Significance
Chickens have been an important animal model in the fields of developmental biology and immunology over the last century and have contributed a number of basic findings in these areas. However, their use has been limited more recently because there has been no way to genetically engineer specific mutations in the chicken. In addition to basic research applications, genetic modification in birds could also be used to mitigate the threat of avian influenza by production of flu-resistant bird stocks in agriculture. Here we describe an efficient way to target genes of interest in the chicken genome and create knockout chickens. We targeted the immunoglobulin heavy chain gene, leading to loss of antibody production and a block in B-cell development.
Keywords: B-cell development, avian immunology, genome editing
Abstract
Gene targeting by homologous recombination or by sequence-specific nucleases allows the precise modification of genomes and genes to elucidate their functions. Although gene targeting has been used extensively to modify the genomes of mammals, fish, and amphibians, a targeting technology has not been available for the avian genome. Many of the principles of humoral immunity were discovered in chickens, yet the lack of gene targeting technologies in birds has limited biomedical research using this species. Here we describe targeting the joining (J) gene segment of the chicken Ig heavy chain gene by homologous recombination in primordial germ cells to establish fully transgenic chickens carrying the knockout. In homozygous knockouts, Ig heavy chain production is eliminated, and no antibody response is elicited on immunization. Migration of B-lineage precursors into the bursa of Fabricius is unaffected, whereas development into mature B cells and migration from the bursa are blocked in the mutants. Other cell types in the immune system appear normal. Chickens lacking the peripheral B-cell population will provide a unique experimental model to study avian immune responses to infectious disease. More generally, gene targeting in avian primordial germ cells will foster advances in diverse fields of biomedical research such as virology, stem cells, and developmental biology, and provide unique approaches in biotechnology, particularly in the field of antibody discovery.
The chicken has historically been an important model vertebrate organism in the fields of developmental biology and immunology and has contributed a number of basic tenets to these fields. For example, B lymphocytes were first recognized in chickens as the antibody-producing cells and are named after the bursa of Fabricius, a gut-associated lymphoid tissue (GALT) that is required for B-cell development in chickens (1). Graft-versus-host response was first described in chicken embryos (2), and the first attenuated vaccine was developed by Louis Pasteur against fowl cholera caused by Pasteurella multocida. Nevertheless, the lack of a robust genome editing technology including knockouts has put the chicken at a distinct disadvantage to mammalian species, especially the mouse, as a vertebrate animal model. The discovery of ES cells provided a powerful method to make desired changes to genes of interest in the mouse using homologous recombination, but interestingly, ES cells have not been as easily derived from other species. In the case of chickens, ES cells can contribute to all somatic lineages in high-grade chimeras, but germ-line transmission has not yet been demonstrated, precluding their use in creating fully transgenic chickens (3). Although ES cells are not germ line competent in chickens, embryo-derived primordial germ cells (PGCs) can be cultured indefinitely, transfected, clonally selected, and reintroduced into the embryo where they colonize the gonad and give rise to fully transgenic progeny in the next generation (4–7). Thus, in chickens, cultured stem cell lines are specific for somatic lineages or the germ line but not both as with mouse ES cells.
Transgenic chicken technology has a number of important applications in the pharmaceutical and agricultural industries. The creation of influenza-resistant chickens could result in a huge benefit to human health. Transgenic chickens overexpressing a short hairpin RNA that acts as a decoy for the influenza polymerase were shown to block the transmission of virus, leading to a strategy for controlling avian influenza outbreaks (8). Transgenic hens have also been made for protein production in the egg using egg white–specific promoters to drive expression in the oviduct (9, 10). Large-scale production of therapeutic proteins that are of interest for the pharmaceutical industry can be produced in a time- and cost-efficient manner. Another human health-related application is creation of transgenic chickens expressing human antibodies, similar to “humanized” mice, rats, and cattle (11–14). Such chickens would take advantage of the phylogenetic distance between chickens and humans for the discovery of human therapeutic antibodies against targets that are highly conserved between mice and humans but immunogenic in avian species.
The first transgenic chicken was produced by direct DNA injection into the fertilized egg (15), a very inefficient process in which the transgene insertion site cannot be controlled. The efficiency increased with the use of retroviral (13, 16, 17) and lentiviral vectors (18), and more recently with transposons (4, 5), but targeting and more subtle mutations are not possible with these approaches. Here we show that classical gene targeting by homologous recombination can be efficiently executed in cultured chicken primordial germ cells to generate knockout birds. In this study we chose to target the Ig heavy chain J gene segment (JH) to create a B cell–less chicken.
Many attempts have been made in the past to generate a B cell–less chicken. Surgical removal of the bursa at an early stage results in depletion of the B-cell lineage and a permanent reduction in antibody production, although results can vary because surgical bursectomy is technically challenging and it is difficult to ensure complete removal of B cells and secreted Ig (19). In chickens, unlike mammals, there is a developmental window during which the Ig genes rearrange, and the adult B-cell population is entirely derived from cells that undergo rearrangement during embryonic and early postembryonic life (20). Also at this time, B-lineage cells colonize the bursa, and there is a rapid expansion of cells expressing surface Ig (sIg). Experiments with chimeric B-cell receptor (BCR) proteins have indicated that basal BCR signaling is required for early stages of B-cell development in the bursa (21–23), although the lack of knockout technologies in chickens has precluded the analysis of deletion of specific BCR components in vivo.
Generation of a B cell–less chicken by knockout of the Ig JH segment now overcomes this problem. The opportunity to study aspects of immunity better in JH knockout chickens will help to improve vaccine technology for the poultry industry. More generally, the availability of knockout chickens demonstrates that targeting technologies long established in mice are now available in birds.
Results
Deletion of the Ig JH Segment.
To create a null allele of the chicken IgH locus we deleted the single known JH segment and its recombination signal sequences by homologous recombination in chicken PGCs. Although the first draft chicken genome was published in 2004, the IgH sequences remain incompletely characterized and are found only in small, unordered contigs (24–26). Regions flanking the JH segment were cloned by PCR using primers spanning the gaps in the genome assembly, from the cell line we used for targeting (derived from a cross of commercial Brown Leghorn and the Minnesota Marker Line), and homology regions totaling ∼8 kb were assembled into an isogenic DNA targeting vector with a puromycin resistance cassette and Enhanced Green Fluorescent Protein (EGFP) flanked by loxP sites (Fig. 1A). To facilitate future insertion of foreign genes such as human Ig sequences into the targeted IgH locus, an attP site and promoterless neo gene were included for site-specific recombination by the phiC31 integrase. The apparent targeting frequency in PGCs with this vector was high; 7 of 25 puromycin-resistant clones screened had a correctly targeted event (28%). This high frequency may reflect the fact that randomly integrated (nontargeted) clones are suppressed, possibly from silencing of the drug selectable marker when inserted in most genomic sites in these germ-line cells (27). The absolute frequency was about one targeted clone per 107 transfected cells, which is in the range of mouse ES cells (10−5–10−8). Germ-line transmission of JH knockout (JH-KO) clones was lower than previously reported with random insertion transgenes (Fig. 1D). Once established in fully transgenic animals, the knockout allele was inherited in Mendelian fashion, and Southern blotting confirmed a single, correct integration of the vector in the IgH locus (Fig. 1C). To easily genotype offspring from heterozygous matings, a PCR assay was established using a primer pair in the deleted J region and a second primer pair spanning from the selectable marker cassette into the 3′ homology region (Fig. 1B).
Fig. 1.
JH segment knockout chickens were produced by gene targeting in primordial germ cells followed by germ-line transmission of injected PGCs. (A) Diagram of the WT IgH locus (top line) with its single functional VH gene, a subset of the D cluster (D cluster is not fully mapped, as indicated by the break), single JH gene, and Cμ constant region. Vector IgH KO2 replaces 390 bp surrounding the JH gene segment with a selectable marker cassette. The JH-KO allele is shown on the bottom line, and positions of primers and Southern blot probes are indicated. R, EcoRI restriction site. (B) Genotyping of cell lines and birds by PCR. Primers were specific for the WT allele (primers 1 and 2) and the JH-KO allele (primers 1 and 3). (C) Southern blot analysis was done on EcoRI-digested gDNA from transgenic birds. (D) Germ-line transmission rates of three of the JH-KO targeted cell lines in chimeric roosters are displayed. Results from six roosters are shown.
Depletion of Peripheral B Cells and Plasma Ig in JH Knockout Birds.
On day 7 after hatch, the homozygous JH-KO birds showed a highly significant reduction in IgM levels in comparison with the control groups (Fig. S1). IgY levels were high for all three genotypes because maternal IgY transmitted via the egg yolk persists for several weeks. Five weeks after hatch, both the IgM and IgY levels of the JH-KO/JH-KO birds were indistinguishable from the PBS control (Fig. 2A). In the heterozygotes, IgM was reduced at day 7 compared with WT chickens (Fig. S1), and by day 35, there was a statistically significant reduction in the IgY levels, although IgM levels were normal (Fig. 2A). Because the process of allelic exclusion guarantees that each B cell expresses only one allele in WT B cells, these data were contrary to our expectation.
Fig. 2.
IgM and IgY expression are eliminated, and B-cell migration to the periphery is blocked in JH-KO/JH-KO birds. (A) Relative amounts of total plasma IgM and IgY from WT (square), JH-KO/+ (triangle), and JH-KO/JH-KO (diamond) chickens were compared by ELISA at day 35 after hatch. Mean and SD of five hens per group are shown. (B) FACS analysis was performed on peripheral blood lymphocytes (PBLs) and spleen of 5-wk-old chickens and the bursa of 3-d-old chickens. B cells were stained with anti–chicken-Bu-1 (AV20), anti-chicken-Cµ (M1), and anti–chicken-lambda (L-1). Gamma/delta T cells were stained with anti–chicken-TCRγδ (TCR1), α/β T cells with anti–chicken-TCRαβ/Vβ1 (TCR2), and anti–chicken-TCRαβ/Vβ2 (TCR3) and chicken thrombocytes with a cross-reactive anti–human-CD51/61 (23C6). Percentages are shown on the y axis. Mean and SD of four birds per group are shown. Significance was calculated by ANOVA followed by Tukey test. **P < 0.01.
We evaluated the cellular composition of the peripheral blood lymphocytes, the bursa, and the spleen 3 wk after hatch by flow cytometry. In the spleen and blood, the B-cell population was essentially deleted in the JH-KO/JH-KO birds [less than 0.2% Bu-1 (a pan-B-cell marker), 0.08% IgM, and 0.2% IgL positive cells were detectable], whereas normal levels were observed in WT and JH-KO/+ chickens (Fig. 2B). No significant difference was measurable in the T-cell or thrombocyte populations (Fig. 2B). At three weeks after hatch, the bursas of JH-KO/JH-KO birds contained few living lymphocytes; as a consequence, we analyzed the bursa 3 d after hatch. Bu-1– and CD45-positive cells were reduced to 67.7% and 84%, respectively, in the homozygotes, compared with ∼98% in the control groups. The reduced proportion of Bu-1+ and CD45+ cells in the bursa could reflect the fact that the cells are undergoing apoptosis and possibly down-regulating expression of these markers. No surface IgM was detected in the homozygotes (Fig. 2B). The B-cell lineage of bursal sIg-negative cells in the homozygotes was confirmed by finding that the light chain locus is rearranged and undergoing gene conversion in these cells, without selection for productive rearrangement (Figs. S2 and S3).
Silencing of eGFP in the Heavy Chain Locus.
Not all somatic cells of the transgenic birds were EGFP positive by FACS, despite flanking copies of the β-globin insulator and expression driven by the β-actin promoter. The proportion of EGFP-positive cells ranged from 30% to 50% in heterozygous and homozygous birds. As there was no evidence for loss of the EGFP gene by Southern blot analysis, we looked for gene specific silencing of the EGFP in chicken embryo fibroblasts (CEFs) from JH-KO/+ birds. After limiting dilution, we compared the CpG methylation status of the EGFP gene in a cell pool with 2.5% EGFP-positive cells vs. a pool with 60% EGFP-positive cells by bisulfite sequencing. We found that the CpG methylation status of specific sites in the EGFP gene correlated with EGFP expression. At one CpG site analyzed, 84% of all sequences showed methylation in the low EGFP CEF pool (Fig. S4), whereas only 7% were methylated in the high EGFP CEF pool (Fig. S5). EGFP silencing has not been reported in other lines of transgenic birds and may be a consequence of its position in the heavy chain locus.
Histological Analysis Shows Depletion of Lymphocytes in the Bursa of JH Knockout Birds.
Progeny of matings between JH-KO heterozygotes hatched normally, and homozygous JH-KO/JH-KO birds grew at the same rate as their hatchmates (Fig. 3A). The weights of the bursa and the spleen were drastically lower in the JH-KO/JH-KO compared with JH-KO/+ and WT birds by 28 d posthatch, although at embryonic day 18, there was no difference in the weights of the spleens and bursas in the mutant genotype (Fig. 3 B–D). Histological sections of bursas were prepared at several stages to evaluate morphology. At embryonic day (ED)14, ED18, and day 1 after hatch, colonization of the epithelial buds was seen in all genotypes, and no defect was apparent in the homozygotes. By 4 wk after hatch, WT and JH-KO/+ knockout birds showed a clear separation of follicle medulla and cortex by cells migrating across the basement membrane that forms the cortico-medullary junction, whereas no such separation was apparent in the JH-KO/JH-KO birds (Fig. 4). This finding was supported by staining the cortico-medullary junction with a cross-reactive anti-human desmin antibody that specifically stains the basement membrane (Fig. 5). Bursal follicles of the JH-KO/JH-KO birds were reduced in size, and although immunohistochemistry with Bu-1 shows that B-lineage cells were still found in the follicles of JH-KO/JH-KO birds at this stage, fewer cells were present compared with follicles from age-matched control birds (Fig. 6A). However, IgM staining was completely absent in bursa and spleen sections of JH-KO/JH-KO birds (Fig. 6). Some Bu-1–positive cells were detectable in spleen sections from JH-KO/JH-KO birds (Fig. 5B); this could be due to the possible cross-reactivity of the antibody to macrophages (28). The CD3-positive T-cell zones in the spleens of JH-KO/JH-KO chickens were well defined even in the absence of B cells (Fig. 6B).
Fig. 3.
JH-KO/JH-KO chickens show reduced weight of lymphoid organs. (A) Gain in body weight was monitored until day 54 after hatch. Mean and SD of five birds per group are shown. Body weights of birds at embryonic day 18 (B), day 28 (C), and day 54 (D) after hatch were measured. Ratio between spleen or bursa weight (mg) and bodyweight (g) was calculated. Mean and SD of three birds per group are shown. Significance was calculated by ANOVA followed by Tukey test. *P < 0.05; **P < 0.01.
Fig. 4.
Effect of the JH segment knockout on the morphology of the bursa of Fabricius. The bursa of Fabricius of WT, JH-KO/+, and JH-KO/JH-KO chickens was prepared at embryonic day 14 and 18 and 1 and 28 d after hatch. Tissue sections of three birds per group were prepared. H&E staining was performed. One representative picture of every development stage and genotype is shown.
Fig. 5.
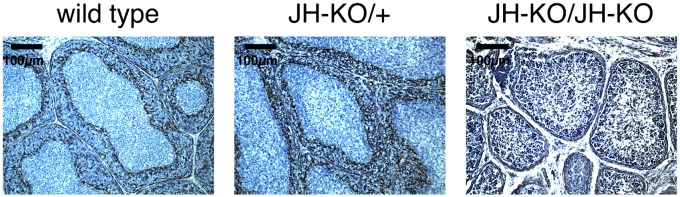
Separation of B-cell follicles by the cortico-medullary junction. Sections from frozen samples of the bursa from birds at 28 d after hatch were prepared. The basement membrane forming the cortico-medullary junction was stained using a cross-reactive anti–human-desmin (D33) antibody. The antibody was detected using the Vector ABC Kit followed by the Vector DAB Kit. One representative picture per group and staining is shown.
Fig. 6.
Distribution of T and B cells in homozygous JH segment knockout birds. Sections from frozen samples of the bursa of Fabricius (A) and the spleen (B) from birds at 28 d after hatch were prepared. B cells were stained with an anti–chicken-Bu-1 (AV20) antibody. IgM was stained using anti–chicken-Cµ (M1). T cells were stained using an anti–chicken-CD3 (CT-3) antibody. All antibodies were detected by an anti–mouse-IgG Cy3. One representative picture per group and staining is shown.
Lack of Antibody Response on Immunization of JH Knockout Chickens.
To determine if JH segment knockout birds can produce antibodies after immunization, hens were immunized at 5 wk of age with keyhole limpet hemocyanin (KLH). WT and JH-KO/+ knockout birds showed increasing IgM titers by ELISA against KLH 3 and 5 d after immunization (Fig. S6). KLH-specific IgY was detectable in WT and JH-KO/+ 5 d after immunization (Fig. S6). No reactivity toward KLH was seen in the IgM or IgY ELISA in plasma from JH-KO/JH-KO chickens (Fig. S6).
Discussion
The ability to culture PGCs that retain germ-line competence once they are injected back into host embryos is unique to chickens. By combining cultured PGCs and classical gene targeting via homologous recombination, it is now possible to introduce a site-specific alteration into the chicken genome. The targeting frequency is high when measured as the ratio of targeted clones to the total number of drug resistant clones, making screening for knockout clones simple and efficient. The absolute targeting frequency requires transfection of at least 107 cells to get one targeted clone. This number of cells is readily obtainable within 40 d following isolation of about 200 PGCs from a single embryo. Because cultures originate from a single embryo, they can be used for targeted modification with isogenic DNA. PGC lines may be grown for more than 150 d in culture without loss of germ-line competence (6), thereby providing a nearly limitless resource of cells. In an initial experiment a shorter 3′ homology region of 3 kb was used, which led to a somewhat lower targeting frequency of 10% (3 targeted clones out of 28 screened). The lower limit of the length of the homology arms has not been established.
EGFP was included in the selectable marker cassette of the knockout vector to facilitate identification of genetically modified cells in culture and to select chimeras for breeding to obtain G1 transgenic offspring by measuring the proportion of GFP-positive sperm in semen via flow cytometry. We also placed an attP site joined to a promotorless neomycin resistance gene into the chicken heavy chain locus (Fig. 1A) to facilitate integrase-mediated targeted insertion into the heavy chain locus to express, for example, V regions derived from the human Ig heavy chain locus. Replacement of both the heavy and light chain V regions would yield chickens from which fully human antigen-specific antibodies could be isolated. A humanized chicken will provide a major advance over mammalian-based antibody discovery platforms. The chicken has not shared a common ancestor with humans in more than 300 million years. As a consequence, many human antigens that are not immunogenic in mammals because of their high homology will give a strong antibody response in chickens. Empirical data from our laboratory have demonstrated that unique epitopes can be accessed from immunized WT chickens. In combination with the ability to make site-specific insertions via the attP site targeted into the IgH locus, it will be possible to evaluate the epitope coverage of chickens making fully human antibodies.
The use of homologous recombination in primordial germ cells has several advantages over nascent technologies such as sequence-specific nucleases for making targeted changes to the genome. Although the in vivo use of zinc finger nucleases and transcription activator-like effector nucleases (TALENs) has been suggested by Tyack et al. (29) their use requires that the genetically modified genotype be identified in hatched chickens and not in cultured cells. From a practical perspective, it is currently more attractive to screen for the genotype in culture than in live chicks, although newer technologies such as clustered regularly interspaced short palindromic repeats (CRISPRs)-Cas9 may change this calculation. The choice of technologies is equally influenced by the flexibility to insert selection cassettes, genetic markers such as GFP and recombination target sequences such as attP, which is best accomplished in cultured cells. There is no advantage in chicken PGCs to use a sequence-specific nuclease when inserting selection cassettes because the targeting frequency is already high. The avian-specific follicular anatomy makes it impractical to directly inject chicken zygotes with genome editing tools, and in vivo transfection of embryos is likely to be very inefficient.
Deletion of the JH gene segment in chickens results in a total loss of heavy chain expression, proving that the chicken genome harbors a single functional heavy chain locus. The strategy of targeting the single JH segment ensures that all heavy chain expression is blocked, because it is required for all heavy chains regardless of V region use or isotype class. In the knockouts, the B-cell receptor complex is not required for population of bursal follicles, in seeming contrast to the previous conclusion that signaling by the B-cell receptor component Igα is required (30). B cells in the JH knockout chickens not only colonize the bursa, but they also proliferate inside the follicles. The epithelial buds are colonized with approximately three B cells per follicle, and these cells proliferate until the bursa contains ∼107 B cells (20). Because bursal morphology and bursal weights at the time of hatch are indistinguishable between KO and WT birds, it appears that B-cell receptor signaling is not necessary for proliferation inside the B-cell follicles. Light chain rearrangement and gene conversion proceed in the bursa of the knockouts, indicating that these diversification processes are also independent of IgH expression. Only one-third of the rearranged light chain genes are in-frame, which is consistent with the absence of selection for productive rearrangement required by a functional receptor. After hatch, expression of the heavy chain becomes obligatory for continued B-cell survival, and by 4 wk of age, bursal follicles in the JH-KO/JH-KO chickens are depleted of B cells. More detailed studies of the molecules required for BCR signaling in B-cell development can now be designed using the IgH knockout birds in experiments that will extend previous work done in WT birds (30–33).
The availability of chickens without B cells will also find utility in both avian and human medicine. In chickens, several diseases including Marek’s disease, infectious bursal disease, avian leukosis, and chicken anemia virus express an immune-compromised phenotype. The IgH knockout chickens described in this paper are currently being developed as a research tool to better understand the etiology of these diseases. In human medicine, avian influenza is a significant zoonotic disease infecting both humans and chickens. The IgH knockout will be incorporated into the toolbox of virologists developing technologies to obviate the anticipated pandemic of increasingly virulent recombinant viruses.
The chicken has a long and distinguished career in fields as diverse as immunology, developmental biology, genetics, animal breeding, agriculture, nutrition, virology, oncology, and biochemistry. In addition, chickens supply approximately one-third of the animal protein in human diets throughout the world. Here we show site-specific changes to the chicken genome, which in combination with the chicken genome sequence and several approaches to nontargeted genetic modification, completes the range of genetic modifications that can be applied to avian models in biomedical research and to poultry production.
Methods
Animal Experiments.
Commercial Brown and White Leghorn chickens and Minnesota Marker Line chickens were used. Birds were housed on short days (8 h) in groups of four birds per cage up to an age of 12 wk. Males and females were moved at 12 and 17 wk of age, respectively, to long days (14 h) into individual cages. All birds received standard diet and water ad libitum. Animal experiments were done in accordance to Institutional Animal Care and Use Committee (IACUC) approved protocols and under supervision of the IACUC.
Construction of Targeting Vector.
To target the chicken JH segment, ∼9 kb of genomic sequence surrounding JH was cloned and sequenced. Genomic DNA was obtained from the cell line used for the gene targeting transfections (Nu69) and used in PCR to amplify heavy chain sequences upstream (2.3 kb) and downstream (6.3 kb) of JH. Extensive polymorphism between the two alleles in Nu69 was observed (one allele is from Brown Leghorn and one is from the Minnesota Marker Line), and care was taken to assemble a single contig from one of the two alleles for targeting. Targeting vector IgH KO2 was assembled with a 5′ homology region of 1938 bp (delineated by primers 5′ HR-for, 5′-TGGGAAATTTGGCCCTCTTGGCC-3′ and 5′ HR-rev, 5′-TCGGGGCGAAAACCGCCCATTT-3), a 3′ homology region of 6,070 bp (delineated by primers 3′ HR-for, 5′- TGGCGGTCTGAGGGGAAAATGTC-3′ and 3′ HR-rev, 5′-ATATTGGCCCCATTTCCCCTCAG-3′), and a selectable marker cassette consisting of β-actin-EGFP and CAGGS-puromycin flanked by duplicated copies of the core 300-bp HS4 insulator from the chicken β-globin gene. LoxP sites flank the HS4-EGFP-puro cassette. Adjacent to the main cassette is a promoterless neomycin resistance gene linked to an attP site for targeted insertion of attB-containing plasmids using phiC31 integrase.
PGC Derivation and Culture.
The parental line Nu69 was derived from a cross of the Minnesota marker line with a commercial Brown Leghorn line. Primordial germ cells were derived from blood collected at stages 13–15 (Hamburger-Hamilton) from a male embryo and cultured in KO-DMEM containing 40% (vol/vol) Buffalo rat liver (BRL) cell conditioned medium, 7.5% (vol/vol) FBS, 2.5% (vol/vol) chicken serum, 2 mM glutamine, 1 mM pyruvate, 1× nonessential amino acids, and 0.1 mM β-mercapto-ethanol and supplemented with 6 ng/mL recombinant mouse stem cell factor and 4 ng/mL recombinant human fibroblast growth factor basic (R&D Systems) on a feeder layer of irradiated BRL cells (6, 7).
Transfection of PGCs.
Targeting construct IgH KO2 was linearized using a unique NotI restriction site at the 3′ end of the 3′ homology region before transfection; 5 × 106 cells were suspended in 100 µl of AmaxaV buffer with 20 µg linearized DNA and transfected as previously described (6, 7). Each transfection was plated on a full 48w plate. Puromycin selection (0.5 µg/mL) was started 3–5 d after transfection, and medium was changed regularly throughout selection. Puromycin-resistant clones were expanded for genotyping and injection.
Injection of PGCs.
Recipient embryos were incubated until stages 13–15 (Hamburger-Hamilton) and transferred to a collection dish. After injection of 3,000 JH-KO PGCs into the vasculature (6, 7), the embryos were transferred to a surrogate shell and incubated until hatch.
Southern Blot.
The 5′ external probe was amplified and confirmed by sequencing from Nu69 primordial germ cell gDNA using the following primers: 5′ CAGTGTCCAAATTCCTTAAATTTCC 3′ and 5′ TCGTTGTGAAATGCGGTGAAAAT 3′. The EGFP probe was prepared from a plasmid containing EGFP by SalI/NotI digest followed by gel purification. For Southern blot analyses, 5 µg gDNA was digested for 12 h with EcoRI. Digested gDNA was separated by gel electrophoresis and visualized by poststaining with GelRed (Biotium). Depurination was done by incubation with 0.2 M HCl for 10 min, followed by denaturation in 0.5 M NaOH and 1.5 M NaCl. The gel was neutralized by two washes in 1 M Tris, pH 7.4, and 1.5 M NaCl. Transfer to Amersham Hybond-N+ blotting membrane (GE Healthcare) was done overnight by capillary transfer. After transfer, the gDNA was cross-linked using a UV light. Membranes were prehybridized at 65 °C for 1 h with Rapid-hyb buffer (GE Healthcare). Probes were labeled using the Prime-a-Gene Labeling System (Promega) following the manufacturer’s protocol. After hybridization for 2 h, blots were washed once with 2× SSC and 0.1% SDS, followed by two washes with 0.1× SSC and 0.1% SDS. Blots were exposed to film for 24–96 h at −80 °C.
Genotyping.
For genotyping, 5 µL blood was used for gDNA isolation using the DNeasy Blood & Tissue Kit (Qiagen). PCR primers specific for the JH-KO were as follows: neo-R1, 5′AGTGACAACGTCGAGCACAGCT 3′; JC-R45, 5′GCCCAAAATGGCCCCAAAAC 3′. PCR primers specific for the deleted WT region were forward primer JH-F3 5′ATGGGGCCACGGGACCGAA 3′ and JC-R45 (above). PCR was performed using 5× FirePol MasterMix (Solis BioDyne) according to the manufacturer’s instructions.
Immunization.
Hens were immunized by intramuscular injection at 5 wk of age with 300 µg KLH (Sigma Aldrich) mixed 1:1 with Freund’s complete adjuvant (Sigma Aldrich). One boost was performed 2 wk after initial immunization using 300 µg KLH mixed 1:1 with Freund’s incomplete adjuvant (Sigma Aldrich).
ELISA.
To measure total plasma IgM and IgY, enzyme immunoassay (EIA) plates (Corning) were coated with 2 µg/mL goat anti–chicken-IgM (Bethyl Laboratories) or rabbit anti–chicken-IgY (Sigma Aldrich) overnight. Plates were blocked with 3% (wt/vol) skim milk and incubated with chicken plasma for 2 h. Bound chicken IgM or IgY was detected with goat anti–chicken-IgM-HRP (Bethyl Laboratories) or rabbit anti–chicken-IgY-HRP (Sigma Aldrich) and developed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. OD was measured at 450 nm wavelength. All samples were measured in duplicate. To measure the IgM or IgY response after immunization with KLH, plates were coated with 10 µg/mL KLH. All subsequent steps were performed as for detection of total plasma IgM or IgY.
FACS.
Cells (1 × 106) were stained for 20 min on ice using the following antibodies: mouse anti–chicken-Bu-1 (AV20), mouse anti-chicken-Cμ (M-1), mouse anti–chicken-lambda (L-1), mouse anti–chicken-TCRγδ (TCR1), anti–chicken-TCRαβ/Vβ1 (TCR2), mouse anti–chicken-TCRαβ/Vβ2 (TCR3), and mouse anti–human-CD51/61 (23C6) (Southern Biotech). Following a wash, all primary antibodies were detected by incubation with a goat anti–mouse-IgG-Cy5 (Bethyl Laboratories) antibody for 20 min. After a final wash, fluorescence was measured using a Beckman-Coulter FC500.
Immunohistochemistry.
Tissue samples were either fixed with 10% (vol/vol) neutral buffered formalin for 24 h or immediately frozen in liquid nitrogen. Formalin-fixed samples were dehydrated and paraffin embedded, and 5-µm sections were collected and stained with H&E (EMS). For antibody staining, 10-µm sections were prepared from fresh frozen samples. Sections were rehydrated and fixed with ice-cold acetone. For the anti-desmin staining, endogenous peroxidase was blocked by incubation with 40% (vol/vol) methanol containing 0.3% H2O2 for 30 min. Nonspecific binding sites were blocked using the Vector ABC Kit and Biotin/Avidin Blocking Kit (Vector) following the manufacturer’s instructions. Mouse anti-desmin (D33) (Thermo Scientific) was used as a primary antibody and detected using the Vector ABC Kit followed by the Vector DAB Kit (Vector). Eukitt mounting medium (EMS) was used to mount the stained sections. For all other staining, sections were blocked with 1% PBS-BSA. The following primary antibodies were used: mouse anti–chicken-Bu-1 (AV20), mouse anti–chicken-Cµ (M-1), and mouse anti–chicken-CD3 (CT-3) (Southern Biotech). After washing, the primary antibodies were detected using a goat anti–mouse-Cy3 (Bethyl Laboratories) antibody. Prolong mounting medium containing DAPI was used to mount the stained sections (Life Technologies). Pictures were taken using a Zeiss AxioImager M1 equipped with a Hamamatsu Orca 03 and QImaging MicroPublisher.
Methylation Analysis.
To analyze methylation in the EGFP gene, CEFs were prepared from 10-d-old heterozygous JH segment knockout embryos by trypsin digest. CEFs were cultured using Iscove’s modified DMEM supplemented with 10% (vol/vol) FBS, 5% (vol/vol) chicken serum, and 1% penicillin/streptomycin. Cells were passaged every 2 d. Limiting dilution was performed, and predominantly EGFP-positive and -negative cultures were grown up. gDNA was isolated using the DNeasy Blood & Tissue Kit (Qiagen) following the manufacturer’s manual. Afterward, gDNA was treated with bisulfite using the EpiTect Fast Bisulfite kit (Qiagen). PCR was performed using the following methylation-specific primers: forward 5′ TTGTTCGTGTTTTGGTTTATTTTC 3′ and reverse 5′GCGAATCTTATAATTACCGTCGT 3′. The PCR product was cloned using a TOPO TA cloning Kit (Life Technologies). Bacterial colonies were sent for sequencing. Sequences were analyzed using DNASTAR Lasergene.
Supplementary Material
Acknowledgments
We thank Connie Cepko (Harvard Medical School, Boston, MA) for the CAG-EGFP construct. This work was supported by National Institutes of Health Small Business Innovation Research Grant GM090626 (to P.A.L.) and Research Fellowship Schu2446/2-1 (to B.S.) from the Deutsche Forschungsgemeinschaft.
Footnotes
Conflict of interest statement: The authors have patents in process regarding the heavy chain knockout in chickens.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317106110/-/DCSupplemental.
References
- 1.Glick B, Chang TS, Jaap RG. The bursa of Fabricius and antibody production. Poult Sci. 1956;35(1):224–225. [Google Scholar]
- 2.Murphy JB. The effect of adult chicken organ grafts on the chick embryo. J Exp Med. 1916;24(1):1–5. doi: 10.1084/jem.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Lavoir MC, et al. High-grade transgenic somatic chimeras from chicken embryonic stem cells. Mech Dev. 2006;123(1):31–41. doi: 10.1016/j.mod.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald J, et al. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proc Natl Acad Sci USA. 2012;109(23):E1466–E1472. doi: 10.1073/pnas.1118715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park TS, Han JY. piggyBac transposition into primordial germ cells is an efficient tool for transgenesis in chickens. Proc Natl Acad Sci USA. 2012;109(24):9337–9341. doi: 10.1073/pnas.1203823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Lavoir MC, et al. Interspecific germline transmission of cultured primordial germ cells. PLoS ONE. 2012;7(5):e35664. doi: 10.1371/journal.pone.0035664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Lavoir MC, et al. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441(7094):766–769. doi: 10.1038/nature04831. [DOI] [PubMed] [Google Scholar]
- 8.Lyall J, et al. Suppression of avian influenza transmission in genetically modified chickens. Science. 2011;331(6014):223–226. doi: 10.1126/science.1198020. [DOI] [PubMed] [Google Scholar]
- 9.Harvey AJ, Speksnijder G, Baugh LR, Morris JA, Ivarie R. Expression of exogenous protein in the egg white of transgenic chickens. Nat Biotechnol. 2002;20(4):396–399. doi: 10.1038/nbt0402-396. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, et al. Production of human monoclonal antibody in eggs of chimeric chickens. Nat Biotechnol. 2005;23(9):1159–1169. doi: 10.1038/nbt1132. [DOI] [PubMed] [Google Scholar]
- 11.Kuroiwa Y, et al. Cloned transchromosomic calves producing human immunoglobulin. Nat Biotechnol. 2002;20(9):889–894. doi: 10.1038/nbt727. [DOI] [PubMed] [Google Scholar]
- 12.Kuroiwa Y, et al. Antigen-specific human polyclonal antibodies from hyperimmunized cattle. Nat Biotechnol. 2009;27(2):173–181. doi: 10.1038/nbt.1521. [DOI] [PubMed] [Google Scholar]
- 13.Lonberg N, et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature. 1994;368(6474):856–859. doi: 10.1038/368856a0. [DOI] [PubMed] [Google Scholar]
- 14.Osborn MJ, et al. High-affinity IgG antibodies develop naturally in Ig-knockout rats carrying germline human IgH/Igκ/Igλ loci bearing the rat CH region. J Immunol. 2013;190(4):1481–1490. doi: 10.4049/jimmunol.1203041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Love J, Gribbin C, Mather C, Sang H. Transgenic birds by DNA microinjection. Biotechnology (N Y) 1994;12(1):60–63. doi: 10.1038/nbt0194-60. [DOI] [PubMed] [Google Scholar]
- 16.Bosselman RA, et al. Germline transmission of exogenous genes in the chicken. Science. 1989;243(4890):533–535. doi: 10.1126/science.2536194. [DOI] [PubMed] [Google Scholar]
- 17.Harvey AJ, Speksnijder G, Baugh LR, Morris JA, Ivarie R. Consistent production of transgenic chickens using replication-deficient retroviral vectors and high-throughput screening procedures. Poult Sci. 2002;81(2):202–212. doi: 10.1093/ps/81.2.202. [DOI] [PubMed] [Google Scholar]
- 18.McGrew MJ, et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 2004;5(7):728–733. doi: 10.1038/sj.embor.7400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzsimmons RC, Garrod EM, Garnett I. Immunological responses following early embryonic surgical bursectomy. Cell Immunol. 1973;9(3):377–383. doi: 10.1016/0008-8749(73)90052-x. [DOI] [PubMed] [Google Scholar]
- 20.Ratcliffe MJ. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev Comp Immunol. 2006;30(1-2):101–118. doi: 10.1016/j.dci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Pike KA, Iacampo S, Friedmann JE, Ratcliffe MJ. The cytoplasmic domain of Ig alpha is necessary and sufficient to support efficient early B cell development. J Immunol. 2004;172(4):2210–2218. doi: 10.4049/jimmunol.172.4.2210. [DOI] [PubMed] [Google Scholar]
- 22.Pike KA, Ratcliffe MJ. Dual requirement for the Ig alpha immunoreceptor tyrosine-based activation motif (ITAM) and a conserved non-Ig alpha ITAM tyrosine in supporting Ig alpha beta-mediated B cell development. J Immunol. 2005;174(4):2012–2020. doi: 10.4049/jimmunol.174.4.2012. [DOI] [PubMed] [Google Scholar]
- 23.Sayegh CE, Ratcliffe MJ. Perinatal deletion of B cells expressing surface Ig molecules that lack V(D)J-encoded determinants in the bursa of Fabricius is not due to intrafollicular competition. J Immunol. 2000;164(10):5041–5048. doi: 10.4049/jimmunol.164.10.5041. [DOI] [PubMed] [Google Scholar]
- 24.International Chicken Genome Sequencing Consortium Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432(7018):695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- 25.Kitao H, et al. Class switch recombination of the chicken IgH chain genes: Implications for the primordial switch region repeats. Int Immunol. 2000;12(7):959–968. doi: 10.1093/intimm/12.7.959. [DOI] [PubMed] [Google Scholar]
- 26.Reynaud CA, Dahan A, Anquez V, Weill JC. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989;59(1):171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- 27.Leighton PA, van de Lavoir MC, Diamond JH, Xia C, Etches RJ. Genetic modification of primordial germ cells by gene trapping, gene targeting, and phiC31 integrase. Mol Reprod Dev. 2008;75(7):1163–1175. doi: 10.1002/mrd.20859. [DOI] [PubMed] [Google Scholar]
- 28.Tregaskes CA, Bumstead N, Davison TF, Young JR. Chicken B-cell marker chB6 (Bu-1) is a highly glycosylated protein of unique structure. Immunogenetics. 1996;44(3):212–217. doi: 10.1007/BF02602587. [DOI] [PubMed] [Google Scholar]
- 29.Tyack SG, et al. A new method for producing transgenic birds via direct in vivo transfection of primordial germ cells. Transgenic Res. 2013 doi: 10.1007/s11248-013-9727-2. 10.1007/s11248-013-9727-2. [DOI] [PubMed] [Google Scholar]
- 30.Aliahmad P, Pike KA, Ratcliffe MJ. Cell surface immunoglobulin regulated checkpoints in chicken B cell development. Vet Immunol Immunopathol. 2005;108(1-2):3–9. doi: 10.1016/j.vetimm.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Kothlow S, et al. Unique and conserved functions of B cell-activating factor of the TNF family (BAFF) in the chicken. Int Immunol. 2007;19(2):203–215. doi: 10.1093/intimm/dxl137. [DOI] [PubMed] [Google Scholar]
- 32.Kothlow S, Schenk-Weibhauser K, Ratcliffe MJ, Kaspers B. Prolonged effect of BAFF on chicken B cell development revealed by RCAS retroviral gene transfer in vivo. Mol Immunol. 2010;47(7-8):1619–1628. doi: 10.1016/j.molimm.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Schneider K, et al. Chicken BAFF—a highly conserved cytokine that mediates B cell survival. Int Immunol. 2004;16(1):139–148. doi: 10.1093/intimm/dxh015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







