Significance
We show that the mandated, subthermoneutral laboratory housing temperature, which is known to cause chronic, metabolic cold stress, induces suppression of the antitumor immune response and promotes tumor growth and metastasis. When mice are housed at thermoneutrality, there are fewer immunosuppressive cells with significantly enhanced CD8+ T cell-dependent control of tumor growth. These findings underscore the fact that investigating mouse models under a single set of environmental temperature conditions may lead to a misunderstanding of the antitumor immune potential. These data also highlight the need for additional study to determine how systemic metabolic stress modulates the functions of immune effector cells, particularly in tumor-bearing mice, and whether cancer therapies, including immunotherapy, are impacted by housing temperature.
Keywords: murine tumor models, metabolism
Abstract
We show here that fundamental aspects of antitumor immunity in mice are significantly influenced by ambient housing temperature. Standard housing temperature for laboratory mice in research facilities is mandated to be between 20–26 °C; however, these subthermoneutral temperatures cause mild chronic cold stress, activating thermogenesis to maintain normal body temperature. When stress is alleviated by housing at thermoneutral ambient temperature (30–31 °C), we observe a striking reduction in tumor formation, growth rate and metastasis. This improved control of tumor growth is dependent upon the adaptive immune system. We observe significantly increased numbers of antigen-specific CD8+ T lymphocytes and CD8+ T cells with an activated phenotype in the tumor microenvironment at thermoneutrality. At the same time there is a significant reduction in numbers of immunosuppressive MDSCs and regulatory T lymphocytes. Notably, in temperature preference studies, tumor-bearing mice select a higher ambient temperature than non-tumor-bearing mice, suggesting that tumor-bearing mice experience a greater degree of cold-stress. Overall, our data raise the hypothesis that suppression of antitumor immunity is an outcome of cold stress-induced thermogenesis. Therefore, the common approach of studying immunity against tumors in mice housed only at standard room temperature may be limiting our understanding of the full potential of the antitumor immune response.
Mouse models are widely used in cancer research to investigate the antitumor immune response and its role in disease progression, as well as to test new therapies. Unfortunately, there is growing appreciation that these models may not accurately predict which new therapies will be effective in the clinic (1, 2). Therefore, identification of factors that impact experimental outcomes could improve our ability to identify the most promising therapies.
One variable that has received little attention in cancer research is the relatively cool ambient housing temperature in research facilities. This factor is important because mice have a high surface area to body mass ratio and lose heat rapidly. In nature, mice seek warm environments and build nests to minimize metabolic demands for heat production (3), and thermal preference studies have clearly shown that healthy mice will select an ambient temperature of 30–31 °C (termed “thermoneutrality”) at which their basal metabolism is sufficient to maintain body temperature (3–7). However, at subthermoneutral temperatures, mice experience cold stress, which induces a systemic sympathetic response involving adaptive metabolic changes and secretion of catecholamines, particularly norepinephrine (8). These changes drive a highly energetically demanding process known as “adaptive thermogenesis” to maintain normal body temperature (8).
For research facilities, the room temperature that the National Research Council Guide for the Care and Use of Laboratory Animals (9) requires is considerably cooler than thermoneutrality to facilitate some aspects of husbandry, to reduce frequency of cage cleaning, and to ensure thermal comfort of animal care technicians (4, 7). Institutes must select and maintain a constant room temperature between 20 °C and 26 °C; until 2011, an even cooler range between 18 °C and 24 °C was permitted. Despite the significant impact of ambient temperature on the metabolism of laboratory mice, the room temperature of mouse colonies has not concerned researchers because mice are able to maintain a normal body temperature. However, cool housing temperature is not always a benign variable and there is a disconcerting possibility that it may affect the outcome of a broad range of experimental endpoints (4, 5, 7). Although researchers interested in measuring fever in LPS-treated rodents have long recognized the importance of ambient temperature (4, 10), more recent studies demonstrate that an expected obesity phenotype in uncoupling protein 1 (UCP1)-deficient mice could only be observed when mice were housed at thermoneutrality (11). In another study, it was shown that adaptation to standard housing temperatures is associated with an increased polarization of macrophages to the alternatively activated state (12). Because there is little or no information on the effect of housing temperature on tumor growth or whether tumor growth affects thermal preference, we began to study the effects of cold stress in mouse tumor models. Here, using several different widely studied tumor models, we compared tumor formation, growth, and metastasis at either subthermoneutral or thermoneutral housing temperatures, and found significant differences that we were able to directly relate to differences in the status of the antitumor immune response.
Results
To address the basic question of whether tumor growth is influenced by ambient temperature, we compared tumor formation and growth rate in several widely used murine models in mice housed at either standard temperature (ST; ∼22–23 °C) or at thermoneutral temperature (TT; ∼30–31 °C). Groups of mice were first acclimated to each ambient temperature for at least 2 wk and we then injected moderate doses (104 to 105) of tumor cells into the appropriate syngeneic strain of mice (BALB/c or C57BL/6). B16.F10, CT26, and Pan02 were injected subcutaneously, and 4T1 was injected orthotopically into a mammary fat pad and then growth rates were monitored. Each tumor type demonstrated typical rapid growth in mice housed at ST. However, in mice maintained at TT, we observed a significant reduction in the growth rate in all four tumor models (Fig. 1 A–D). Although most of the work presented here was done in mice that were allowed to acclimate at each temperature for at least 2 wk, acclimation may not be a critical factor because in at least one model (Pan02), a reduced growth rate still occurred when tumor-bearing mice were separated into ST and TT after tumors were first palpable (Fig. S1).
Fig. 1.
Maintaining mice at TT slows tumor growth in immunocompetent mice but not in immunodeficient mice. Tumor growth was monitored following injection of (A) 1 × 105 B16.F10 cells into C57BL/6 mice, (B) 1 × 104 4T1 cells into BALB/c mice, (C) 1 × 105 CT26 cells into BALB/c mice or (D) 1 × 105 Pan02 cells into C57BL/6 mice, (E) 1 × 104 4T1 cells into SCID mice, and (F) 1 × 104 CT26 cells into NUDE mice. Data presented as mean ± SEM (n = 5–10; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Two-way ANOVA with Bonferroni posttests). (G) Tumor incidence was monitored in mice after injection of 1 × 104 CT26 into BALB/c mice or 1 × 103 CT26 cells into SCID mice. Data presented as percent of mice tumor-free (n = 5; #P < 0.05; Mantel-Cox test). (H) Isolated lung weight was measured in 4T1 tumor-bearing (average volume: ST = 537.6 ± 119.3 mm3; TT = 128.1 ± 54.07 mm3) or age-matched tumor-free BALB/c mice. Data presented as mean ± SEM (n = 6; *P < 0.05; Student t test). (I) Representative photographs of lungs stained with India Ink; tumor nodules appear white. (J) Tumor incidence was monitored after injection of 50 µg MCA into BALB/c mice. Data presented as percent of mice tumor-free (n = 5; #P < 0.05; Mantel-Cox test).
We compared basic white blood cell counts in tumor-free mice housed under each condition and found identical levels of all major leukocytes populations (Fig. S2A). However, when examined shortly after tumor cells were injected we saw a significant increase in leukocytes, specifically lymphocytes and monocytes, in mice housed at TT compared with ST (Fig. S2B). In sharp contrast to the data obtained in immunocompetent mice (Fig. 1 A–D), when we compared tumor growth rates at ST and TT in immunodeficient mice, we observed that the improved control of tumor growth seen at TT was lost in both SCID (Fig. 1E; data shown for 4T1) and NUDE mice (Fig. 1F; data shown for CT26). These data strongly suggest that the impact of ambient temperature on tumor growth was largely indirect, and involved the antitumor adaptive immune response. We also compared the time it took for a palpable tumor to form in immunocompetent and immunodeficient hosts. We observed a significant delay in tumor development in BALB/c mice housed at TT compared with ST which was not observed in SCID mice (Fig. 1G).
The 4T1 model affords an excellent opportunity to examine the impact of ambient temperature on metastasis from orthotopically implanted mammary tumors (13), a process known to involve a clinically relevant route of lung invasion that is sensitive to the status of antitumor immune activity (14). Using this model, we observed that the lung weights of tumor-bearing mice at ST were significantly greater than those of tumor-bearing mice at TT, which were comparable to those of the nontumor-bearing mice (Fig. 1H). Correlating with this difference in lung weight, significantly fewer numbers of metastatic lesions were seen in lungs from mice at TT compared with ST (Fig. 1I), strongly suggesting an improvement of metastatic tumor growth control.
We next asked if the difference in tumor growth at ST vs. TT seen in implantable tumor models would occur in a carcinogen-induced tumor model in which tumors develop in situ. We selected the 3-methylcholanthrene (MCA) model for which a critical role for T lymphocytes in control of tumor growth has been previously reported (15–17). Here, we again found that the incidence of MCA-induced fibrosarcomas was markedly delayed when mice were maintained at TT (Fig. 1J) compared with those maintained at ST.
To explore further the role of cytotoxic or helper T-cell subsets in this phenomenon, we depleted either CD8+ or CD4+ T cells in CT26 and B16 tumor-bearing mice and examined tumor growth in mice housed at each temperature. These experiments revealed that the presence of CD8+ T cells was critical for the tumor growth delay seen at TT in both B16.F10 and CT26 tumor models (Fig. 2 A and C). Isotype control antibody-depletion experiments did not impact the difference in tumor growth seen at ST vs. TT (Fig. 2 B and C). The role of CD4+ cells appeared to depend upon the model tested. In B16.F10-bearing C57BL/6 mice, depletion of CD4+ cells had no effect on the tumor growth delay seen at TT (Fig. S3A), but in CT26-bearing BALB/c mice, we found that depletion of CD4+ cells resulted in loss of improved tumor control at TT (Fig. S3B), suggesting an important role for CD4+ cells in the CT26 tumor model. Because CD8+ T cells were consistently identified as critical effector cells in these experiments, we next asked whether there was a difference in CD8+ T-cell frequency in the tumor microenvironment in mice housed at ST and TT. Using flow cytometry, we saw significantly higher numbers of CD3+CD8+ T cells in 4T1 tumors at TT compared with tumors from ST mice (Fig. 3A). Additionally, by immunohistochemistry (IHC), we saw increases in CD8+ cells in CT26 tumors excised from mice maintained at TT and ST (Fig. 3 B and C). These differences were evident even when tumors were small and similarly sized between the two groups. We also looked at the frequency of CD4+ cells by IHC in the tumors of mice housed at ST and TT but we found no difference (Fig. 3B), suggesting that the functional role of CD4+ T cells in mediating improved tumor control at TT in this model occurs independently of their accumulation within the tumor microenvironment.
Fig. 2.
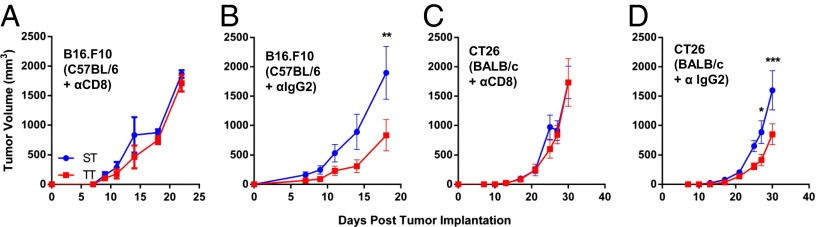
Tumor growth suppression at TT is dependent on CD8+ T cells. Starting on the day of tumor implantation, (A and B) B16.F10 or (C and D) CT26, and every 4 d after, anti-CD8 was injected intraperitoneally to deplete CD8+ cells (A and C) or anti-IgG2 isotype control was injected intrapteritoneally (B and D). Data presented as mean ± SEM (n = 5; *P < 0.05, **P < 0.01, ***P < 0.001; Two-way ANOVA with Bonferroni posttests).
Fig. 3.
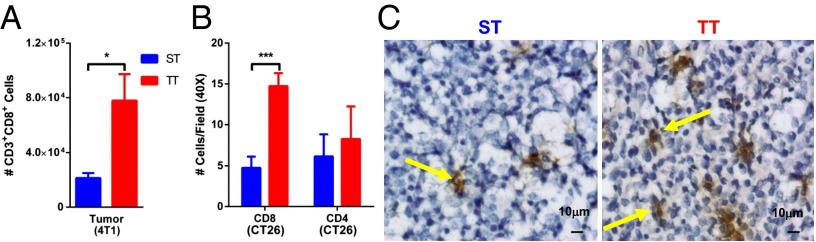
CD8+ T cells are increased at TT. (A) Single-cell suspensions of 4T1 tumors (average volume: ST = 242 ± 20.41 mm3; TT = 222.2 ± 49.94 mm3) were stained for CD3 and CD8 for flow cytometry. Data shown as mean ± SEM (n = 5; *P < 0.05; Student t test). (B and C) CT26 tumors were excised (average volume: ST = 616 ± 133 mm3; TT = 199 ± 67 mm3), frozen and stained for CD4 and CD8 for IHC. Data shown as mean ± SEM (n = 5; ***P < 0.001; Student t test). (C) Representative views of CD8-stained micrographs quantified in B.
To test whether the increased CD8+ T cells at TT were antigen-specific, we used a well-characterized tumor antigen (gp70) in the CT26 model (18) and the H-2Ld pentamer complexed with a gp70423–431 peptide. We observed a significant increase in antigen-specific, pentamer-positive CD8+ T cells in both the tumor (Fig. 4 A and B) and draining lymph node (Fig. 4 D and E) from mice at TT vs. ST. We also observed that the increased numbers of pentamer-positive CD8+ T cells were not influenced by tumor volume (Fig. 4C).
Fig. 4.
Housing mice at TT increases antigen-specific T cells in the tumor and lymph nodes. Single-cell suspensions of (A and B) CT26 tumors (average volume: ST = 746.9 ± 133.3 mm3; TT = 436.8 ± 195.9 mm3) and (D and E) tumor draining lymph nodes were stained with for CD8 and a pentamer specific to H-2Ld presenting a gp70 peptide and analyzed with flow cytometry. Data presented as mean ± SEM (n = 9–10; *P < 0.05; Student t test). (C) Relationship between pentamer-positive T cells analyzed in B and tumor volume (n = 9–10; P < 0.01; ANCOVA).
To explore further the potential mechanism by which T-cell function could be influenced by thermal stress, we examined changes in the CD8+ T-cell subset within the lymphocytic cell population in 4T1 tumors from mice housed at ST and TT. In this experiment, we observed an increase in the CD3+CD8+ T cells (Fig. 5 A and B). We also examined CD69, a marker of early activation, and observed an increase in CD69+CD8+ T cells (Fig. 5C). Next, we quantified the percentage of IFN-γ–producing CD8+ T cells in tumors from mice housed at TT and ST and found that IFN-γ production was significantly increased in mice at TT compared with ST (Fig. 5D). Additionally, we saw that the surface expression of Glut-1 was also increased in CD8+ T cells from TT vs. ST mice, suggesting that CD8+ T cells in mice housed at TT have a more metabolically active phenotype than that seen at ST (Fig. 5E).
Fig. 5.
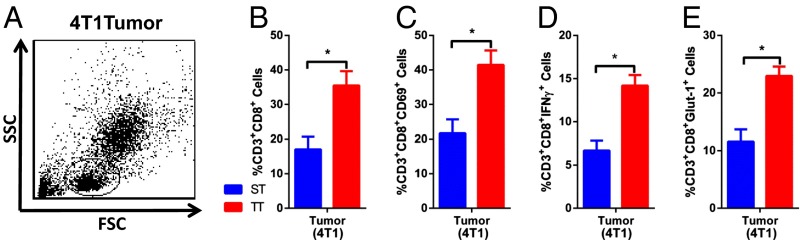
CD8+ T-cell activation is increased at TT. (A) The lymphocyte population identified in single-cell suspensions of 4T1 tumors (average volume: ST = 737.5 ± 117.6 mm3; TT = 394.2 ± 35.69 mm3) was gated on forward and side scatter. The percentage of lymphocytes that were (B) CD3+CD8+, (C) CD69+CD3+CD8+, (D) IFN-γ–producing CD3+CD8+, and (E) Glut-1–expressing CD3+CD8+ were measured. Data presented as mean ± SEM (n = 4–5; *P < 0.05; Student t test).
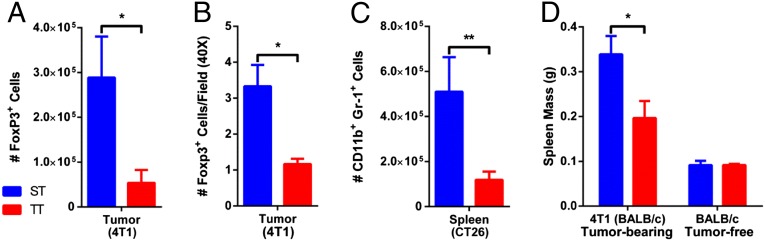
The efficacy of antitumor immune activity is now known to be determined not only by effector (CD8+ T) cell activity, but also the presence of suppressor/regulatory immune cells, including myeloid-derived suppressor cells (MDSCs) and T regulatory cells (Treg), which can inhibit CD8+ T-cell function and facilitate tumor growth (19). We compared the frequency of these immunosupressive cells in tumors and spleens of tumor-bearing mice. Flow cytometry of 4T1 tumors revealed a significant reduction in the number of Treg cells (FoxP3+ cells) in the tumors of mice at TT vs. ST (Fig. 6A and Fig. S4A). By immunohistochemistry, we saw FoxP3+ cells scattered throughout the tumors of ST mice, but saw far fewer cells in the tumor microenvironment of TT mice (Fig. 6B and Fig. S4B).
Fig. 6.
Fewer immunosuppressive cells seen at TT. (A) Single-cell suspensions from 4T1 tumors (average volume: ST = 537.6 ± 119.3 mm3; TT = 128.1 ± 54.07 mm3) were stained for FoxP3 for flow cytometry. (B) 4T1 tumors (average volume: ST = 274 ± 71.43 mm3; TT = 145.6 ± 39.5 mm3) were formalin fixed, sectioned, and stained for FoxP3 for IHC. (C) Single-cell suspensions of spleens from CT26 tumor-bearing mice were stained for Gr-1 and CD11b for flow cytometry. (D) Spleen weight was compared between 4T1 tumor-bearing mice and age-matched controls. Data presented as mean ± SEM (n = 6; *P < 0.05, **P < 0.01; Student t test).
Previous studies (20) have demonstrated that the spleen is a critical repository for MDSCs before their trafficking to the tumor microenvironment. Therefore, we evaluated their numbers in the spleens of tumor-bearing mice housed at ST and TT. There were far fewer splenic MDSCs (Gr-1+CD11b+ cells) in CT26 tumor-bearing mice at TT vs. ST (Fig. 6C). This reduction in MDSCs at TT may also explain, at least in part, our observation that the spleen, although slightly larger than in tumor-free mice, is consistently smaller in tumor-bearing mice at TT compared with ST (Fig. 6D). We also examined Gr-1+CD11b+ cells from tumors, but here we found no difference between ST and TT (Fig. S5). However, as others have pointed out, it can be problematic to analyze MDSC accumulation in the tumor microenvironment, which favors rapid differentiation of MDSCs, thus compromising accurate quantification (21). Overall, these data support the hypothesis that housing tumor-bearing mice at ST shifts the balance of immune cells such that there is a greater potential for systemic immunosuppression, which is consistent with more rapid tumor growth compared with that seen at TT.
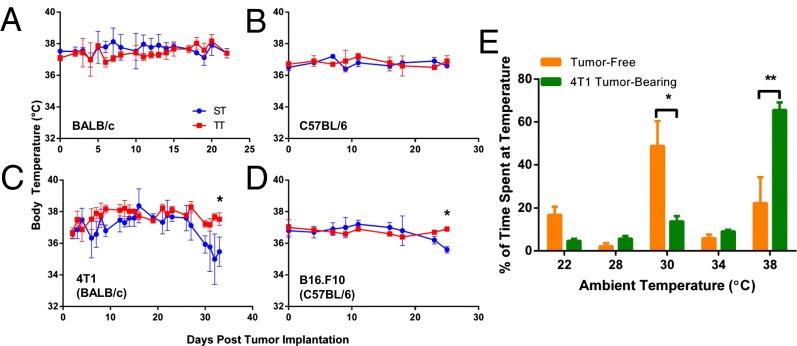
We monitored core temperature in several animals per cage and it should be noted that the body temperature of nontumor (Fig. 7 A and B) and tumor-bearing (Fig. 7 C and D) mice remained normal at ST and TT. However, we observed that when tumors approached the maximal allowable volume (which occurred beyond the time-frame used in all of the analyses presented here), body temperature fell by 1–2° in the ST but not TT mice (Fig. 7 C and D) suggesting that tumor growth eventually exhausted the ability of mice to maintain normal body temperature under subthermoneutral conditions. It is well known that when mice are required to increase their metabolic heat production via thermogenesis, they exhibit long-conserved behavioral changes associated with “feeling cold,” provoking them to move to warmer ambient temperatures, thus minimizing thermogenesis (3, 4, 8, 22). Because caged mice are unable to carry out this behavioral activity, a thermal preference determination is frequently used to measure the degree of cold stress/heat-seeking activity in laboratory mice (6). To address whether the presence of tumors impacts thermal preference, we first placed normal, nontumor-bearing BALB/c mice individually into a thermal preference apparatus (23), which permitted them to move between different chambers maintained at 22, 28, 30, 34, or 38 °C. We observed that the mice spent the most time in the 30 °C chamber (rather than the 22 °C chamber), confirming many previous studies (reviewed in ref. 4) showing that normal mice prefer a thermoneutral environment. In contrast, tumor-bearing mice preferred to spend the majority of their time in the warmest chamber available, at 38 °C (Fig. 7E). This shift in preferred temperature is 8° warmer than that seen in nontumor-bearing mice and, remarkably, 16° warmer than the temperature at which tumor-bearing mice are routinely housed. Thus, tumor-bearing mice “feel colder” than nontumor-bearing mice, selecting ambient temperatures that are even greater than thermoneutrality.
Fig. 7.
Tumor-bearing mice demonstrate increased heat-seeking behavior despite maintaining a normal body temperature for a prolonged period. (A–D) The body temperature of nontumor-bearing (A and B) and tumor-bearing (C and D) mice was monitored over several weeks in either ST or TT housing conditions. (E) 4T1 cells were orthotopically injected into the mammary fat pad. Mice (average volume in the tumor-bearing group was 483 ± 102 mm3) and tumor-free age-matched controls were placed individually into a thermal preference apparatus for 1 h and the time a spent at each temperature was calculated. Data presented as mean ± SEM (n = 5; *P < 0.05, **P < 0.01; Student t test).
Discussion
These observations demonstrate that tumor growth control is significantly impaired by housing mice at ST compared to TT, even though core body temperatures are similar, reflecting changes in the balance between immune effector (antitumor) and immunosuppressive (protumor) immune cells. Not only are there more CD8+ T cells (and fewer immune suppressive cells) in the tumor microenvironment of mice housed at TT vs. ST, these T cells exhibit increased IFN-γ production and a higher expression of the activation markers CD69 and Glut-1. Our observation that tumor-bearing mice prefer higher temperatures than nontumor-bearing mice supports the idea that the presence of a tumor exacerbates the effects of cold stress. In other words, the presence of metabolic stress associated with tumor growth may compound the impact of mild cold stress on the immune system since only tumor-bearing mice exhibited obvious differences in endogenous immune cell populations at ST vs. TT. Our data raise the possibility that our current understanding of the ability of laboratory mice to control tumor growth has been limited by conducting experiments in an environment of chronic cold stress. Recent research highlights the large bio-energetic cost of generating and maintaining an effective T-cell–mediated immune response (24, 25). It has been suggested that when immunological defenses are too energetically costly, they are selectively “traded off” in favor of higher-priority functions, such as thermoregulation (26–28). An increase in the activity of norepinephrine-driven stress responses likely is one of the underlying mechanisms involved here since cold exposure has long been used to study the activation of thermogenesis via norepinephrine production (8, 12), and this, as well as other, stress pathways have been linked to immunosuppression (29).
However, the interrelationships between stress signals and immune cells in the tumor microenvironment remain unclear. For example, what are the underlying mechanisms by which the antitumor immune cell function is modulated by systemic stress? Key intracellular mediators that should be evaluated include target of rapamycin and molecules that activate (PI3K and AKT) and antagonize (AMPK) its regulation. PIM1 and PIM2 may also serve as target molecules that may be impacted by cold stress (24). Further, studies should examine how immune cell proliferation compares between mice at ST and TT. It would also be important to determine how a more vigorous, endogenous host immune response at TT affects immunoediting of tumor cells in mouse models (30), and to examine to what extent ambient temperature impacts models of vaccination, adoptive cellular therapy, and other forms of immunotherapy as well as the potential for autoimmunity. Addressing these questions will require a greater dissection of functional changes among various immune cell subsets.
Determining how the balance of proinflammatory and suppressive cellular and cytokine/chemokine networks is affected by ambient temperature will also be informative. As recently demonstrated (12), increased norepinephrine produced as a result of cold stress originates from an alternatively activated subset of macrophages to help facilitate heat production. Importantly, this phenotype is induced by increased levels of IL-4 and IL-13, both of which can contribute to a more immunosuppressive microenvironment. Norepinephrine is also known to drive adaptive thermogenesis by a process that depends upon induction of UCP1 in the mitochondria of brown fat cells and other cell types for heat generation. Thus, we might expect to see more UCP1 in the mitochondria of tumor-bearing cold-stressed mice, and this is an important question to explore in the future.
This study does not aim to identify an optimal housing condition for all laboratory mice. Unlike the constant temperature caged mice experience, in nature, mice are free to move about over a wide range of temperatures and build warm nests that numerous mice occupy for long periods of time (6). Our data do suggest that tumor-bearing mice may be experiencing an underappreciated degree of metabolic cold stress, and changes to their caging conditions might be considered for assessment of tumor growth and antitumor immunity. Further, when mice are caged in groups of four or five, their huddling helps somewhat to reduce thermal stress; however, mice are often removed during the course of an experiment, reducing the potential for heat generation through huddling. Given the fact that the result of testing a new therapy in mouse models often fails to predict what will happen in humans (1, 2), our study highlights the importance of establishing a more accurate “baseline” of endogenous immune responsiveness. This would be important not only for studies of immunotherapy, but also for radiation and chemotherapy, which are increasingly recognized to be dependent upon antitumor immune responses (31). A practical alternative to adjusting actual ambient temperature may be to add more appropriate nesting material to cages (6) or to use cabinets in which environmental temperature can be manipulated, as used in this study. In line with this theory, our laboratory and that of others, have long been interested in the positive effects of temporarily inducing a mild (fever range, ∼39 °C) hyperthermia on various immune cells in mice (32), where (unlike the experiments reported here) body temperature is transiently elevated for several hours. Because short-term mild hyperthermia causes a similar increase of CD8+ T cells and a decrease of Treg cells (32, 33) in the tumor microenvironment, an open question is whether these therapeutically beneficial effects are functionally linked to alleviation of cold stress.
More tumor models, such as transgenic cancer-prone mice, must be evaluated to fully appreciate how cold stress affects therapeutic outcome. Further, each of the models we tested here (transplantable solid tumor models, metastases and carcinogen-induced tumors) grow as solid tumors. Therefore disseminated hematological malignancies, or cancers that grow as ascites in the peritoneal cavity should examined for their responsiveness to cold stress since the type of tumor microenvironment could be critical for how sympathetic nerve endings or metabolic factors associated with cold stress interact with immune cells.
In summary, while the precise mechanistic pathways linking metabolic cold stress and antitumor immunity are not yet defined, this study demonstrates that it is important to consider ambient temperature when cancer or metabolic disorders are modeled in mice. But do these data also have implications for cancer patients? While it is already clear that an abnormal energy balance in humans is linked to obesity, inflammatory disease, and increased risk of several cancers (34), a role for thermogenesis is much less appreciated. Unlike mice, humans are able to easily manipulate their environment to achieve thermal comfort. However, cold stress is among other stressors more commonly experienced by humans including psychological or emotional stress that are mediated through the sympathetic arm of the nervous system and which may be significantly increased after cancer. Moreover, while until quite recently, a significant role for brown fat thermogenesis in humans has not been well recognized, new studies have demonstrated the presence of brown fat in humans, it has now been demonstrated to be quite metabolically active as a result of cold stress (35, 36). Whether thermogenesis is playing a role in regulation of antitumor immunity in patients is not known, but in light of this recent data and the findings reported here, it is intriguing to consider that symptoms of deep “chills” (often independent of fever) that are well known to occur in cancer patients (37), including those receiving various therapies including immunotherapies such as IL-2 (38, 39), could be affecting metabolism associated with thermogenesis. Clearly a better understanding of the physiological interactions between stress responses, thermoregulation, and immune regulation could reveal important new strategies for strengthening antitumor immunity.
Materials and Methods
Mouse Housing at ST and TT.
Mice were maintained in specific pathogen-free facilities and were treated in accordance with the guidelines established by the Institutional Animal Care and Use Committee at Roswell Park Cancer Institute (Buffalo, NY). Cages containing experimental mice were housed five to a cage in Precision Refrigerated Plant-Growth Incubators (Thermo Fisher Scientific) maintained at 22° or 30 °C. Several experiments were repeated (with no differences observed) by placing cages in standard Division of Laboratory Animal Resources rooms with the thermostat set to 22° or 30 °C. Humidity was controlled using a Top Fin® Air Pump AIR 1000 with Top Fin® airline tubing.
Body Temperature Measurements.
Body temperature was measured with a DAS-7007S reader (Bio Medic Data Systems) detecting signal from an IPTT-300 transponder (Bio Medic Data Systems) implanted beneath the skin.
Additional details are provided in SI Materials and Methods.
Thermal Preference.
The experimental design has been previously described (23). Movement was traced using an Auto-Track Open Field Activity Monitor Opto-Varimex-3 (Columbus Instruments). Each mouse was tracked for 60 min per day.
Supplementary Material
Acknowledgments
We thank Drs. Kelvin Lee, John Subjeck, Sharon Evans, Aimin Jiang, and Carl Anderson, Chunmei Fu, Chelsey Reed and Jeanne Prendergast for their discussions or technical and editorial assistance. This research was supported by National Institute of Heath Grants R01 CA135368 (to E.A.R.), R01 CA140622 (to S.I.A.), and T32 CA085183; the Roswell Park Cancer Institute Alliance Foundation; the James L. Desiderio Endowment Fund; and used Shared Resources supported by the Roswell Park Cancer Institute’s Comprehensive Cancer Center Support Grant CA016056. Dr. Christopher Gordon, an author who works at the US Environmental Protection, adds the following disclaimer: The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304291110/-/DCSupplemental.
References
- 1.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9(11):4227–4239. [PubMed] [Google Scholar]
- 2.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terrien J, Perret M, Aujard F. Behavioral thermoregulation in mammals: A review. Front Biosci (Landmark Ed) 2011;16:1428–1444. doi: 10.2741/3797. [DOI] [PubMed] [Google Scholar]
- 4.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Therm Biol. 2012;37:654–685. [Google Scholar]
- 5.Lodhi IJ, Semenkovich CF. Why we should put clothes on mice. Cell Metab. 2009;9(2):111–112. doi: 10.1016/j.cmet.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Gaskill BN, et al. Heat or insulation: Behavioral titration of mouse preference for warmth or access to a nest. PLoS ONE. 2012;7(3):e32799. doi: 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karp CL. Unstressing intemperate models: How cold stress undermines mouse modeling. J Exp Med. 2012;209(6):1069–1074. doi: 10.1084/jem.20120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th Ed. Washington, DC: National Academies; 2011. [PubMed] [Google Scholar]
- 10.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: Dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1244–R1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- 11.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen KD, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480(7375):104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pulaski BA, Ostrand-Rosenberg S (2001) Mouse 4T1 breast tumor model. Curr Protoc Immunol May;Chapter 20:Unit 20.2. [DOI] [PubMed]
- 14.Danna EA, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64(6):2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 15.Swann JB, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA. 2008;105(2):652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 17.Teng MW, et al. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc Natl Acad Sci USA. 2010;107(18):8328–8333. doi: 10.1073/pnas.1003251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang AY, et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93(18):9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostrand-Rosenberg S. Immune surveillance: A balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18(1):11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortez-Retamozo V, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci USA. 2012;109(7):2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corzo CA, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overton JM. Phenotyping small animals as models for the human metabolic syndrome: Thermoneutrality matters. Int J Obes (Lond) 2010;34(Suppl 2):S53–S58. doi: 10.1038/ijo.2010.240. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu I, et al. Enhanced thermal avoidance in mice lacking the ATP receptor P2X3. Pain. 2005;116(1-2):96–108. doi: 10.1016/j.pain.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: Energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5(11):844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 25.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- 27.Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: Mediation by physiological trade-offs. Philos Trans R Soc Lond B Biol Sci. 2008;363(1490):321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauw WM. Immune response from a resource allocation perspective. Front Genet. 2012;3:267–280. doi: 10.3389/fgene.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24(8):444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 31.Zitvogel L, et al. The anticancer immune response: Indispensable for therapeutic success? J Clin Invest. 2008;118(6):1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res. 2013;1(4):1–7. doi: 10.1158/2326-6066.CIR-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher DT, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121(10):3846–3859. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28(26):4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannon B, Nedergaard J. Yes, even human brown fat is on fire! J Clin Invest. 2012;122(2):486–489. doi: 10.1172/JCI60941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouellet V, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122(2):545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kokolus KM, Hong CC, Repasky EA. Feeling too hot or cold after breast cancer: Is it just a nuisance or a potentially important prognostic factor? Int J Hyperthermia. 2010;26(7):662–680. doi: 10.3109/02656736.2010.507235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fyfe G, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 39.Lotze MT, et al. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985;135(4):2865–2875. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.