliar); DSC: left peak MNI coordinates −6, −10, −5; t(114) = 2.56, PFWE = 0.03; right peak MNI coordinates 0, −10, −5; t(114) = 2.33, PFWE = 0.051; RPL: left peak MNI coordinates 0, −16, −8; t(152) = 1.93, PFWE = 0.12; right peak MNI coordinates 6, −13, −5; t(152) = 2.89, PFWE = 0.02; display threshold P < 0.05 uncorrected). Analyses of percent signal change in the bilateral VTA showed the greatest response to the mate after OXT administration. Error bars indicate SEM. DSC, discovery; L, left hemisphere; OXT, oxytocin; PLC, placebo; R, right hemisphere; RPC, replication; VTA, ventral tegmental area.
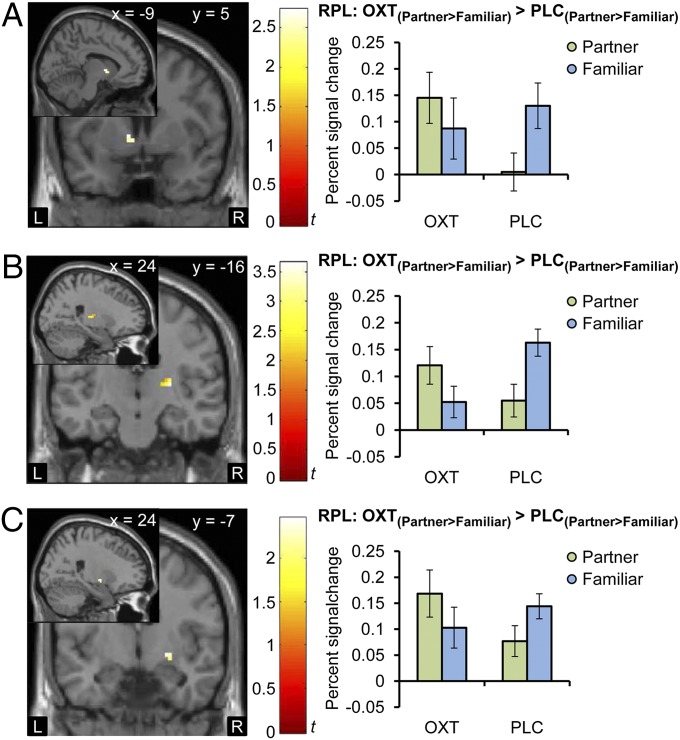
To further elucidate the specificity of these findings, we applied the ROI analyses to the contrast [PartnerOXT > FamiliarOXT] > [PartnerPLC > FamiliarPLC] in the RPL study and again detected an increased activation in the NAcc (left peak MNI coordinates −9, 5, 1; t(152) = 2.73, PFWE = 0.02; right peak MNI coordinates 12, 5, 1; t(152) = 2.09, PFWE = 0.099; Fig. 4A). Interestingly, OXT also enhanced the activation in response to the partner compared with the familiar control in the right putamen (peak MNI coordinates 27, −19, 7; t(152) = 3.65, PFWE < 0.01; Fig. 4B) and right globus pallidus (peak MNI coordinates 24, −7, −5; t(152) = 2.46, PFWE = 0.04; Fig. 4C). For the contrast [FamiliarOXT > UnfamiliarOXT] > [FamiliarPLC > UnfamiliarPLC], there was no effect of OXT in either the NAcc or VTA, even at very liberal significance thresholds (uncorrected P < 0.05). However, we observed a greater activation in the caudate body (right: MNI x, y, z: 21, −13, 25, t(152) = 2.58, PFWE = 0.03), possibly suggesting that OXT may also influence nonpartner bonding via distinct striatal nuclei. As expected, neither the whole-brain nor the ROI-based analyses showed any OXT effect on responses to nonsocial control stimuli ([HouseOXT > HousePLC] or [HouseOXT < HousePLC]).
Fig. 4.
OXT effects on responses to the partner compared with a familiar woman. OXT increased the response to the female partner’s face compared with a highly familiar woman in the left NAcc (A; peak MNI coordinates −9, 5, 1; t(152) = 2.73, PFWE = 0.02), right putamen (B; peak MNI coordinates 27, −19, 7; t(152) = 3.65, PFWE < 0.01), and right globus pallidus (C; peak MNI coordinates 24, −7, −5; t(152) = 2.46, PFWE = 0.04, display threshold P < 0.05 uncorrected). Error bars indicate SEM. DSC, discovery; L, left hemisphere; NAcc, nucleus accumbens; OXT, oxytocin; PLC, placebo; R, right hemisphere.
Discussion
In this study, we examined the effect of an intranasal OXT administration on the behavioral ratings of and the neural response to face stimuli of long-term romantic partners. Our results confirmed the hypothesis that OXT also contribute to romantic pair bonding in humans by showing that OXT treatment enhanced a positive bias in men to perceive their female partner's face as more attractive than that of other unfamiliar women, an effect paralleled by increased activation of reward neurocircuitry.
The notion that OXT may play a specific role in maintaining long-term pair bonding in humans is also substantiated by our previous findings that it makes men in a relationship keep a greater social distance from attractive female strangers (29) and reinforces the suggestion that OXT effects are both context and person dependent (32, 33). An OXT-mediated positive bias in the perception of the female partner can be adaptive and may serve as a long-term resilience factor because such idealization can predict sustained relationship satisfaction over the first 3 y of marriage (34). In the left NAcc, OXT augmented the neural response to the partner both in comparison with unfamiliar and familiar women. However, we also observed an increased neural response to nonpartner related bonding stimuli (familiar women) in the caudate body, intriguingly suggesting that OXT may influence different kinds of attachments via distinct striatal areas. Future studies are warranted to disentangle this complex interaction between OXT and different types of bonding. Importantly, three different phases in romantic relationships have been defined that share common biological substrates but also involve different brain systems (11, 35, 36). The relative short first phase of “being in love” usually lasts around half a year and evolves into a longer phase of “passional love.” Eventually, after several years, the third phase of a relationship can be characterized as “companionate love” similar to friendships. The mean relationship durations in the present study were 29 and 36 mo (DSC and RPL, respectively), indicating that the participants were in the second phase of their relationship in which the initial euphoria and excitation decline but the passion remains high. Thus, OXT may still play a substantial role in the process of establishing a pair bond in the first stage of romantic love. Attractiveness and arousal ratings may not be sufficiently sensitive to detect nonsexual bonding effects, but OXT also enhanced caudate responses to familiar control stimuli. Consequently, OXT may not exclusively affect long-term romantic partner- and parent-infant bonds (37), but also influence other kinds of social bonds.
Enhanced activation of the NAcc and VTA is likely to reflect an increased reward value of the female partner and suggests that OXT may affect these dopaminergic areas in the context of bonds in a similar manner to monogamous voles. Facial attractiveness is known to elicit striatal activation (38), but by choosing face stimuli matched for overall attractiveness, we avoided any nonspecific contribution from this. Likewise, our results cannot be accounted for by nonspecific conscious effects of OXT because it did not affect state anxiety or mood ratings, and the subjects could not identify whether they had received OXT or PLC (Tables S4 and S5). Furthermore, reduced VTA activation in response to the faces of other women after OXT treatment indicates it could further contribute to long-term pair bonding by making interactions with other women less rewarding, although we found no corresponding behavioral support for this. If the OXT effect on the VTA activation in response to the partner reflects a devaluation of other unfamiliar women the man has no social bond with, then this could explain why we did not find a similar response to other familiar women they do have a social bond with in the RPL study. Recently, it has been suggested that the VTA is the human brain site where oxytocin acts to attach salience to socially relevant cues (39), and it is also likely that the comparison of the partner with an unfamiliar woman involves a larger salience difference than the contrast with a familiar one.
The absence of a stronger neural response to the female partner during PLC treatment indicates that the presentation of a partner's neutral expression face is not more rewarding per se than that of a novel female of equivalent attractiveness (40). Thus, findings in previous fMRI studies of increased activation in brain reward areas in response to a partner's face may reflect the instruction to think about the person at the same time (11–14). In fact, another non–pharmaco-fMRI study in which no additional instruction was given also failed to detect any striatal activation in response to partner faces (41). Furthermore, previous studies differed with regard to sample characteristics (e.g., a higher mean relationship duration) and task design (e.g., a longer duration of stimulus presentation) (11–14).
For men in a relationship, increased endogenous OXT signaling in the brain following experience of proximity, social support, intimate contact, or sex with their romantic partner might make these behaviors even more rewarding via an engagement of related neurocircuitry. In this way, a feed-forward loop would be initialized, resulting in a progressive increase in desire for the partner, similar to a drug addict's increased craving for drug consumption (42). This notion suggests the intriguing possibility that OXT may serve as a potential treatment for love-related withdrawal syndromes, including lovesickness and pathological grief from loss of a loved one. However, against the background of mounting evidence for anxiogenic and fear-enhancing OXT effects (33, 43, 44), it is necessary that the mechanisms determining the outcome of an OXT treatment are completely identified.
Although we report here a very specific OXT effect on pair-bond–related behavioral ratings and neural substrates, several limitations of our study should be acknowledged. In accordance with the majority of previous intranasal OXT studies, we did not observe an OXT effect on state anxiety and mood ratings, but it is conceivable that more sensitive measures might have detected subtle alterations. Most importantly, we cannot rule out that low-level, unconscious changes in affective state occurred because we did not collect psychophysiological data. Furthermore, it is likely that social desirability contributed to the positive partner bias, and future studies should try to elucidate to what extent OXT influences the perception of the partner per se. However, the fact that OXT had no effect on the positive bias toward more socially desirable familiar as opposed to unfamiliar women indicates that social desirability alone cannot explain our behavioral findings.
Taken together, our findings suggest that OXT may contribute to romantic bonds by making men perceive their female partner as increasingly attractive and rewarding compared with other women. By keeping an exclusive devotion to a single mate, it may open an evolutionary path to biparental care of offspring and shift a potentially destructive competition between males for mates toward more beneficial in-group cooperation (45).
Materials and Methods
Participants.
Twenty healthy, nonsmoking male adults participated in the DSC study, and a completely independent sample of 20 healthy, nonsmoking male adults volunteered in the RPL study after giving written informed consent. The studies were approved by the institutional review board of the Medical Faculty of the University of Bonn and were carried out in compliance with the latest revision of the Declaration of Helsinki. Subjects received monetary compensation for study participation. Screening of the subjects was conducted before the test sessions (Table S6). Subjects were free of current and past physical or psychiatric illness, as assessed by medical history and a structured clinical interview for axis I and axis II disorders according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) IV (46). In addition, they were naive to prescription-strength psychoactive medication and had not taken any over-the-counter psychoactive medication in the last 4 wk.
All subjects were in a romantic heterosexual relationship for more than 6 mo, were unmarried, and had no children. The subjects reported to be passionately in love, and the time intervals since they last saw their partners and had intimate contact were comparable between the OXT and PLC sessions, as well as between the DSC and RPL study (Table S1). We also controlled if the subjects had an argument with their partners in the week before both test sessions and if anything important in their relationship changed between the two test sessions. The familiar control in the RPL study was a nonrelated woman whom the participants had known for at least 30 mo.
Experimental Design.
In both the DSC and RPL studies, we applied a randomized, placebo-controlled, double-blind, within-group design. Subjects were randomly assigned to either intranasal administration of OXT (24 IU; Syntocinon-Spray; Novartis; three puffs per nostril, each with 4 IU OXT) or PLC (sodium chloride solution) 30 min before the start of the fMRI. The mean interval between the two fMRI sessions was 33 d (minimum, 21 d; maximum, 56 d) in the DSC study and 12.7 d (minimum, 6 d; maximum, 35 d) in the RPL study. Details on the tasks, fMRI procedure, and analyses can be found in SI Materials and Methods.
fMRI Paradigm.
We adopted a modified version of an established face perception task (47) in the DSC study involving passive viewing of photographs of the partner, a matched, unknown woman, and houses. In the RPL study, we used photographs of the partner and of a highly familiar woman. As control stimuli, a photograph of an unknown woman matched to the partner and another photograph matched to the familiar woman were used in the RPL study. Subjects were asked to send several photographs of their partners and of a familiar, but nonrelated, woman, arranged as passport photographs, with a neutral, but friendly facial expression. The selected photographs were equated for facial expression, size, and luminance. An independent sample of 10 heterosexual men (age: 25.50 ± 2.99 y; an ANOVA with the factor group yielded no significant age difference between the DSC, RPL, or pilot samples, P = 0.38) rated the arousal and attractiveness of the women and the quality of the photographs on a visual analog scale (0 = minimum, 100 = maximum) before the first fMRI session. Based on these three dimensions, the Euclidian distances between all photographs were computed, and the photograph with the lowest Euclidian distance was chosen as control stimulus for a partner photograph. The same procedure was applied to find matched photographs for the familiar women in the RPL study. We ensured that no subject knew the woman whose photograph was used as his control stimulus. To avoid confounding factors in the photographs, the backgrounds were masked in black, and the photographs were gray-scaled. Pictures of houses were used as nonfacial control stimuli. Houses serve as an appropriate control stimuli for faces because they share similar spatial and visual features (48). Moreover, houses are everyday objects with nonarousing properties. All houses were gray-scaled and equated for size and luminance.
Using Presentation 14 (Neurobehavioral Systems), stimuli were presented blockwise, via liquid crystal display (LCD) video goggles (Nordic NeuroLab). In total, there were six blocks for each stimulus category, and each block included four repetitions of the same stimulus (e.g., four times the photograph of the partner). Stimuli were presented for 2,625 ms on-screen, and the interstimulus interval varied between 250 and 1,500 ms to create jitter, resulting in a mean block length of 14.5 s. The sequence of blocks was randomized, and blocks were separated from each other by a low-level baseline period of 14.5-s duration, where a fixation cross was depicted in the center of the screen. To assure attentive stimulus processing, subjects were asked to press a keypad button whenever a stimulus was presented (percent correct responses DSC: OXT 97.64 ± 7.49, PLC 95.90 ± 10.16; RPL: OXT 98.62 ± 3.19, PLC 98.62 ± 4.5; all P ≥ 0.33). In addition, the subjects were asked to look attentively at the pictures and to avoid cognitive processing; there were no further instructions.
Behavioral Task.
After scanning, subjects were seated in front of a computer and were asked to rate photographs showing their own partner, the familiar woman, and partners of the other subjects on a visual analog scale for attractiveness (ranging from 0, most unattractive, to 100, most attractive) and arousal (ranging from 0, not arousing, to 100, most arousing). The sequence of the photographs was randomized.
Acquisition of fMRI Data.
The MRI data of the DSC and RPL study were acquired with the same system and scanning parameters. A Siemens Trio MRI system (Siemens) operating at 3 T was used to obtain T2*-weighted echoplanar (EPI) images with BOLD contrast (repetition time = 3,000 ms, echo time = 35 ms, matrix size: 64 × 64, pixel size: 3 × 3 × 3 mm, slice thickness = 3.0 mm, distance factor = 10%, field of view = 192, flip angle = 90°, 36 axial slices). In addition, high-resolution anatomical images were acquired on the same scanner using a T1-weighted 3D MPRAGE sequence (imaging parameters: repetition time = 1,570 ms, echo time = 3.42 ms, matrix size: 256 × 256, pixel size: 1 × 1 × 1 mm, slice thickness = 1.0 mm, field of view = 256, flip angle = 15°, 160 sagital slices).
Analysis of fMRI Data.
fMRI data were preprocessed and analyzed using SPM8 software (Wellcome Trust Centre for Neuroimaging, www.fil.ion.ucl.ac.uk/spm) implemented in Matlab 7 (MathWorks). On the first level, six conditions (PartnerOXT, ControlOXT, HouseOXT, PartnerPLC, ControlPLC, and HousePLC) in the DSC study and eight conditions (PartnerOXT, Control_PartnerOXT, FamiliarOXT, Control_FamiliarOXT, PartnerPLC, Control_PartnerPLC, FamiliarPLC, and Control_FamiliarPLC) in the RPL study were modeled by a boxcar function convolved with a hemodynamic response function (49). The movement parameters were included as confounds in the design matrix. Main effects of treatment and bonding were analyzed by comparing the conditions relative to the low-level baseline. Parameter estimates for each contrast were subjected to one-sample t tests on the second level for the whole brain with a significance threshold of P < 0.05 corrected for multiple comparisons [familywise error (FWE)]. To specifically examine the modulatory effects of OXT, a flexible factorial model was designed with the factors treatment (OXT vs. PLC) and bond (DSC: partner vs. unfamiliar; RPL: partner, unfamiliar and familiar) to test the contrasts [PartnerOXT > ControlOXT] > [PartnerPLC > ControlPLC], [PartnerOXT > HouseOXT] > [PartnerPLC > HousePLC], [FamiliarOXT > Control_FamiliarOXT] > [FamiliarPLC > Control_FamiliarPLC], and [PartnerOXT > FamiliarOXT] > [PartnerPLC > FamiliarPLC]. The Wake Forest University Pickatlas (Version 3.0) was used to generate 5-mm ROI masks for NAcc, VTA, caudate, and lentiform nucleus (SI Materials and Methods), and the threshold for significance was set at P < 0.05 and was FWE corrected for multiple comparisons based on the size of the ROI.
Supplementary Material
Acknowledgments
R.H. was supported by German Research Foundation Grant HU1302/2-2 and a starting independent researcher grant (Neuromodulation of Emotion) jointly provided by the Ministry of Innovation, Science, Research and Technology of the German State of North Rhine-Westphalia and the University of Bonn. K.M.K. was supported by National Natural Science Foundation of China Grant 91132720.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314190110/-/DCSupplemental.
References
- 1.Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52(1):39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- 2.Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32(1):53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394(2):146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro LE, Insel TR. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Ann N Y Acad Sci. 1992;652:448–451. doi: 10.1111/j.1749-6632.1992.tb34380.x. [DOI] [PubMed] [Google Scholar]
- 5.Ophir AG, Gessel A, Zheng DJ, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm Behav. 2012;61(3):445–453. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aragona BJ, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9(1):133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121(3):537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115(4):910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 9.Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125(1):35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 11.Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Soc Cogn Affect Neurosci. 2012;7(2):145–159. doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aron A, et al. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94(1):327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- 13.Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11(17):3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- 14.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Love TM, et al. Oxytocin gene polymorphisms influence human dopaminergic function in a sex-dependent manner. Biol Psychiatry. 2012;72(3):198–206. doi: 10.1016/j.biopsych.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero-Fernandez W, Borroto-Escuela DO, Agnati LF, Fuxe K. Evidence for the existence of dopamine d2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol Psychiatry. 2013;18(8):849–850. doi: 10.1038/mp.2012.103. [DOI] [PubMed] [Google Scholar]
- 17.Insel TR. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurlemann R, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30(14):4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32(4):426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol Psychol. 2005;69(1):5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67(4):531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- 22.Morhenn V, Beavin LE, Zak PJ. Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Altern Ther Health Med. 2012;18(6):11–18. [PubMed] [Google Scholar]
- 23.Borrow AP, Cameron NM. The role of oxytocin in mating and pregnancy. Horm Behav. 2012;61(3):266–276. doi: 10.1016/j.yhbeh.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Schneiderman I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin during the initial stages of romantic attachment: Relations to couples’ interactive reciprocity. Psychoneuroendocrinology. 2012;37(8):1277–1285. doi: 10.1016/j.psyneuen.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Churchland PS, Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm Behav. 2012;61(3):392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walum H, et al. Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biol Psychiatry. 2012;71(5):419–426. doi: 10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav. 2009;56(1):128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Ditzen B, et al. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65(9):728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Scheele D, et al. Oxytocin modulates social distance between males and females. J Neurosci. 2012;32(46):16074–16079. doi: 10.1523/JNEUROSCI.2755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatfield E, Sprecher S. Measuring passionate love in intimate relationships. J Adolesc. 1986;9(4):383–410. doi: 10.1016/s0140-1971(86)80043-4. [DOI] [PubMed] [Google Scholar]
- 31.Lee JA. Colours of Love: An Exploration of the Ways of Loving. Toronto: New Press; 1973. [Google Scholar]
- 32.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Striepens N, et al. Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci USA. 2012;109(44):18144–18149. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray SL, et al. Tempting fate or inviting happiness?: Unrealistic idealization prevents the decline of marital satisfaction. Psychol Sci. 2011;22(5):619–626. doi: 10.1177/0956797611403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Boer A, van Buel EM, Ter Horst GJ. Love is more than just a kiss: A neurobiological perspective on love and affection. Neuroscience. 2012;201:114–124. doi: 10.1016/j.neuroscience.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Fisher HE, Aron A, Brown LL. Romantic love: A mammalian brain system for mate choice. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2173–2186. doi: 10.1098/rstb.2006.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman R. Oxytocin and social affiliation in humans. Horm Behav. 2012;61(3):380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Aharon I, et al. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32(3):537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 39.Groppe SE, et al. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2013;74(3):172–179. doi: 10.1016/j.biopsych.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Bunzeck N, Düzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51(3):369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Taylor MJ, et al. Neural correlates of personally familiar faces: Parents, partner and own faces. Hum Brain Mapp. 2009;30(7):2008–2020. doi: 10.1002/hbm.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224(1):1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guzmán YF, et al. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 2013;16(9):1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grillon C, et al. Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 2013;18(9):958–960. doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gavrilets S. Human origins and the transition from promiscuity to pair-bonding. Proc Natl Acad Sci USA. 2012;109(25):9923–9928. doi: 10.1073/pnas.1200717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, Washington), 4th Ed.
- 47.Goossens L, et al. Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp. 2009;30(10):3332–3338. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yovel G, Kanwisher N. Face perception: Domain specific, not process specific. Neuron. 2004;44(5):889–898. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Friston KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2(4):189–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.