Significance
The somatosensory barrels are a unique feature of the rodent cortex. Each barrel represents a functional unit in which clustered innervation from an individual whisker connects with a ring of cortical neurons. This study reports that when a single transcription factor, LIM homeobox 2, is deleted specifically in the cortex, neither the barrel cores nor the cortical barrel walls are able to form, although a rudimentary functional mapping of the somatosensory innervation does occur. Understanding how barrels form will shed light on how functional neurocircuitry is assembled in its final stages, and this insight may be broadly applicable in the nervous system.
Abstract
LIM homeodomain transcription factors are critical regulators of early development in multiple systems but have yet to be examined for a role in circuit formation. The LIM homeobox gene Lhx2 is expressed in cortical progenitors during development and also in the superficial layers of the neocortex in maturity. However, analysis of Lhx2 function at later stages of cortical development has been hampered by severe phenotypes associated with early loss of function. We identified a particular Cre-recombinase line that acts in the cortical primordium after its specification is complete, permitting an analysis of Lhx2 function in neocortical lamination, regionalization, and circuit formation by selective elimination of Lhx2 in the dorsal telencephalon. We report a profound disruption of cortical neuroanatomical and molecular features upon loss of Lhx2 in the cortex from embryonic day 11.5. A unique feature of cortical circuitry, the somatosensory barrels, is undetectable, and molecular patterning of cortical regions appears disrupted. Surprisingly, thalamocortical afferents innervate the mutant cortex with apparently normal regional specificity. Electrophysiological recordings reveal a loss of responses evoked by stimulation of individual whiskers, but responses to simultaneous stimulation of multiple whiskers were present, suggesting that thalamic afferents are unable to organize the neurocircuitry for barrel formation because of a cortex-specific requirement of Lhx2. We report that Lhx2 is required for the expression of transcription factor paired box gene 6, axon guidance molecule Ephrin A5, and the receptor NMDA receptor 1. These genes may mediate Lhx2 function in the formation of specialized neurocircuitry necessary for neocortical function.
The formation of a functional brain structure is a stepwise process starting with the specification of a particular region of neuroepithelium, followed by the production of the correct types and numbers of neurons, and finally the assembling of the circuitry so that innervation to and from other structures is connected properly. The mammalian neocortex is unique because of its complex six-layer architecture, and its development is particularly complex because not only do neurons in different layers have unique identities and innervation patterns, but also the cortex as a whole is patterned into discrete regions subserving distinct functions. Several transcription factors known to have roles in neocortical patterning display graded expression in the dorsal telencephalon. Paired box 6 (Pax6), empty spiracles homeobox 2 (Emx2), Nuclear receptor subfamily 2, group f, member 1 (NR2f1; also known as Coup transcription factor 1, COUP-TFI), and Specificity protein 8 (Sp8) are expressed in graded pattern in the cortical ventricular zone. Sp8 and Pax6 are expressed in a rostral (high) to caudal (low) gradient and impart rostral identity (1–3), whereas Emx2 and NR2f1 are expressed in the opposite pattern, caudal (high) to rostral (low), and impart caudal areal identity to the cortical primordium (4–6). LIM homeobox 2 (Lhx2) is expressed in a gradient similar to that of Emx2 and NR2f1, but its role in cortical patterning remains to be investigated because early loss of Lhx2 function results in severe defects that prevent the formation of the neocortex (7–9).
Lhx2 plays a fundamental role as a cortical selector gene, permitting the specification of the cortical primordium as a whole by suppressing alternative fates corresponding to the hem, antihem, and the paleocortex. In the Lhx2-null mutant, two noncortical structures, the hem and the antihem, expand at the expense of the cortical primordium (7, 9, 10). Conditional deletion of Lhx2 at embryonic day (E) 10.5 using an Emx1Cre driver (11) produces ectopic paleocortex instead of neocortex (8). NestinCre acts from E11.5 and spares the neocortex (8), but it also drives recombination in subcortical regions such as the thalamus (12), preventing an analysis of Lhx2 loss of function exclusively in the cortex.
We were able to circumvent these constraints using another Emx1Cre line (13) which we found to act a day later (E11.5) than the one commonly (11). This later-acting Emx1Cre line permits the neocortex to form despite the loss of Lhx2, permitting an analysis of Lhx2 function in neocortical lamination and regionalization by selective elimination of Lhx2 in the dorsal telencephalon. We report that loss of Lhx2 in the dorsal telencephalon results in a profound disruption of neocortical regional characteristics. Molecular and neuroanatomical features that distinguish the somatosensory cortex—the barrels—are not detectable when Lhx2 is deleted in the cortical primordium. Surprisingly, thalamocortical fibers extend to the cortex and demonstrate apparently normal region specificity with respect to the somatosensory and visual projections, indicating that a broad areal map is formed in the absence of Lhx2. Consistent with this observation, stimulation of multiple whiskers together is able to drive cortical neurons in the mutant. However, responses evoked by the stimulation of an individual whisker are not seen in the mutant cortex, suggesting that the refinement of thalamocortical connectivity to form barrels fails to occur. The barrels are a prominent example of what may be a broader role for Lhx2 in the cortex, the organization of normal neuroanatomical and connectional features of mature cortical circuitry.
Results
Emx1Cre transgenic mice have been used extensively to study the functions of important developmental control molecules in the cortical primordium. The advantage of such lines is that the recombinase expression, like that of Emx1 itself, is limited to the cortical primordium in the forebrain. The timing of this expression generally is considered to be effective at E10.5, although in the most extensively used Emx1Cre line, in which Cre recombinase is knocked into the 3′ UTR as an internal ribosome entry site (IRES)-Cre construct, the initiation of recombinase activity has been reported as early as E9.5 (11). We compared the Emx1Cre line with another such line available to us in which an IRES-Cre construct is knocked into exon 1 of the Emx1 gene, creating a null allele of Emx1 (13). These lines henceforth are referred to as Emx1CreKJ (for the 3′ UTR knockin) and Emx1CreYL (for the exon 1 knockin).
Animals homozygous for the floxed Lhx2 allele (conditional knockout, cKO) were crossed with Emx1CreKJ or Emx1CreYL mice. Embryos from each cross were harvested at different ages and examined for the expression of Lhx2 exon 2/3, which is lost in floxed cells. Emx1CreKJ and Emx1CreYL mice also were crossed to the membrane targeted tomato and membrane targeted GFP (mTmG) reporter line in which GFP expression is seen upon successful floxing (Fig. 1).
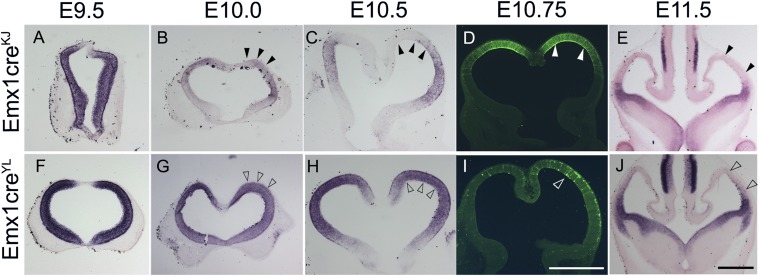
Fig. 1.
Time points of action of two different Emx1Cre lines. (A–E) Brains from embryos carrying Emx1CreKJ together with Lhx2 cKO (A–C and E) or mTmG reporter (D), harvested at different stages. Lhx2 is expressed in the dorsal telencephalon at E9.5 but decreases by E10.0 and is lost by E10.5 (arrowheads in B and C). The mTmG reporter reveals extensive GFP expression in an E10.75 brain, an indication of Cre activity (arrowheads in D). An E11.5 brain shows complete loss of Lhx2 expression in the dorsal telencephalon (arrowheads in E). (F–J) Brains from embryos carrying Emx1CreYL together with Lhx2 cKO (F–H and J) or mTmG reporter (I), harvested at different stages. Lhx2 is expressed in the dorsal telencephalon at E9.5, E10.0, and E10.5 (open arrowheads in G and H). The mTmG reporter reveals only a sparse sprinkling of floxed GFP-expressing cells at E10.75 (open arrowhead in I). The dorsal telencephalon displays extensive floxing and loss of Lhx2 expression only by E11.5 (open arrowheads in J). (Scale bars: 500 μm.)
Embryos from both Emx1CreKJ and Emx1CreYL crosses reveal intense Lhx2 exon 2/3 expression at E9.5. Emx1CreKJ;Lhx2 cKO embryos reveal a dramatic decline in Lhx2 exon 2/3 expression in the dorsal telencephalon from E10.0 onwards. Complete floxing of Lhx2 in the dorsal telencephalon is seen by E10.5–E10.75, corroborated by the mTmG reporter line which displays strong GFP expression in the dorsal telencephalon (Fig. 1D).
In contrast, Emx1CreYL;Lhx2 cKO embryos reveal strong expression of Lhx2 exon 2/3 in the dorsal telencephalon up to E10.5. The mTmG reporter line shows only minimal GFP expression at 10.75. It is at E11.5 that the dorsal telencephalon displays floxing of Lhx2 exon 2/3. Therefore, there is at least a 1-d difference in the timing of action of the two Emx1Cre lines, with the Emx1CreKJ line acting earlier than the Emx1CreYL line (Fig. 1). Control embryos display intense expression of Lhx2 in the dorsal telencephalon from E9.5 to E12.5 (Fig. S1).
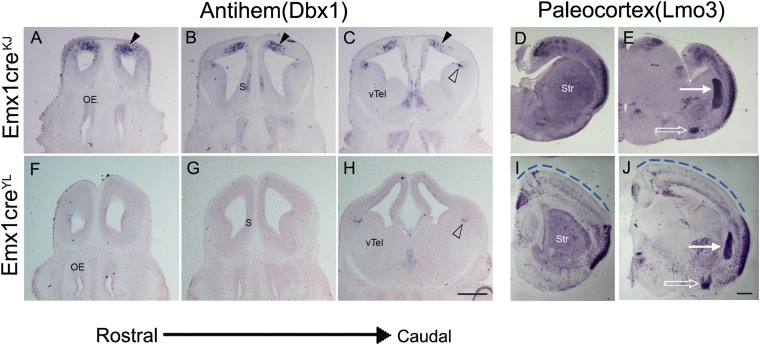
This difference in timing has important consequences for the Lhx2 cKO phenotype, because there are distinct critical periods for different functions of Lhx2 in the cortical primordium: suppression of hem/antihem fate (up to E10.5) (9) and suppression of paleocortical fate (up to E11.5) (8). Therefore, the temporal difference in the activity of the two Emx1Cre lines would be expected to give different Lhx2 cKO phenotypes. We tested this hypothesis by examining Emx1CreKJ;Lhx2 cKO and Emx1CreYL;Lhx2 cKO embryos (Fig. 2) and littermate controls (Fig. S2) for the expression of antihem marker developing brain homeobox protein 1 (Dbx1) at E12.5 and the paleocortex marker LIM domain only 3 (Lmo3) at P0. Emx1CreKJ;Lhx2 cKO embryos display ectopic antihem dorsally in locations that normally would correspond to the neocortical primordium. In contrast, Emx1CreYL;Lhx2 cKO embryos do not display ectopic antihem (Fig. 2 F–H). There is no specific marker at E12.5 for the neuroepithelial domain that will give rise to the paleocortex, but we examined the postmitotic paleocortex using Lmo3 as a marker (14). In Emx1CreKJ;Lhx2 cKO mice, as previously described, the paleocortex appears ectopically, and the neocortex is greatly shrunken (Fig. 2 D and E) (8). In contrast, in Emx1CreYL;Lhx2 cKO brains, an entire stretch of neocortex is present (blue dashed line, Fig. 2 I and J), and there is no ectopic paleocortex. In summary, the Emx1CreYL line offers a unique tool to examine the effects of cortex-specific deletion of Lhx2 after the critical periods for hem, antihem, and paleocortical fate restriction are past.
Fig. 2.
Ectopic antihem and paleocortex appear when Lhx2 is deleted using Emx1CreKJ but not Emx1CreYL. (A–E) Emx1CreKJ;Lhx2 cKO brains reveal ectopic antihem in the dorsal telencephalon at E12.5 (arrowheads in A–C) and ectopic paleocortex instead of neocortex at P0 (D and E). (F–J) Emx1CreYL;Lhx2 cKO brains do not reveal ectopic antihem (F–H), and the neocortex is spared (dashed line in I and J). Open arrowheads in C and H identify the normal antihem. In E and J, the white arrow indicates the basolateral amygdaloid complex, and the white open arrow indicates the nucleus of the lateral olfactory tract, layer 2/3. (Scale bars: 500 μm.) OE, olfactory epithelium; S, septum; Str, striatum; vTel, ventral telencephalon.
We examined Emx1CreYL;Lhx2 cKO brains at P5–P10 for two major features of neocortical development, regional patterning of the cortex into distinct areas and the production of layer-specific neuronal fates. A unique feature of cortical area patterning is the barrel cortex, consisting of an array of barrels that receive sensory input from the whiskers. These barrels consist of neuropil formed by the terminal arbors of thalamocortical afferents (the “barrel core”) synapsing onto the dendrites of spiny stellate neurons in layer 4 that form the cellular “wall” of each barrel. Barrels appear in early postnatal life, with each barrel representing a unique one-to-one association between input from each whisker on the contralateral snout of the animal and the corresponding barrel (15).
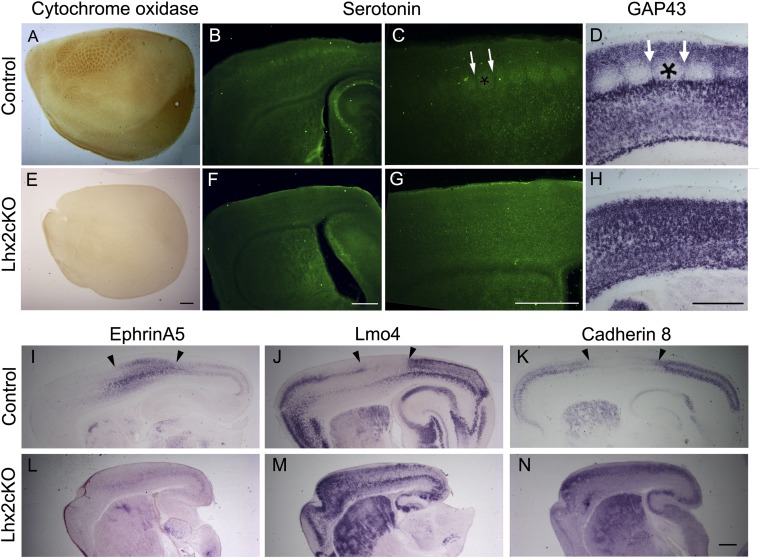
Cytochrome oxidase staining in tangential sections of control cortices at P7 reveals the barrels in the somatosensory cortical area (Fig. 3A). These patches, corresponding to the barrel cores, appear to be completely missing in Lhx2 cKO brains (Fig. 3E). Each barrel core also contains high levels of serotonin seen in control brains (Fig. 3 B and C) but missing in the Lhx2 cKO brains (Fig. 3 F and G). In control brains, growth-associated protein 43 (GAP43) expression is specifically excluded from the barrel core and is expressed in the cellular septae in between them, but no such segregation is seen in Lhx2 cKO brains (Fig. 3 D and H). Molecular markers of cortical patterning also reveal apparently disrupted patterning, with the somatosensory cortex marker EphrinA5 being nearly undetectable in the Lhx2 cKO brains (Fig. 3L). LIM domain only 4 (Lmo4) and Cadherin8, markers that in controls delineate the somatosensory cortex by a sharp boundary and a gap in expression (Fig. 3 J and K), display no such gap in the Lhx2 cKO brain (Fig. 3 M and N).
Fig. 3.
The somatosensory cortical barrels are missing upon loss of cortical Lhx2 function. Emx1CreYL;Lhx2 cKO and littermate controls were examined at P5–P7. (A–D) Control brains display characteristic cytochrome oxidase staining in tangential sections (A) and serotonin immunostaining in sagittal sections (B and C) in the barrel cores (asterisk in C). In situ hybridization for GAP43 (D) identifies cortical neurons that form the cellular barrel walls (arrows in D) and are excluded from the cell-poor barrel core (asterisks in C and D). (E–H) In Lhx2 cKO brains, neither cytochrome oxidase histochemistry nor serotonin immunostaining reveals detectable barrels, and GAP43 expression shows cortical neurons uniformly distributed with no sparing of barrel cores. (I–K) In sagittal sections of control brains, the somatosensory cortex is marked by expression of EphrinA5 and also is delineated by a gap in the expression of Lmo4 and Cadherin8 (the region between the arrowheads in J and K). (L–N) Lhx2 cKO sections reveal disrupted molecular regionalization with loss of EphrinA5 from the superficial layers and reduced expression of EphrinA5 in the deep layers and no apparent boundaries in Lmo4 and Cadherin8 expression. A is a montage of three tangential section images that have been assembled to display the barrel cortex. (Scale bars: 500 μm.)
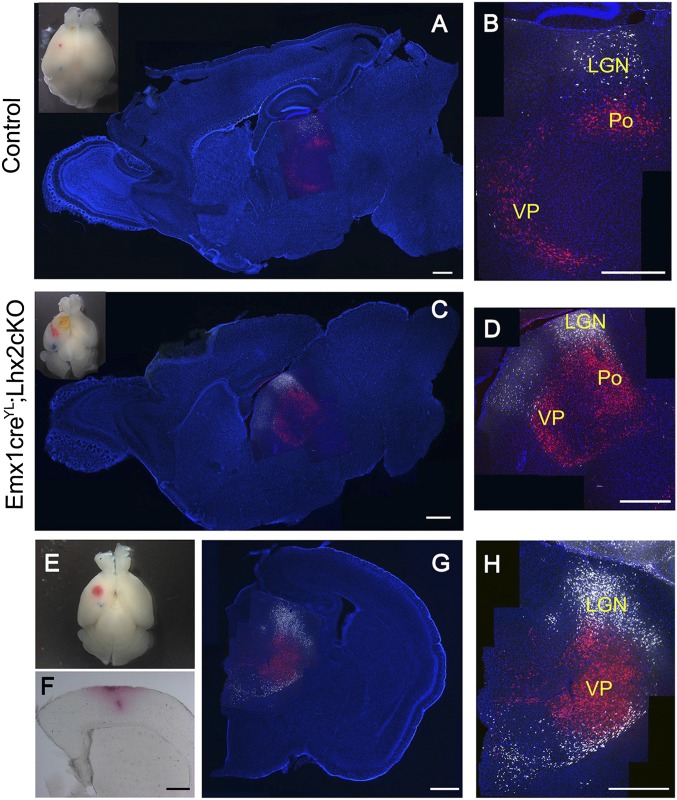
Because barrel formation is an interactive process between thalamocortical afferents and cortical neurons, we examined projections between the ventrobasal nucleus of the thalamus and the presumptive somatosensory area. We injected 0.5% DiI and DiD in the somatosensory and visual cortex, respectively, of live control pups at postnatal day (P) 9–P12, when barrel formation is complete, and made similar placements in Emx1CreYL;Lhx2 cKO pups. After 3 d of active transport, the brains were sectioned coronally or sagittally. A total of six controls and five Lhx2cKO animals were examined. In control brains, the DiI label was detected broadly in both parts of the somatosensory nuclei, the ventroposterior and posterior nuclei. The DiD label was seen in the lateral geniculate nucleus (Fig. 4). Surprisingly, a very similar pattern was seen in all 5 Lhx2 cKO brains. Thus, despite the disruption of molecular patterning of the cortex and the loss of the barrels, the projections between the thalamus and cortex maintained their area-specific patterns (Fig. 4).
Fig. 4.
Area-specific projections are formed between the thalamus and the Lhx2 cKO cortex. (A–H) DiI and DiD injections were made in discrete locations in the cortex of P9–P12 Emx1CreYL;Lhx2 cKO pups and littermate controls under anesthesia, and the brains were harvested after 3 d. Whole-brain images (E and Insets in A and C) indicate the injection sites. Sagittal (A–D) and coronal (G and H) sections were counterstained with DAPI. Confocal images of the thalamus reveals DiI (red label) in the ventroposterior nucleus (VP) and posterior (Po) nucleus and DiD (white label) in the lateral geniculate nucleus (LGN) of both control (A and B) and mutant (C, D, G, and H) brains. (F) A bright-field image of a section of the Lhx2 cKO brain in E and G, revealing the injection site of DiI in cortex. A–D, G, and H are montages of multiple images that have been assembled to display the entire brain section in A, C, and G (low-magnification epifluorescence images) and the entire labeled region in B, D, and H (high-magnification confocal images). In each low-magnification image (A, C, and G), the corresponding high-magnification confocal image (B, D, and H, respectively) is overlaid in the appropriate location of the thalamus to indicate the region in which the label was detected. (Scale bars: 500 μm.)
This finding prompted us to examine whether the thalamocortical arbors made functional synaptic contacts onto the cortical neurons. We performed extracellular, multineuron recordings in adult animals under urethane anesthesia to determine spontaneous and stimulus-driven activity from the region that was shown to be innervated by the neurons from the somatosensory thalamic nuclei. Three Emx1CreYL;Lhx2 cKO and three control animals were examined. Penetrations were made in the barrel column of controls and compared with recordings from Lhx2 cKO animals at comparable depths. Spontaneous discharges of neurons in the Lhx2 cKO cortex displayed high burst rates, with large amplitude spike, whereas controls displayed a more uniform rate of spontaneous activity (Fig. S3).
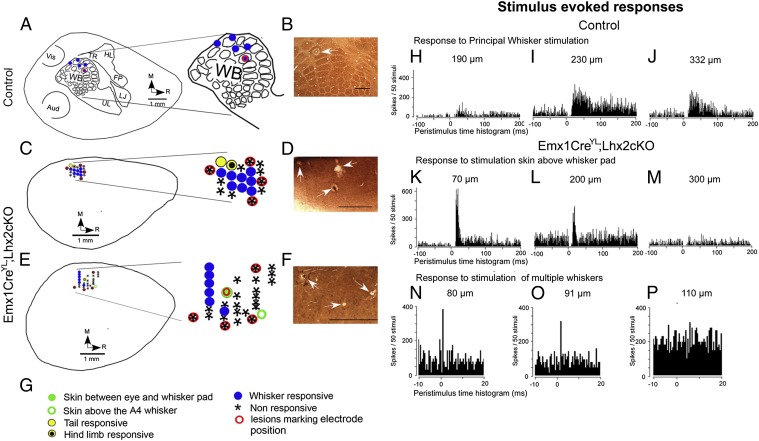
We mapped the receptive fields of neurons at various depths in multiple radial penetrations in control brains, in the area ∼1.5 mm posterior and ∼3 mm lateral to bregma, corresponding to area S1. The schema in Fig. 5A shows the somatotopic map in control animals that has been well described in the literature (16). Stimulation of individual whiskers in control mice generated responses that mapped to the whisker barrels (blue circles in Fig. 5A). In contrast, no responses to stimulation of individual whiskers were obtained in the Lhx2 cKO brain. Responses were obtained only when all the large whiskers on the contralateral whisker pad were stimulated together (blue circles in Fig. 5 C, E, and N–P). Furthermore, these responses were restricted to a small region of the Lhx2 cKO cortex. All three Emx1CreYL;Lhx2 animals from the laboratory of Yuqing Li gave similar results. In addition, we found robust responses to stimulation of different parts of the animals’ body, such as parts of the face, tail, and hind limb (Fig. 5 C and E). These responses were in appropriate somatotopic locations with respect to the whisker-responsive region.
Fig. 5.
Aberrant evoked responses and a rudimentary functional map in the adult Lhx2 cKO cortex. (A, C, and E) Schematic representations of a flattened control cortex (A) and Lhx2 cKO cortices from two different animals (C and E). The lesion sites with reference to which the recording sites were localized are marked. The key for the symbols in A, C, and E is shown in G. (B, D, and F) Cytochrome oxidase staining in tangential sections of the same cortices, showing the lesion sites (white arrows). (H–J) Evoked responses to principal whisker stimulation in control brains at different depths of penetration. (Scale bars: 500 μm.) (K–P) Electrophysiological recordings in adult control and Emx1CreYL;Lhx2 cKO mice. (K–M) Evoked responses to stimulation of skin above the whisker pad. (N–P) Stimulation of multiple whiskers simultaneously in Lhx2 cKO animals at different depths of penetration. Note the peristimulus time histogram reveals responses at short latencies (<10 ms) after stimulus onset in control and mutants, indicative of direct input from the thalamus. Aud, auditory; FP, forepaw; HL, hindlimb; LJ, lower jaw; TR, trunk; UL, upper lip; Vis, visual; WB, whisker barrels.
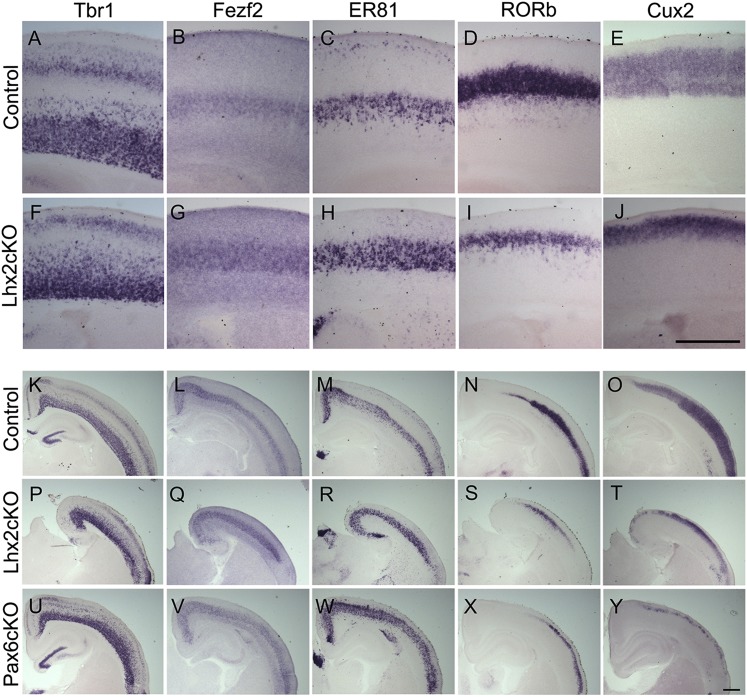
In control brains, stimulus-evoked responses were obtained at expected depths of penetration, consistent with the location of layer 4 (Fig. 5 H–J). A surprising feature of evoked responses in Lhx2 cKO brains was that activity usually was seen in very superficial levels of penetration (Fig. 5 K, L, N, and O). The short latencies of these responses (<10 ms after stimulus onset) are indicative of functional thalamocortical inputs to the mutant cortex, seen at levels of penetration more superficial than the expected depth for layer 4. To understand the nature of this defect, we first ascertained whether layer 4 neurons and other cortical laminar-specific fates are specified and normally positioned in the Emx1CreYL;Lhx2 cKO brain. All layer-specific markers were seen in the appropriate order, with deep-layer markers T-box brain 1 (Tbr1), forebrain embryonic zinc finger protein 2 (Fezf2), and Ets-related protein 81 (ER81) displaying comparable expression in the mutant brains (Fig. 6). The expression of the layer 4 marker RAR-related orphan receptor B (RORb) and the layer 2/3 marker cut-like homeobox 2 (Cux2) reveals that, although these molecular identities are indeed specified, these layers are thinner in the absence of Lhx2 (Fig. 6), as is consistent with a recent report (17). Therefore it is reasonable that layer 4 neurons reside more superficially in the Lhx2 cKO brain than in the control brain, and this more superficial location could explain the functional responses seen in the mutant. These deficiencies in the thickness of the superficial layers are reminiscent of the phenotype reported for the loss of Pax6 (18). We examined Pax6 cKO brains using the same Emx1CreYL driver. The laminar expression of Tbr1, Fezf2, ER81, RORb, and Cux2 in Emx1CreYL;Pax6 cKO brains is strikingly similar to the Emx1CreYL;Lhx2 cKO phenotype (Fig. 6).
Fig. 6.
Loss of Lhx2 produces cortical lamination phenotypes similar those seen with loss of Pax6. P7 brains were examined with a panel of layer-specific markers. Control (A–E) and Emx1CreYL;Lhx2 cKO (F–J) sections reveal layer-specific markers expressed in appropriate relative positions, but the superficial layers in the Lhx2 cKO cortex are significantly reduced. (K–Y) The same panel of markers was used to compare control sections (K–O) with Emx1CreYL;Lhx2 cKO (P–T) and Emx1CreYL;Pax6 cKO (U–Y) sections. Lhx2 cKO and Pax6 cKO brains display a similar thinning of the Cux2- and RORb-expressing superficial layers. Deep layers, marked by Tbr1, Fezf2, and ER81, appear similar to controls. (Scale bars: 500 μm.)
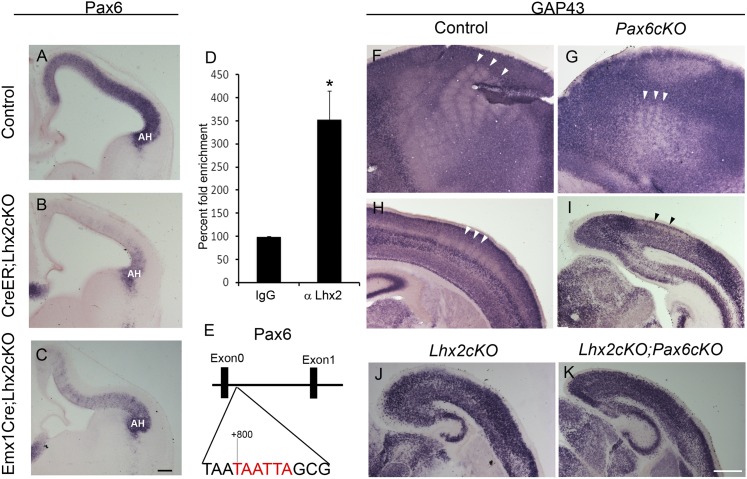
This finding motivated an examination of whether Lhx2 and Pax6 may interact in an epistatic relationship. We examined Lhx2 expression in Pax6-null mutant embryos (Pax6sey/sey) at E12.5 and found it to be comparable to that in control brains (Fig. S4). In contrast, Pax6 expression is dramatically reduced in the absence of Lhx2. Pax6 expression is depleted in much of the dorsal telencephalon in both CreER;Lhx2 cKO brains administered tamoxifen at E10.5 and Emx1CreYL;Lhx2 cKO brains. Only the extreme lateral antihem region is spared and continues to express high levels of Pax6 even though Lhx2 has been floxed in this region (Fig. 7B). These data indicate an interaction between Lhx2 and Pax6 in the cortical primordium, with Lhx2 acting upstream of Pax6. To test whether this interaction may be direct, we performed ChIP from E12.5 cortex tissue. We focused on a conserved Lhx2-binding site TAATTA within the Etel region, a well-characterized telencephalon-specific enhancer of Pax6 transcription located between exon 0 and exon 1 of the Pax6 gene (19). Lhx2 binding to this site has been demonstrated in human embryonic stem cells (20). We found a 3.5-fold enrichment of Lhx2 binding at this site in E12.5 cortex tissue (Fig. 7D, n = 4). These results, together with the strikingly similar reduced upper-layer phenotypes seen in Lhx2 cKO and Pax6 cKO animals, suggest that Lhx2 may act via Pax6 to regulate the production of cells in the superficial layers of the cortex.
Fig. 7.
Lhx2 regulates Pax6 in the dorsal telencephalon. Sections of control (A) and Lhx2 cKO (B and C) brains at E12.5. (A) In control brains, Pax6 is expressed in a medial (low) to lateral (high) gradient. (B and C) When Lhx2 is removed by tamoxifen administration to CreER;Lhx2 cKO animals at E10.5 (B) or by crossing to Emx1CreYL (C), much of the Pax6 expression in the dorsal telencephalon is lost or greatly reduced by E12.5, except in the antihem (AH) region at the lateral edge of the pallium which is maintained (B and C). (D) Lhx2 binding to its conserved site within the Etel enhancer region of Pax6 in E12.5 cortical tissue in vivo. ChIP using Lhx2 antiserum displays 3.5-fold enrichment over control IgG. Error bars represent the mean ± SEM. *P < 0.05. (E) A schematic representation of the Lhx2-binding site in the Etel enhancer. (F–K) GAP43 expression reveals barrel walls with unstained barrel cores (white arrowheads) in tangential (F) and coronal (H) sections of control P7 brains. In Emx1CreYL;Pax6 cKO mutant brains, a reduced barrel field is seen which displays GAP43 expression in barrel walls in tangential (G) and coronal (I) sections. In contrast, there is a complete absence of barrel-like cytoarchitecture in P7 Emx1CreYL;Lhx2 cKO (J) and double-mutant Emx1CreYL;Lhx2 cKO;Pax6 cKO (K) brains. (Scale bars: 100 μm in A–C and 500 μm in F–K.)
When Pax6 is conditionally deleted in the cortex, cytochrome oxidase patches are seen in the barrel cortex (21). We examined Emx1CreYL;Pax6 cKO brains for evidence of cellular barrel walls and found that GAP43 expression does indeed display a barrel-like expression pattern in an appropriate region of the Pax6 cKO cortex (Fig. 7 G and I), reduced in size as has been previously reported (21, 22). Because both barrel walls and barrel cores form in the absence of Pax6, it is unlikely that Pax6 is a major target of Lhx2 with respect to the regulation of barrel formation. We confirmed this notion by comparing GAP43 expression at P7 in Emx1CreYL-driven single- and double-cKO mutant brains. As predicted, the double Lhx2 cKO;Pax6 cKO phenotype (Fig. 7K) closely resembled that seen in Lhx2 cKO (Fig. 7J) but not in Pax6 cKO brains (Fig. 7 G and I).
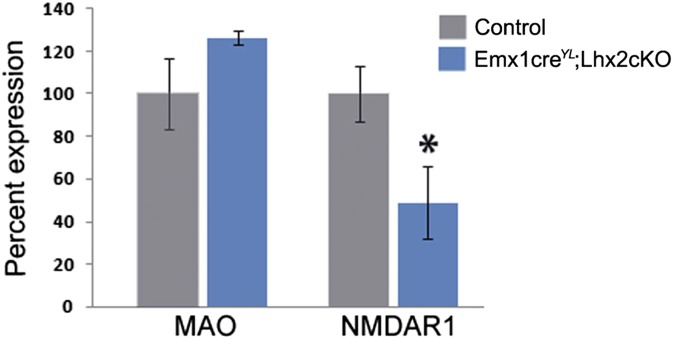
We examined mechanisms that are known to regulate synaptic maturation, NMDA receptor (NMDAR)- and serotonin-mediated signaling. Barrel formation is known to require functional NMDARs (23, 24). It also is sensitive to enhanced levels of serotonin (25), which may act via 5HT1b receptors expressed by thalamocortical afferents (25, 26). We examined NMDAR1 and monoamine oxidase A (MAOA) mRNA levels in the Emx1CreYL;Lhx2 cKO cortex at P3, when the barrels have not yet formed (Fig. 8). NMDAR1 mRNA levels are reduced to 48% of control levels, but the expression of MAO is unaffected, suggesting that Lhx2 may specifically control NMDA-dependent signaling mechanisms.
Fig. 8.
Lhx2 regulates the expression of synaptic plasticity molecules in the dorsal telencephalon. MAO and NMDAR1 levels were determined by quantitative real-time PCR analysis in tissue harvested at P3 (control, n = 3; Emx1CreYL;Lhx2 cKO, n = 4). Statistical analysis was performed using the Student t test. MAO levels were comparable in control and mutant cortices, whereas NMDAR1 levels in the mutant were 48% of the control levels. Error bars represent SEM. *P < 0.05.
Discussion
LIM homeodomain (LIM-HD) genes regulate key steps in the development of many systems, and the LIM-HD family members LIM homeobox transcription factor 1α (Lmx1a), Lhx2, and Lhx5 are known to be critical for the development of different components of the dorsal telencephalon. Broadly, these genes have roles in early development, such as the specification of a particular cell fate, with parallels across vertebrate and invertebrate species: apterous is a dorsal selector gene in the Drosophila wing disk (27); in a parallel role, Lhx2 acts as a cortical selector in the mammalian telencephalon (9); Lmx1a regulates the development of the cortical hem (28); Lhx5 is required for the development of the hippocampus (29); and Lmx1b has a parallel role in the development of the isthmic organizer (30). Lhx6, Lhx7, and islet 1 (Isl1) are necessary for the proper specification of striatal interneurons (31, 32); mec3 is required for the specification of touch receptor neurons in Caenorhabditis elegans (33). A complex code of Isl1, Isl2, and Lhx3 controls motor neuron subtype identity in the vertebrate spinal cord (34). A second set of roles identified for LIM-HD transcription factors involve axon guidance: Lhx2 itself in thalamocortical pathfinding (35, 36) and Apterous and Isl in axon guidance of Drosophila ventral nerve cord interneurons (37, 38).
A notable feature of this family is that its members subserve multiple roles in different systems, and in some cases the same gene plays distinct roles at different times in the development of a particular system. For example, Isl1 is necessary first for specification of motoneurons (39) and then participates in a combinatorial code to specify the identity of particular motoneuronal subtypes within this pool (40). The diverse roles of Lhx2 are striking in this regard: It is required not only for erythropoiesis (41) but also for multiple stages of optic development (42). In the dorsal telencephalon, there are distinct critical periods for different functions of Lhx2. Before E10.5, Lhx2 suppresses alternative fates corresponding to the hem and antihem in the cortical primordium (7, 9), and up to E11.5 it prevents the neocortex from being transformed into paleocortex (8). Later, during the period of neurogenesis in the hippocampal primordium, it acts in the ventricular-zone progenitors to suppress astrogliogenesis (43). In the neocortical primordium, it maintains ventricular-zone progenitors in a proliferative state (17). Lhx2 also regulates thalamocortical pathfinding (35, 36), which is an important regulator of cortical arealization (44), thereby making it difficult to examine whether Lhx2 has a role in the development of area-specific features in the cortex independent of its role in the thalamus. We have uncovered a novel function of Lhx2 using a cKO strategy combined with a spatio-temporally controlled Cre line that acts in the dorsal telencephalon from E11.5 (Emx1CreYL). Our results highlight the Emx1CreYL line as a valuable tool that permits fine temporal dissection of gene function in cortical development.
Graded Transcription Factors in Cortical Arealization.
Transcription factors expressed in gradients in the cortical primordium are well positioned to regulate the patterning of the cortex into distinct areas subserving different functions. Pax6, Emx2, Sp8, and NR2f1 each are required to position these areas properly; in the absence of any, the area map is shifted (1–6). Lhx2, which also is expressed in a gradient, thus far has not been examined for this role. In Emx1CreYL;Lhx2 cKO animals, the expression of molecular markers of arealization is disrupted profoundly in a manner that is not easy to interpret, except that the molecular characteristics of the somatosensory area appear to be missing. Nonetheless, it is clear that thalamocortical/corticothalamic axons are able to interpret broad areal identities in appropriate positions relative to each other in the Lhx2 cKO cortex. Furthermore, despite gross aberrations in the expression of molecular markers that call into question the molecular identity of the presumptive somatosensory region, stimulation of different body parts is able to drive neurons in the Lhx2 cKO cortex. A significant feature of these evoked responses is that they display a topography that roughly parallels that in controls. Responses to stimulation of the tail and hind limb are seen in sites medial to the whisker-responsive region in the Lhx2 cKO, similar to the representation of the trunk and hindlimb in controls. Likewise, stimulation of parts of the face evoked responses in sites rostral to the whisker-responsive area in Lhx2 cKO animals, similar to the location of “upper lip” responses in control animals. It is the whisker responses themselves that are profoundly disrupted in the Lhx2 cKO animals, in that stimulation of individual whiskers failed to evoke any detectable response. However, responses were obtained to stimulation of multiple whiskers, supporting the interpretation that this site is indeed the presumptive barrel field of the Lhx2 cKO cortex, in which the barrels have failed to form. In summary, the evidence indicates that in cortical neurons Lhx2 is necessary for the circuitry that enables the formation of whisker-specific barrels rather than for the specification of the somatosensory area itself.
Transcription Factors in Cortical Lamination.
Several transcription factors play key roles in the specification of particular layer-specific neuronal identities. In Emx1CreYL;Lhx2 cKO animals, all cortical layers appear to be specified. However, superficial layer neurons are reduced in number upon cortex-specific loss of Lhx2, apparently because the early exit of ventricular-zone progenitors from the cell cycle depletes the progenitor pool (17). Recently, it has been shown that Lhx2 regulates neuronal differentiation in human embryonic stem cells by promoting the expression of Pax6 as well as Cerberus1, an antagonist of Wnt and bone morphogenic protein signaling (20). We find Pax6 expression in the cortical primordium to be critically dependent on Lhx2. Together with the similar reduction of superficial layers seen in the Emx1CreYL;Lhx2 cKO and the Emx1CreYL;Pax6 cKO phenotypes, this finding suggests that Lhx2 may act via Pax6 for the control of neurogenesis in the cortical ventricular zone.
One explanation for the lack of cortical barrels in the Lhx2 cKO brain might be that barrel formation requires a minimum number of layer 4 cortical neurons. Comparison with the Pax6 cKO brain is useful in this regard. Upon cortex-specific loss of Pax6, a reduction in superficial layer neurons, similar to the defect in the Lhx2 cKO brain (17), is seen because of the premature exit of ventricular zone progenitors from the cell cycle (18). However, whisker-specific cytochrome oxidase-expressing barrel cores (21, 22) as well as cellular barrel walls (this study) do form in the absence of Pax6. Therefore, a decrease in cortical neuronal number may not by itself explain the loss of barrel formation in the Lhx2 cKO. Furthermore, because barrels do form despite the loss of Pax6, this transcription factor may not be the critical mediator for the function of Lhx2 in regulating barrel formation.
The Regulation of Somatosensory Barrel Formation.
The barrel field is an organizational hallmark of the rodent cortex. Layer 4 spiny stellate neurons form the cellular wall of each barrel and extend dendrites in a polarized manner into the barrel core, which is innervated by whisker-specific, clustered thalamocortical afferents. Thalamocortical axons are thought to presegregate into barrel-specific clusters just as they enter the cortex (45). This segregation can be independent of cellular barrel-wall formation. For example, barrel formation critically requires NMDA-mediated signaling, so that neither cellular barrel walls nor thalamocortical afferent clusters are seen in NMDAR-null mutants (24). However, cortex-specific NMDAR1 cKO mutants (Emx1Cre;NMDAR1 cKO) display cytochrome oxidase patches corresponding to the large whiskers but no cellular barrel walls (23). This observation indicates that NMDAR1 function in the cortex is critical for the formation of the barrel walls but not for the segregation of whisker-specific thalamocortical afferents.
Our results show that Lhx2 is required for normal levels of NMDAR expression in the cortex. However, in Emx1CreYL;Lhx2 cKO animals, cortex-specific deletion of Lhx2 causes the loss of both cellular barrel walls and cytochrome oxidase-positive patches, suggesting that NMDA-regulated mechanisms may mediate Lhx2 function only partially in barrel formation. What mechanisms might mediate the role of Lhx2 in the clustering of thalamocortical axons? The expression of EphrinA5, an axon-guidance molecule specifically expressed in the somatosensory cortex, is greatly reduced upon loss of Lhx2. In particular, expression of EphrinA5 appears to be lost almost completely in the superficial layer. EphrinA5 has been shown to regulate thalamocortical axon branching in cortical slices (46), and this mechanism may contribute to the loss of cytochrome oxidase-positive patches in the Lhx2 cKO.
Barrel formation is dependent on many synaptic proteins and activity-regulated molecules such as the receptor mGlur5, adenylate cyclase1, phospholipase C β1, synaptic Ras GTPase activating protein 1, and Rab3-interacting molecule 1 and 2 (47, 48). Cortex-specific knockouts of particular transcription factors such as CCCTC-binding factor (CCTF), neurogenic differentiation 2 (NeuroD2), and DNA methyltransferase 1 (Dnmt1) (49–51) also display impaired or deficient barrel formation. In particular, CCTF regulates several members of the protocadherin (Pcdh) cluster, many of which have been implicated in the control of dendritic morphogenesis and synapse formation that may be critical to barrel formation (49). A link between Lhx2 and Pcdh10b has been discovered in the zebrafish diencephalon, where Lhx2 and Lhx9 suppress Wnt signaling and the expression of Pcdh10b is critical for patterning and boundary formation (52). These findings motivate further studies aimed at examining whether Lhx2 interacts with CCTF or members of the protocadherin cluster to regulate cortical barrel formation.
In summary, we report a cortex-specific role for Lhx2 in the formation of area-specific neurocircuitry specializations, of which the somatosensory barrels may be the most prominent example in rodents. Multiple direct or indirect downstream targets may mediate this function of Lhx2, such as Pax6 (this study), the Notch signaling pathway (17, 43), axon guidance molecules such as Robo1 (36) and Ephrin A5 (this study), and the receptor NMDAR1 (this study), a major regulator of synaptic plasticity. This work extends the known functions of Lhx2 in fundamental stages of corticogenesis, positioning it as a master regulator of forebrain development.
Materials and Methods
Mice.
The different mice mutant strains along with their sources are detailed in SI Materials and Methods.
Histochemistry.
In situ hybridization was performed as described in ref. 7. Cytochrome oxidase histochemistry was done as previously described (53).The sources, concentrations, and protocols for the antibodies used in this study (rabbit anti-serotonin, biotinylated goat anti-GFP, and goat anti Lhx2) are detailed SI Materials and Methods.
Imaging.
The different epifluorescence and confocal microscopes and the image-analysis procedures used are described in SI Materials and Methods.
Lipophilic Dye Labeling.
Dye labeling was performed via injections of lipophilic carbocyanine dye in the cortex and is detailed in SI Materials and Methods.
Electrophysiology.
Multineuronal activity from adult control and Emx1CreYL;Lhx2 cKO mice was recorded using standard protocols (54, 55) as detailed in SI Materials and Methods.
ChIP.
Mouse E12.5 cortical tissue was used for ChIP using Lhx2 antibody as detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank B. Anderson (Lmo4), A. Pierani (Dbx1), E. Grove (Cadherin 8), R. Hevner (Tbr1), J. Macklis (Fezf2), R. Neve (GAP 43), S. McConnell (ER81), T. Rabbitts (Lmo3), C. Ragsdale (RORb), E. Monuki (Cux2), and G. Saunders (Pax6) for gifts of plasmid DNA; Elizabeth Grove, Pushkar Joshi, Lakshmi Subramanian, and Vidita Vaidya for suggestions and input; Raghu Ram Katreddi and Kuldeep Tripathi for help with histology; Vidita Vaidya and Ankit Sood for help with dye tracing; Rachel Cinco for assistance with harvests; Shital Suryavanshi and the animal house staff of the Tata Institute for Fundamental Research (TIFR) for excellent support; and Sanjeev Galande for guidance with the ChIP experiments performed at the Center for Excellence in Epigenetics facility at the Indian Institute of Science Education and Research-Pune. This work was supported by intramural funds from TIFR (to S.T.), a Sarojini Damodaran travel award (TIFR Endowment Fund) (to A.S.S.), and Wellcome Trust-Department of Biotechnology India Alliance Early Career Fellowships (to B.M. and G.G.). S.T. is a recipient of the Shanti Swarup Bhatnagar award (Council of Scientific and Industrial Research, Government of India).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311158110/-/DCSupplemental.
References
- 1.Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288(5464):344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- 2.Sahara S, Kawakami Y, Izpisua Belmonte JC, O’Leary DD. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zembrzycki A, Griesel G, Stoykova A, Mansouri A. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armentano M, et al. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10(10):1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- 5.Faedo A, et al. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-catenin signaling. Cereb Cortex. 2008;18(9):2117–2131. doi: 10.1093/cercor/bhm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamasaki T, Leingärtner A, Ringstedt T, O’Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43(3):359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Bulchand S, Grove EA, Porter FD, Tole S. LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech Dev. 2001;100(2):165–175. doi: 10.1016/s0925-4773(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 8.Chou SJ, Perez-Garcia CG, Kroll TT, O’Leary DD. Lhx2 specifies regional fate in Emx1 lineage of telencephalic progenitors generating cerebral cortex. Nat Neurosci. 2009;12(11):1381–1389. doi: 10.1038/nn.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangale VS, et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319(5861):304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32(4):591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 11.Gorski JA, et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22(15):6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23(1):99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 13.Jin XL, et al. Emx1-specific expression of foreign genes using “knock-in” approach. Biochem Biophys Res Commun. 2000;270(3):978–982. doi: 10.1006/bbrc.2000.2532. [DOI] [PubMed] [Google Scholar]
- 14.Bulchand S, Subramanian L, Tole S. Dynamic spatiotemporal expression of LIM genes and cofactors in the embryonic and postnatal cerebral cortex. Dev Dyn. 2003;226(3):460–469. doi: 10.1002/dvdy.10235. [DOI] [PubMed] [Google Scholar]
- 15.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 16.Chapin JK, Lin CS. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol. 1984;229(2):199–213. doi: 10.1002/cne.902290206. [DOI] [PubMed] [Google Scholar]
- 17.Chou SJ, O’Leary DD. Role for Lhx2 in corticogenesis through regulation of progenitor differentiation. Mol Cell Neurosci. 2013;56:1–9. doi: 10.1016/j.mcn.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuoc TC, et al. Selective cortical layering abnormalities and behavioral deficits in cortex-specific Pax6 knock-out mice. J Neurosci. 2009;29(26):8335–8349. doi: 10.1523/JNEUROSCI.5669-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kammandel B, et al. Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev Biol. 1999;205(1):79–97. doi: 10.1006/dbio.1998.9128. [DOI] [PubMed] [Google Scholar]
- 20.Hou PS, et al. LHX2 regulates the neural differentiation of human embryonic stem cells via transcriptional modulation of PAX6 and CER1. Nucleic Acids Res. 2013;41(16):7753–7770. doi: 10.1093/nar/gkt567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piñon MC, Tuoc TC, Ashery-Padan R, Molnár Z, Stoykova A. Altered molecular regionalization and normal thalamocortical connections in cortex-specific Pax6 knock-out mice. J Neurosci. 2008;28(35):8724–8734. doi: 10.1523/JNEUROSCI.2565-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zembrzycki A, Chou SJ, Ashery-Padan R, Stoykova A, O’Leary DD. Sensory cortex limits cortical maps and drives top-down plasticity in thalamocortical circuits. Nat Neurosci. 2013;16(8):1060–1067. doi: 10.1038/nn.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasato T, et al. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406(6797):726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasato T, et al. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19(6):1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 25.Cases O, et al. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: Role of a serotonin excess during the critical period. Neuron. 1996;16(2):297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 26.Salichon N, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21(3):884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Benjumea FJ, Cohen SM. Interaction between dorsal and ventral cells in the imaginal disc directs wing development in Drosophila. Cell. 1993;75(4):741–752. doi: 10.1016/0092-8674(93)90494-b. [DOI] [PubMed] [Google Scholar]
- 28.Chizhikov VV, et al. Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc Natl Acad Sci USA. 2010;107(23):10725–10730. doi: 10.1073/pnas.0910786107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, et al. Control of hippocampal morphogenesis and neuronal differentiation by the LIM homeobox gene Lhx5. Science. 1999;284(5417):1155–1158. doi: 10.1126/science.284.5417.1155. [DOI] [PubMed] [Google Scholar]
- 30.Adams KA, Maida JM, Golden JA, Riddle RD. The transcription factor Lmx1b maintains Wnt1 expression within the isthmic organizer. Development. 2000;127(9):1857–1867. doi: 10.1242/dev.127.9.1857. [DOI] [PubMed] [Google Scholar]
- 31.Fragkouli A, van Wijk NV, Lopes R, Kessaris N, Pachnis V. LIM homeodomain transcription factor-dependent specification of bipotential MGE progenitors into cholinergic and GABAergic striatal interneurons. Development. 2009;136(22):3841–3851. doi: 10.1242/dev.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20(16):6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Way JC, Chalfie M. mec-3, a homeobox-containing gene that specifies differentiation of the touch receptor neurons in C. elegans. Cell. 1988;54(1):5–16. doi: 10.1016/0092-8674(88)90174-2. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchida T, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79(6):957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 35.Lakhina V, Falnikar A, Bhatnagar L, Tole S. Early thalamocortical tract guidance and topographic sorting of thalamic projections requires LIM-homeodomain gene Lhx2. Dev Biol. 2007;306(2):703–713. doi: 10.1016/j.ydbio.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Marcos-Mondéjar P, et al. The lhx2 transcription factor controls thalamocortical axonal guidance by specific regulation of robo1 and robo2 receptors. J Neurosci. 2012;32(13):4372–4385. doi: 10.1523/JNEUROSCI.5851-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundgren SE, Callahan CA, Thor S, Thomas JB. Control of neuronal pathway selection by the Drosophila LIM homeodomain gene apterous. Development. 1995;121(6):1769–1773. doi: 10.1242/dev.121.6.1769. [DOI] [PubMed] [Google Scholar]
- 38.Thor S, Thomas JB. The Drosophila islet gene governs axon pathfinding and neurotransmitter identity. Neuron. 1997;18(3):397–409. doi: 10.1016/s0896-6273(00)81241-6. [DOI] [PubMed] [Google Scholar]
- 39.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84(2):309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 40.Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110(2):237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 41.Porter FD, et al. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development. 1997;124(15):2935–2944. doi: 10.1242/dev.124.15.2935. [DOI] [PubMed] [Google Scholar]
- 42.Roy A, et al. LHX2 is necessary for the maintenance of optic identity and for the progression of optic morphogenesis. J Neurosci. 2013;33(16):6877–6884. doi: 10.1523/JNEUROSCI.4216-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian L, et al. Transcription factor Lhx2 is necessary and sufficient to suppress astrogliogenesis and promote neurogenesis in the developing hippocampus. Proc Natl Acad Sci USA. 2011;108(27):E265–E274. doi: 10.1073/pnas.1101109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vue TY, et al. Thalamic control of neocortical area formation in mice. J Neurosci. 2013;33(19):8442–8453. doi: 10.1523/JNEUROSCI.5786-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agmon A, Yang LT, Jones EG, O’Dowd DK. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15(1 Pt 2):549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann F, Peuckert C, Dehner F, Zhou R, Bolz J. Ephrins regulate the formation of terminal axonal arbors during the development of thalamocortical projections. Development. 2002;129(16):3945–3955. doi: 10.1242/dev.129.16.3945. [DOI] [PubMed] [Google Scholar]
- 47.Narboux-Nême N, et al. Neurotransmitter release at the thalamocortical synapse instructs barrel formation but not axon patterning in the somatosensory cortex. J Neurosci. 2012;32(18):6183–6196. doi: 10.1523/JNEUROSCI.0343-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu CS, Ballester Rosado CJ, Lu HC. What can we get from ‘barrels’: The rodent barrel cortex as a model for studying the establishment of neural circuits. Eur J Neurosci. 2011;34(10):1663–1676. doi: 10.1111/j.1460-9568.2011.07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirayama T, Tarusawa E, Yoshimura Y, Galjart N, Yagi T. CTCF is required for neural development and stochastic expression of clustered Pcdh genes in neurons. Cell Rep. 2012;2(2):345–357. doi: 10.1016/j.celrep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Ince-Dunn G, et al. Regulation of thalamocortical patterning and synaptic maturation by NeuroD2. Neuron. 2006;49(5):683–695. doi: 10.1016/j.neuron.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 51.Golshani P, Hutnick L, Schweizer F, Fan G. Conditional Dnmt1 deletion in dorsal forebrain disrupts development of somatosensory barrel cortex and thalamocortical long-term potentiation. Thalamus Relat Syst. 2005;3(3):227–233. doi: 10.1017/S1472928807000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peukert D, Weber S, Lumsden A, Scholpp S. Lhx2 and Lhx9 determine neuronal differentiation and compartition in the caudal forebrain by regulating Wnt signaling. PLoS Biol. 2011;9(12):e1001218. doi: 10.1371/journal.pbio.1001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- 54.Welker E, Armstrong-James M, Van der Loos H, Kraftsik R. The mode of activation of a barrel column: Response properties of single units in the somatosensory cortex of the mouse upon whisker deflection. Eur J Neurosci. 1993;5(6):691–712. doi: 10.1111/j.1460-9568.1993.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Seif I, Armstrong-James M. Differences in somatosensory processing in S1 barrel cortex between normal and monoamine oxidase A knockout (Tg8) adult mice. Cereb Cortex. 2001;11(1):26–36. doi: 10.1093/cercor/11.1.26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.