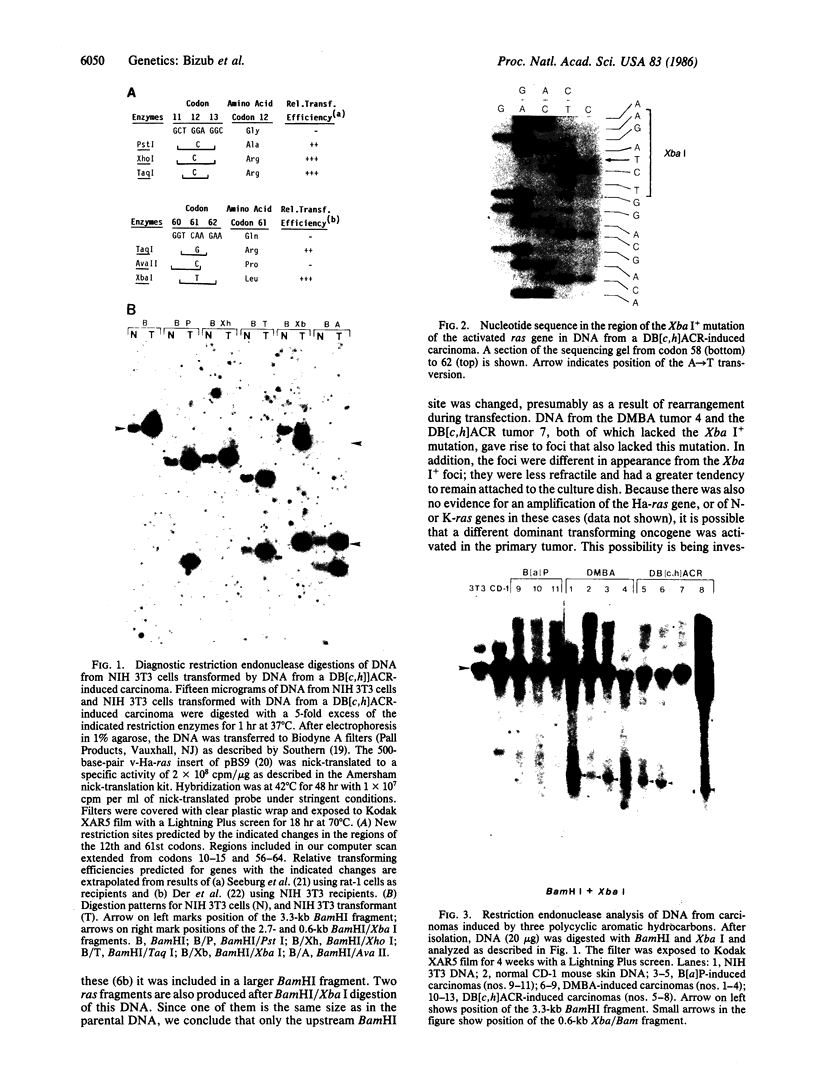
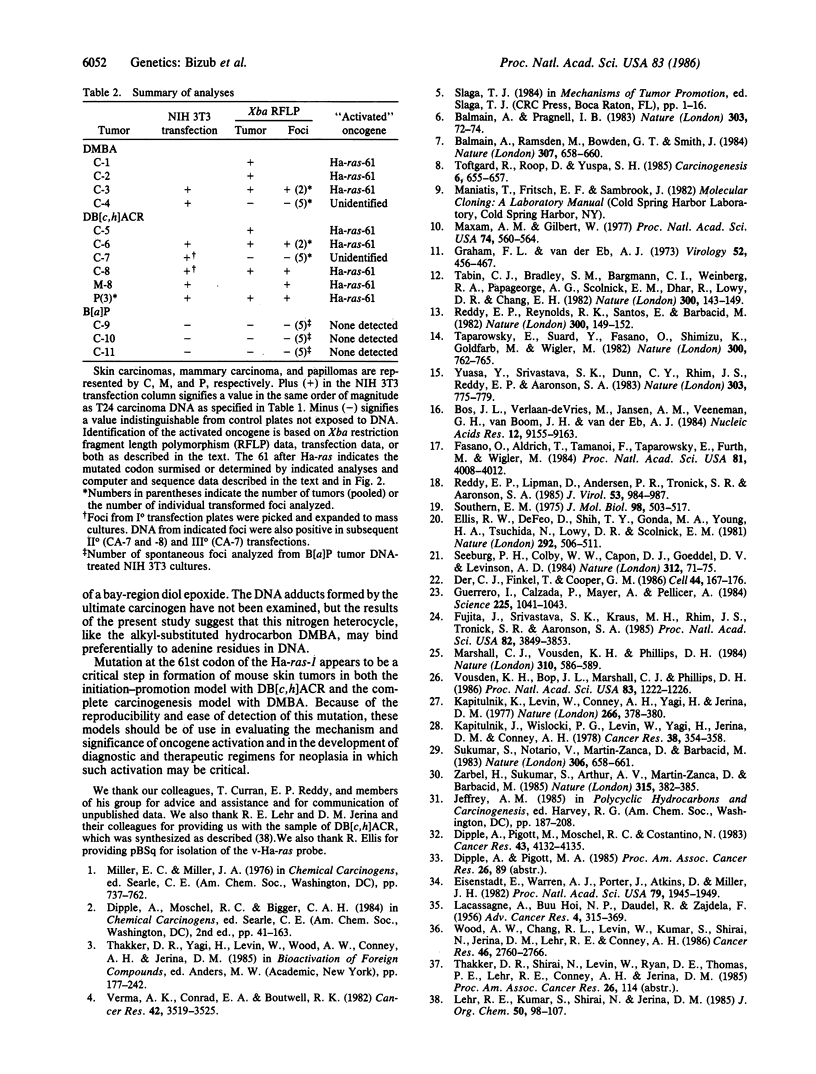
Abstract
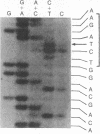
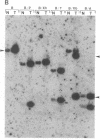
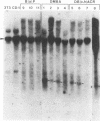
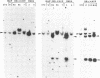
The importance of mutational activation of the Ha-ras protooncogene in polycyclic aromatic hydrocarbon-induced mouse skin tumors was investigated in a complete carcinogenesis model using repetitive applications of 7,12-dimethylbenz[a]anthracene (DMBA), or in an initiation-promotion model using a single application of dibenz[c,h]acridine (DB[c,h]ACR) or benzo[a]pyrene (B[a]BP) followed by chronic treatment with phorbol 12-myristate 13-acetate. DNA isolated from carcinomas induced by DMBA or DB[c,h]ACR, but not by B[a]P, efficiently transformed NIH 3T3 cells, and a high percentage of the transformed foci had an amplified Ha-ras gene. Restriction enzyme Southern blot analysis and DNA sequencing revealed that the amplified Ha-ras genes of the transformants had an A----T transversion in the second position of the 61st codon. The same mutation was also detected in primary tumor DNA in a high percentage of the DMBA- or DB[c,h]ACR-induced carcinomas. Identification of the mutation in NIH 3T3 cells transformed with DNA from DB[c,h]ACR-induced benign skin papillomas suggests that it is an early event in skin carcinogenesis. Thus, mutation of the 61st codon of the Ha-ras-1 gene appears to be a critical step in the formation of mouse skin tumors induced in both of the two models tested. Our analyses also delineate two other classes of hydrocarbon-induced carcinomas--namely, tumors whose DNAs efficiently transform 3T3 cells but do not contain mutated ras genes and tumors whose DNAs do not transform 3T3 cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUU-HOI N. P., DAUDEL R., LACASSAGNE A., ZAJDELA F. The relation between carcinogenic activity and the physical and chemical properties of angular benzacridines. Adv Cancer Res. 1956;4:315–369. doi: 10.1016/s0065-230x(08)60727-7. [DOI] [PubMed] [Google Scholar]
- Balmain A., Pragnell I. B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Balmain A., Ramsden M., Bowden G. T., Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Verlaan-de Vries M., Jansen A. M., Veeneman G. H., van Boom J. H., van der Eb A. J. Three different mutations in codon 61 of the human N-ras gene detected by synthetic oligonucleotide hybridization. Nucleic Acids Res. 1984 Dec 11;12(23):9155–9163. doi: 10.1093/nar/12.23.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Dipple A., Pigott M., Moschel R. C., Costantino N. Evidence that binding of 7,12-dimethylbenz(a)anthracene to DNA in mouse embryo cell cultures results in extensive substitution of both adenine and guanine residues. Cancer Res. 1983 Sep;43(9):4132–4135. [PubMed] [Google Scholar]
- Eisenstadt E., Warren A. J., Porter J., Atkins D., Miller J. H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Fasano O., Aldrich T., Tamanoi F., Taparowsky E., Furth M., Wigler M. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4008–4012. doi: 10.1073/pnas.81.13.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita J., Srivastava S. K., Kraus M. H., Rhim J. S., Tronick S. R., Aaronson S. A. Frequency of molecular alterations affecting ras protooncogenes in human urinary tract tumors. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3849–3853. doi: 10.1073/pnas.82.11.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., D'Eustachio P., Pellicer A. Isolation, characterization, and chromosome assignment of mouse N-ras gene from carcinogen-induced thymic lymphoma. Science. 1984 Sep 7;225(4666):1041–1043. doi: 10.1126/science.6089339. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Levin W., Conney A. H., Yagi H., Jerina D. M. Benzo[a]pyrene 7,8-dihydrodiol is more carcinogenic than benzo[a]pyrene in newborn mice. Nature. 1977 Mar 24;266(5600):378–380. doi: 10.1038/266378a0. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Wislocki P. G., Levin W., Yagi H., Jerina D. M., Conney A. H. Tumorigenicity studies with diol-epoxides of benzo(a)pyrene which indicate that (+/-)-trans-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene is an ultimate carcinogen in newborn mice. Cancer Res. 1978 Feb;38(2):354–358. [PubMed] [Google Scholar]
- Marshall C. J., Vousden K. H., Phillips D. H. Activation of c-Ha-ras-1 proto-oncogene by in vitro modification with a chemical carcinogen, benzo(a)pyrene diol-epoxide. Nature. 1984 Aug 16;310(5978):586–589. doi: 10.1038/310586a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Lipman D., Andersen P. R., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of the BALB/c murine sarcoma virus transforming gene. J Virol. 1985 Mar;53(3):984–987. doi: 10.1128/jvi.53.3.984-987.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Colby W. W., Capon D. J., Goeddel D. V., Levinson A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984 Nov 1;312(5989):71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Toftgard R., Roop D. R., Yuspa S. H. Proto-oncogene expression during two-stage carcinogenesis in mouse skin. Carcinogenesis. 1985 Apr;6(4):655–657. doi: 10.1093/carcin/6.4.655. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Conrad E. A., Boutwell R. K. Differential effects of retinoic acid and 7,8-benzoflavone on the induction of mouse skin tumors by the complete carcinogenesis process and by the initiation-promotion regimen. Cancer Res. 1982 Sep;42(9):3519–3525. [PubMed] [Google Scholar]
- Vousden K. H., Bos J. L., Marshall C. J., Phillips D. H. Mutations activating human c-Ha-ras1 protooncogene (HRAS1) induced by chemical carcinogens and depurination. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1222–1226. doi: 10.1073/pnas.83.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. W., Chang R. L., Levin W., Kumar S., Shirai N., Jerina D. M., Lehr R. E., Conney A. H. Bacterial and mammalian cell mutagenicity of four optically active bay-region 3,4-diol-1,2-epoxides and other derivatives of the nitrogen heterocycle dibenz[c,h]acridine. Cancer Res. 1986 Jun;46(6):2760–2766. [PubMed] [Google Scholar]
- Yuasa Y., Srivastava S. K., Dunn C. Y., Rhim J. S., Reddy E. P., Aaronson S. A. Acquisition of transforming properties by alternative point mutations within c-bas/has human proto-oncogene. Nature. 1983 Jun 30;303(5920):775–779. doi: 10.1038/303775a0. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]