Significance
Targeting the Wnt pathway in cancer is an attractive therapeutic approach. However, success has been limited because of the lack of effective therapeutic agents and the lack of biomarkers to define the patient population that would benefit from such a therapy. Herein, we report the discovery of LGK974, a drug that targets Porcupine, a Wnt-specific acyltransferase. We show that LGK974 potently inhibits Wnt signaling, has strong efficacy in rodent tumor models, and is well-tolerated. We also show that head and neck cancer cell lines with loss-of-function mutations in the Notch signaling pathway have a high response rate to LGK974. Together, these findings provide a strategy and tools for targeting Wnt-driven cancer.
Keywords: Wnt inhibition, β-catenin, HNSCC
Abstract
Wnt signaling is one of the key oncogenic pathways in multiple cancers, and targeting this pathway is an attractive therapeutic approach. However, therapeutic success has been limited because of the lack of therapeutic agents for targets in the Wnt pathway and the lack of a defined patient population that would be sensitive to a Wnt inhibitor. We developed a screen for small molecules that block Wnt secretion. This effort led to the discovery of LGK974, a potent and specific small-molecule Porcupine (PORCN) inhibitor. PORCN is a membrane-bound O-acyltransferase that is required for and dedicated to palmitoylation of Wnt ligands, a necessary step in the processing of Wnt ligand secretion. We show that LGK974 potently inhibits Wnt signaling in vitro and in vivo, including reduction of the Wnt-dependent LRP6 phosphorylation and the expression of Wnt target genes, such as AXIN2. LGK974 is potent and efficacious in multiple tumor models at well-tolerated doses in vivo, including murine and rat mechanistic breast cancer models driven by MMTV–Wnt1 and a human head and neck squamous cell carcinoma model (HN30). We also show that head and neck cancer cell lines with loss-of-function mutations in the Notch signaling pathway have a high response rate to LGK974. Together, these findings provide both a strategy and tools for targeting Wnt-driven cancers through the inhibition of PORCN.
Wnt signaling is a key oncogenic pathway in multiple cancers (1, 2). On binding to its receptors LRP5/6 and Frizzled (FZD) at the plasma membrane, Wnt ligand triggers the disruption of the β-catenin degradation machinery (consisting of AXIN2, GSK3β, APC, and others), leading to the accumulation of β-catenin in the cytoplasm (3). Elevated levels of β-catenin ultimately lead to its translocation into the nucleus to form a complex with LEF/TCF and drive downstream gene expression (3). Dysregulation of Wnt signaling (1) can occur through mutations of downstream components, such as APC and β-catenin, which are well-documented in colon cancer (1). In addition, overexpression of Wnt ligands or costimulants, such as R-Spondin 2/3 (RSPO2/3), or silencing of Wnt inhibitor genes has been reported in various cancers (1, 4). Mutations of pan-Wnt pathway components, such as AXIN1/2 or the RSPO coreceptors RNF43/ZNFR3, may potentially play key roles in pancreatic, colon, and hepatocellular carcinoma (4–6).
The Wnt gene was originally identified as an oncogene in murine mammary tumors 30 y ago (2) and confirmed to be a key oncogenic pathway in many studies, including an unbiased insertional mutagenesis screen, with Wnt1, Wnt3, and Wnt3A comprising 38% of all unbiased hits (7). In human breast cancer, overexpression of Wnt or silencing of Wnt inhibitor genes has been observed in up to one-half of breast cancer samples (8). Cytoplasmic and nuclear β-catenin have also been correlated with triple-negative and basal-like breast cancer subtypes (9, 10), and Wnt signaling has also been implicated in cancer-initiating cells in multiple cancer types (11–14). Wnt pathway signaling activity is dependent on Wnt ligand. During the biosynthesis of Wnt ligands, Wnt undergoes posttranslational acylation (palmitoylation) that is mediated by Porcupine (PORCN), a membrane bound O-acyltransferase (3, 15). PORCN is specific and dedicated to Wnt posttranslational acylation, which is required for subsequent Wnt secretion (16). Loss of PORCN leads to inhibition of Wnt ligand-driven signaling activities in KO mouse models (17, 18). In humans, loss-of-function (LoF) mutations in the PORCN gene cause focal dermal hypoplasia, an X-linked dominant disorder associated with a variety of congenital abnormalities in both heterozygotes and those individuals with mosaicism for the PORCN gene. This phenotype is consistent with the role of the Wnt signaling pathway during embryogenesis and development (15).
Given the key role of Wnt signaling in cancer, targeting this pathway has been an attractive therapeutic approach. However, success has been limited because of the lack of effective therapeutic agents for targets in the Wnt pathway and the lack of a defined patient population that would be sensitive to a Wnt inhibitor. Herein, we describe a cellular high-throughput screen for small molecules that block Wnt secretion. This effort led to the discovery of LGK974, a potent and specific PORCN inhibitor. We show that LGK974 potently inhibits Wnt signaling in vitro and in vivo and has strong efficacy in tumor models in vivo. We also show that head and neck squamous cell carcinoma cell lines with LoF mutations in the Notch signaling pathway have a high response rate to LGK974. These findings provide a path forward to target Wnt-driven cancer through the inhibition of PORCN.
Results
Cellular High-Throughput Screen for Wnt Secretion Inhibitors.
To identify small-molecule Wnt signaling inhibitors, a cellular Wnt pathway-based screen was performed against ∼2.4 million compounds. In this assay, Wnt-secreting cells, a stable L-cell line overexpressing Wnt3A, were cocultured with the Wnt reporter cells, a TM3 cell line harboring a luciferase reporter gene (19) driven by Wnt-responsive promoter elements. The level of the luciferase reporter gene readout was used as an indicator for Wnt ligand-driven, LEF/TCF-dependent transcriptional activities. Compounds showing the greatest activity in the screen were reconfirmed and further triaged through a set of secondary assays as follows. To distinguish the hits from functioning in a parallel signaling pathway, a Hedgehog (HH) coculture assay analogous to the Wnt assay was set up as a counterscreen. The HH pathway bears much similarity to the Wnt pathway, including biosynthesis and posttranslational modification of the ligand, receptor stimulation, and downstream transcriptional activation (20). To distinguish hits that function upstream or downstream of Wnt secretion, Wnt3A conditioned medium (CM) was added back into the cell culture medium in the context of the Wnt reporter cells. Specific Wnt secretion inhibitors should be active in the Wnt coculture assay but not the HH coculture or Wnt3A CM assays. These compounds were further characterized to elucidate their potential targets, including the two known dedicated nodes required for Wnt secretion: Wntless, a 7-pass transmembrane protein that is responsible for Wnt intracellular trafficking, and PORCN (3, 15).
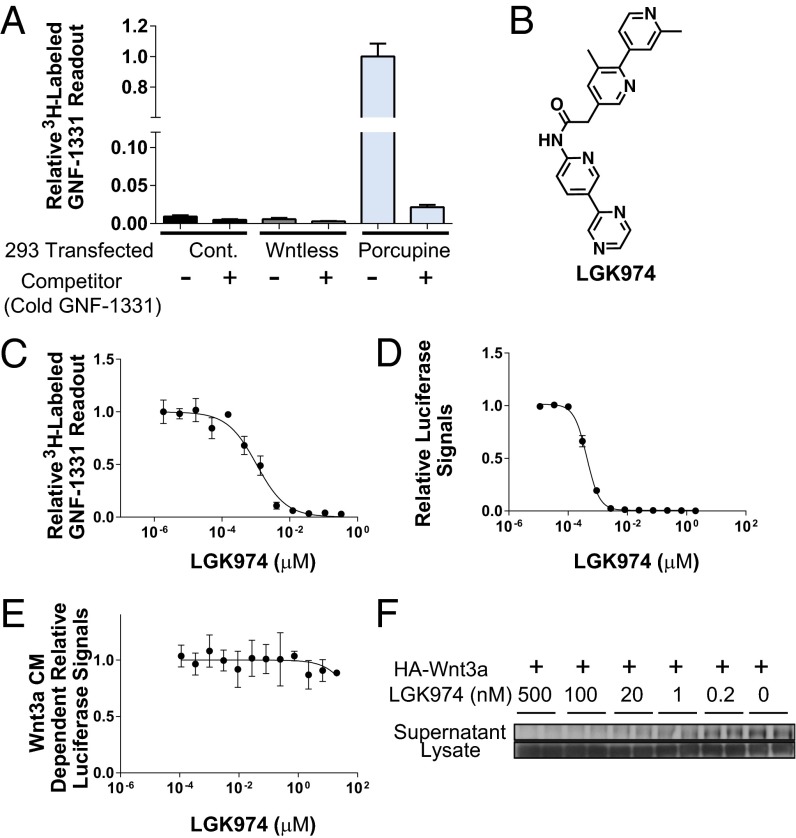
GNF-1331 (Fig. S1A), one of the chemical classes identified from the screen, was selected for tritium labeling and subsequently used in a radioligand binding assay. As shown in Fig. 1A, [3H]-GNF-1331 bound well to the membrane preparations from 293 cells transfected with PORCN but not to the membrane preparations from cells transfected with Wntless or a vector control, suggesting that GNF-1331 specifically interacted with PORCN. Furthermore, the specific interaction between GNF-1331 and PORCN could be competed off by unlabeled GNF-1331 (Fig. 1A).
Fig. 1.
Biochemical and cellular activities of LGK974. (A) PORCN identified as the target of Wnt secretion inhibitor, GNF-1331. [3H]-GNF-1331 binds to the membrane preps made from PORCN-transfected 293T cells but not from Wntless or vector control-transfected 293T cells. The binding signals could be competed off by 70-fold unlabeled GNF-1331. (B) The structure of LGK974. (C) LGK974 interacts with PORCN. LGK974 competes off [3H]-GNF-1331 in a dose-dependent manner. (D) LGK974 inhibits Wnt signaling reporter assay with an IC50 of 0.4 nM. (E) The cellular activities of LGK974 were rescued in the presence of Wnt3A CM. (F) LGK974 strongly inhibited Wnt secretion in 293A cells transfected with human HA-Wnt3A.
Inhibition of PORCN by LGK974 Blocks Wnt Signaling in Vitro.
Medicinal chemistry optimization of GNF-1331 was carried out to improve potency and pharmacokinetic properties. This effort led to the discovery of LGK974 (Fig. 1B), a highly specific and potent PORCN inhibitor. LGK974 effectively displaced [3H]-GNF-1331 with an IC50 of 1 nM in the PORCN radioligand binding assay (Fig. 1C and Fig. S1B). LGK974 also potently inhibited Wnt signaling in the aforementioned Wnt coculture assay with an IC50 of 0.4 nM (Fig. 1D). This inhibitory effect was rescued by the addition of exogenous Wnt3A CM (Fig. 1E). Additionally, LGK974 showed no major cytotoxicity in cells up to 20 µM (Fig. S1C). To further confirm the activity of LGK974 in blocking PORCN-dependent Wnt secretion, 293A cells were transfected with HA-tagged Wnt3A and treated with various doses of LGK974. As shown in Fig. 1F and Fig. S1D, LGK974 potently decreased levels of HA-Wnt3A in the supernatant with slightly increased levels of HA-Wnt3A in the cell lysate, suggesting that Wnt3A secretion was substantially inhibited by LGK974 in a dose-dependent manner. In Wnt-responsive cells, secreted Wnts cause phosphorylation of the Wnt coreceptor LRP6. In L-Wnt3A cells, a mouse cell line overexpressing Wnt3A, LGK974, strongly blocked Wnt-dependent phosphorylation of LRP6 (Fig. S1E).
Inhibition of PORCN by LGK974 Blocks All Wnts Tested.
Based on genetic KOs, PORCN is known to affect posttranslational palmitoylation of all Wnt ligands (3, 15). Indeed, the residues around the universal Wnt palmitoylation site, Ser209, are conserved among all 19 Wnts (Fig. S1F), and the CHG×SGSC palmitoylation motif was not identified in any other protein throughout the proteome (http://www.genome.jp/tools/motif/MOTIF2.html). To test if LGK974 can recapitulate the consequences of genetic loss of PORCN on Wnt processing, we tested a set of canonical Wnts, including Wnt1, -2, -3, -3A, -6, -7A, and -9A, in Wnt-dependent reporter assays. As shown in Fig. S1G, LGK974 showed comparable inhibitory activities against all tested Wnts, which is consistent with the genetic loss of PORCN phenotype.
LGK974 Induces Tumor Regression at a Well-Tolerated Dose in a Wnt-Driven Murine Tumor Model.
Given the pivotal role of Wnt signaling in tissue stem cell renewal, achieving an acceptable therapeutic index with targeted inhibition of Wnt signaling poses an important clinical challenge (3). The discovery of the potent, selective, and orally bioavailable PORCN inhibitor LGK974 enabled us to investigate this issue using a well-established Wnt-dependent murine breast tumor model, the mouse mammary tumor virus (MMTV)-driven Wnt1 model (2).
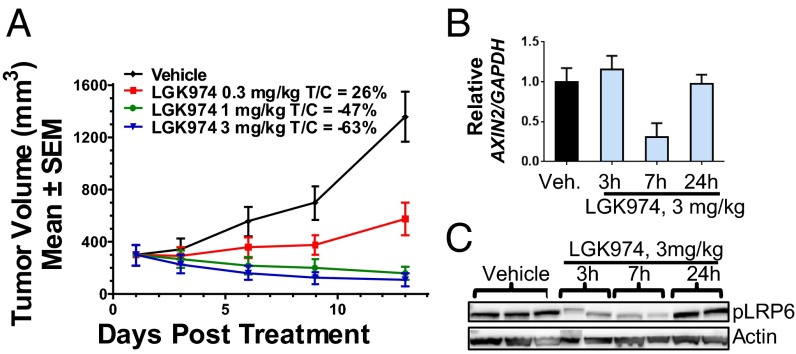
In a murine MMTV-Wnt1 tumor model using s.c. implanted tumor fragments derived from MMTV-Wnt1 transgenic mice, LGK974 exhibited strong dose-dependent efficacy when administered daily (Fig. 2A). Briefly, changes in tumor volume for each of the treated (T) and control (C) groups were measured and used to calculate growth delay as expressed by the T/C ratio. A dose of 0.3 mg/kg LGK974 led to tumor growth delay (T/C: 26%), whereas a dose of 1 or 3 mg/kg induced very significant tumor regression (T/C: −47% or −63%, respectively) on day 13 of treatment. As shown in Fig. S2A, the regimen was well-tolerated without significant body weight loss in the mice. Similar efficacy was observed with LGK974 in a murine MMTV-Wnt3 model (Fig. S2B).
Fig. 2.
LGK974 inhibits Wnt signaling in vivo and induces tumor regression in a mechanistic MMTV-Wnt1 tumor model. (A) LGK974 showed a strong efficacy in a Wnt tumor model (MMTV-Wnt) in nude mice. Spontaneous tumors from the MMTV-Wnt1 transgenic mice were implanted in nude mice. LGK974 was dosed at 0.3, 1.0, and 3.0 mg/kg per day for 13 d. LGK974 induced robust tumor regression at 1.0- and 3.0-mg/kg doses. (B) A PD study was performed in the murine MMTV-Wnt tumor model. LGK974 significantly inhibited AXIN2 expression 7 h after the last dose, and the effect diminished 24 h after the dose. (C) In the same PD study, pLRP6 expression level showed a very similar pattern to the PD response of AXIN2. LGK974 inhibitory effect peaked at 7 h after the last dose and diminished 24 h after the last dose.
To correlate the observed antitumor activity with the inhibition of Wnt signaling, both distal (AXIN2 mRNA) and proximal (phospho-LRP6) Wnt signaling events were examined by quantitative RT-PCR through TaqMan assays and Western blot analysis, respectively. As shown in Fig. 2 B and C, a dose of 3 mg/kg LGK974 inhibited both AXIN2 expression and phospho-LRP6 (pLRP6) levels 7 h after the last dose to a greater degree than the inhibition seen at 3 h. Both AXIN2 expression and pLRP6 levels were back to baseline levels 24 h after treatment, suggesting that sustained pathway inhibition was not required to achieve antitumor activities. Consistent with our results, a recent publication using a different PORCN inhibitor from our patent (21) also showed good efficacy in this model at a well-tolerated dose (22).
LGK974 Is Well-Tolerated In Wnt-Dependent Tissues at the Efficacious Dose in Rats.
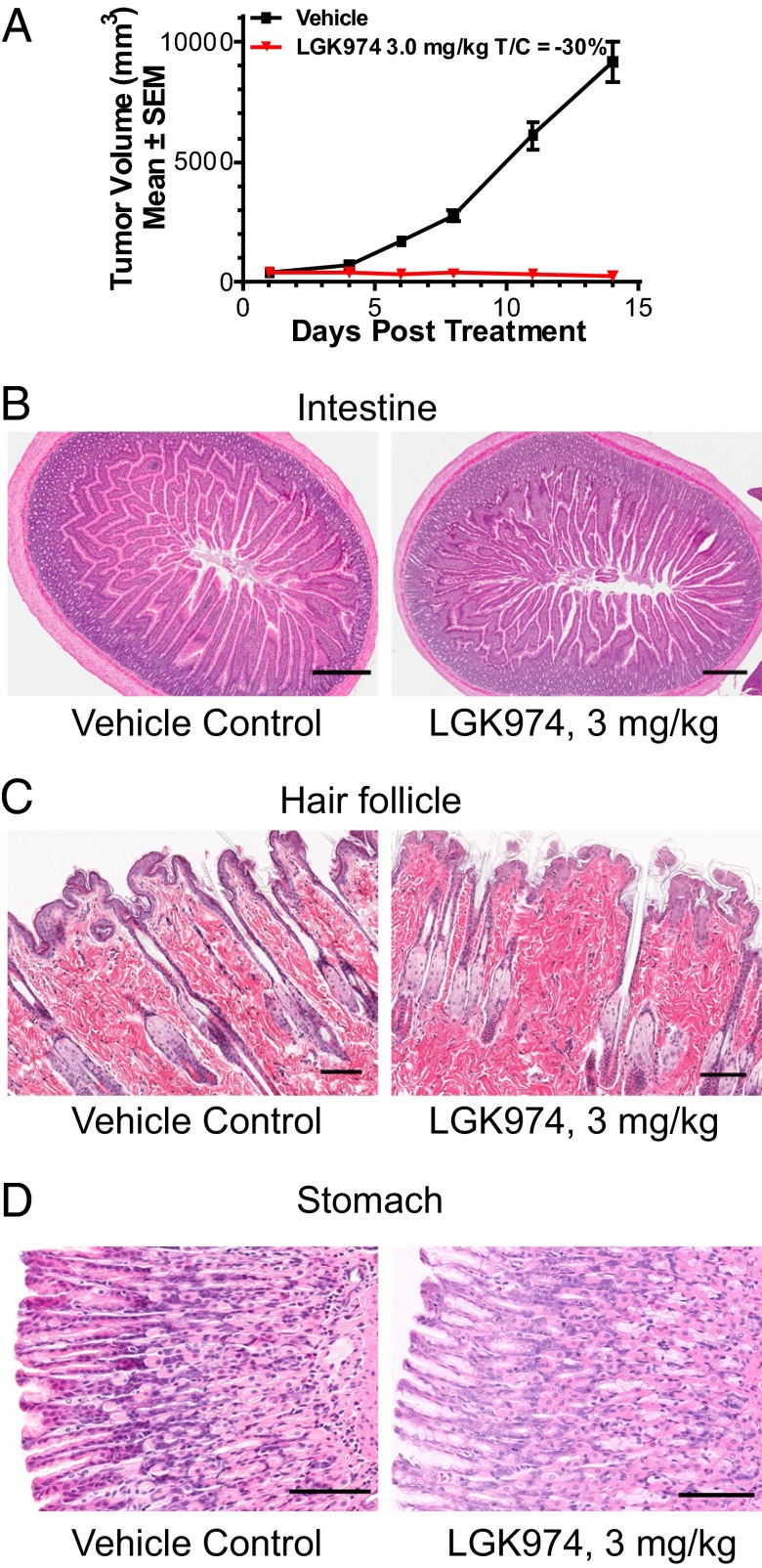
To further examine the effect of LGK974 on normal tissue homeostasis, we carried out a set of rat efficacy and toxicology studies. We confirmed that LGK974 induced tumor regression when dosed at 3 mg/kg per day in a rat MMTV-Wnt1 xenograft tumor model (Fig. 3A). Toxicology studies were performed on nontumor bearing rats at 3 and 20 mg/kg. As shown in Fig. 3 B–D, at the efficacious dose of 3 mg/kg per day for 14 d, LGK974 was well-tolerated without abnormal histopathological findings in Wnt-dependent tissues, including the intestine, stomach, and skin (23–26). When rats were administrated a very high dose of 20 mg/kg per day for 14 d, loss of intestinal epithelium was observed (Fig. S2C), consistent with the concept that Wnt is required for intestinal tissue homeostasis. This study shows a therapeutic window for LGK974, promoting tumor regressions at doses that spare normal tissues.
Fig. 3.
LGK974 is well-tolerated in Wnt-dependent tissues at an efficacious dose in rats. (A) LGK974 induced tumor regression when dosed at 3 mg/kg per day in a rat MMTV-Wnt1 xenograft tumor model. (B) Intestine, (C) skin/hair follicle, and (D) stomach tissues from rats treated with vehicle control or LGK974 at the dose of 3 mg/kg per day were subjected to H&E staining. (Magnification: intestine, 4×; hair follicle and stomach, 20×. Scale bars: intestine, 500 μM; hair follicle and stomach, 100 μM.)
Cellular Functional Effects of LGK974 in Human Head and Neck Cancer Cell Lines.
To identify human cancer cell lines that respond to porcupine inhibition, we surveyed cell lines from different cancer types using AXIN2 mRNA expression as a readout for Wnt pathway activity. We defined a responsive cell line as one that achieved greater than 50% AXIN2 mRNA reduction after treatment with 10–100 nM LGK974 for 48 h. We found that head and neck cancer (HNSCC) cell lines were responsive to LGK974, with 31 of 96 HNSCC cell lines showing pathway inhibition on treatment with LGK974 (Table S1), in contrast to brain cancer, small cell lung cancer, lymphoma/leukemia, or colon cancer cell lines, where no responsive lines were identified (Fig. S3A).
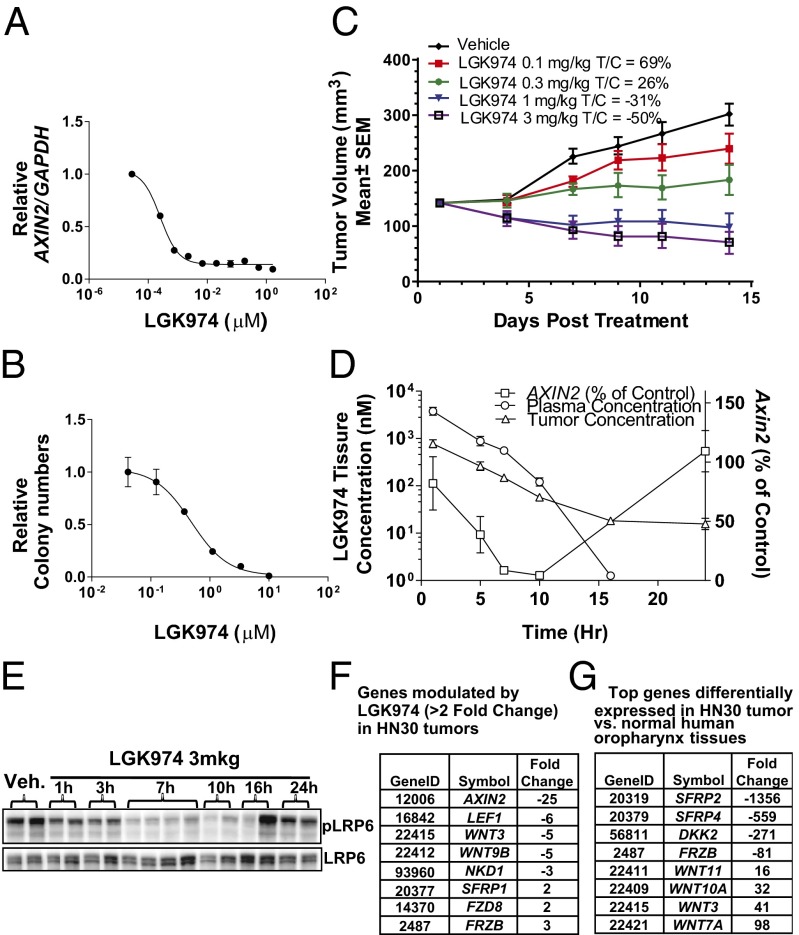
To correlate pathway inhibition with cellular function, the human HNSCC cell line HN30 was used for additional in vitro and in vivo characterization. LGK974 potently reduced Wnt-dependent AXIN2 mRNA levels in HN30 cells with an IC50 of 0.3 nM (Fig. 4A). It strongly attenuated HN30 colony formation, albeit with a right-shifted IC50 vs. the IC50 seen for pathway inhibition in vitro (Fig. 4B). Similar AXINX2 mRNA reduction and colony formation attenuation effects were also observed with the aforementioned structurally different PORCN inhibitor, GNF-1331 (Fig. S3 B and C). The reduced colony formation caused by LGK974 could be partially rescued with overexpression of dominant β-catenin (Fig. S3D), which suggests that LGK974 functions, at least in part, through inhibiting the β-catenin–dependent activities. To further confirm whether the cellular effect of LGK974 was caused by the inhibition of PORCN-dependent Wnt signaling activities, shRNA experiments were performed. shRNAs against PORCN potently knocked down PORCN expression levels (Fig. S3E) and substantially inhibited both the expression of the Wnt target gene AXIN2 (Fig. S3F) and the colony formation of HN30 cells in vitro (Fig. S3G), consistent with the LGK974 data.
Fig. 4.
Human HNSCC cell line profiling with LGK974. (A) LGK974 inhibited Wnt signaling in HN30 cells with an IC50 of 0.3 nM. (B) LGK974 reduced HN30 cell colony formation in vitro. (C) LGK974 single agent-induced tumor regression in the human HNSCC tumor model HN30 in vivo. (D) A multitime points pharmacokinetic/PD study was done in the HN30 xenograft model. The 3-mg/kg dose inhibited the expression of a Wnt signaling target gene, AXIN2, by ∼60–95% between 5 and 10 h after the dose, and the effect was completely absent at 24 h after the dose. (E) LGK974 showed similar inhibitory activities on pLRP6 in Western blot analysis, peaking at around 7–10 h after the dose. (F) Using TaqMan GeneCard analysis, LGK974 down-regulated the known Wnt target genes, including AXIN2, LEF1, and NKD1, as well as Wnt ligands, including Wnt3 and Wnt9B. (G) Compared with the gene expression patterns of normal human oropharynx tissues, Wnt ligands, including Wnt3, -7A, -10A, and -11, were substantially overexpressed, whereas the known Wnt inhibitory genes, such as SFRP2, FRZB, SFRP4, and DKK2, were substantially down-regulated.
Efficacy of LGK974 in a Mouse Model of Wnt-Dependent Human HNSCC Cell Line.
To test the antitumor activity of LGK974, a mouse xenograft model of the HNSCC HN30 cell line was established. As shown in Fig. 4C, LGK974 had strong efficacy when administered one time per day. Changes in tumor volume for each of the treated T and C groups were measured and used to calculate growth delay, which was expressed by the T/C ratio. After 14 d of treatment, a dose of 0.1 mg/kg per day led to moderate tumor growth delay (T/C: 69%), a dose of 0.3 mg/kg per day significantly inhibited tumor growth (T/C: 26%), and doses of 1.0 and 3.0 mg/kg resulted in substantial tumor regression (T/C: −31% and −50%, respectively) (Fig. 4C). The regimen was well-tolerated, and there was no significant body weight loss in the mice. A similar result was obtained with twice daily treatment in this model with −44% T/C at a dose of 0.5 mg/kg two times per day (Fig. S3H).
Like in the MMTV-Wnt1 model, both AXIN2 mRNA and pLRP6 levels were used as pharmacodynamic markers to link with the observed antitumor activity in the HN30 mouse xenograft model. After a single 3-mg/kg dose LGK974, the level of AXIN2 mRNA expression in tumors was reduced by ∼60–95% between 5 and 10 h postdose, and the effect started to diminish at 16 h in correlation with decreasing drug concentrations (Fig. 4D). A time delay was observed between the peak drug concentration (at 1 h) and the maximum AXIN2 mRNA inhibition (at 10 h). Additionally, as shown in Fig. 4E, pLRP6 levels in the HN30 tumors were substantially reduced in a time-dependent manner. The maximum effect was achieved at 7–10 h postdose, and the pLRP6 levels were largely back to baseline levels by 24 h. The delayed pharmacodynamics (PD) are presumably caused by the mechanism of POCRN inhibition, which blocks the secretion of newly formed Wnts while having no effect on preexisting Wnts. The time needed for preexisting Wnts to become unavailable or inactive to their receptors is presumably the reason for the delay between the peak time of drug exposure and PD effects. This pharmacokinetic, PD, and efficacy relationship is consistent with aforementioned results from the MMTV-Wnt1 model. Sustained pathway inhibition is not required for inducing tumor regression in the HN30 xenograft model, which may yield a safety margin by differentiating tumor cells from normal cells, such as Wnt-dependent stem cells.
To examine other Wnt-related genes that might be regulated by treatment with LGK974 in vivo, a TaqMan GeneCard analysis revealed down-regulation of several other known Wnt target genes, including LEF1 and NKD1, as well as Wnt ligands, including Wnt3 and Wnt9B (Fig. 4F). Comparing the gene expression patterns between HN30 tumors and normal human oropharynx tissues using this GeneCard analysis (Fig. 4G), Wnt ligands, including Wnt3, 7A, 10A, and 11, were substantially overexpressed in HN30 tumors, whereas the known Wnt inhibitory genes, such as SFRP2, FRZB, SFRP4, and DKK2, were substantially down-regulated, which together, is consistent with the hypothesis that Wnt signaling aberrations might be an underlying driver of this tumor-derived cell line. Using these types of Wnt pathway-related gene signatures may be a useful means to ultimately inform patient selection in support of the clinical development of PORCN inhibitors, like LGK974.
Correlation of Notch Mutations with PORCN Inhibitor Responsiveness.
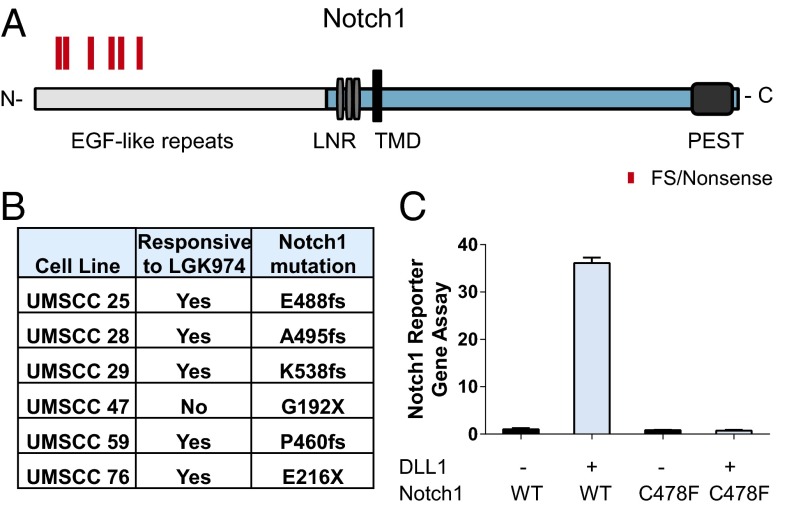
To further understand the mechanism of action in the cells that responded to LGK974 treatment, exome sequencing was performed on 40 HNSCC cell lines, including 25 responsive lines and 15 nonresponsive lines (a responsive cell line was defined as one that achieves greater than 50% AXIN2 mRNA reduction after treatment with 10–100 nM LGK974 for 48 h) (Table S2). Consistent with two independent epidemiologic studies on HNSCC cell lines reported recently (27, 28), TP53, CDKN2A, Notch1/2/3, PTEN, HRAS gene, and PIK3CA were among the top oncogenes or tumor suppressor genes mutated in this set of cell lines (Fig. S4A). To correlate genetic changes with LGK974 response, we focused on aggregated LoF mutations, which include stop codon gain, start codon loss, frameshift, and splicing site mutations (details in Material and Methods). One of the most striking results of our study was the correlation of aggregated LoF Notch1 mutations with responsiveness to LGK974. As shown in Fig. 5 A and B, five frameshift/nonsense mutations have been identified among the responsive cell lines, with only one Notch1 nonsense mutation among the nonresponsive cell lines. Interestingly, all cells with Notch1 mutations harbored at least one mutant allele that affected the N terminus of Notch1. This result is consistent with the notion that the N terminus of Notch1 is required for its function, with the N-terminal EGF repeats responsible for ligand–receptor interaction (29), such that mutations in this region are more likely to be LoF mutations.
Fig. 5.
Highly enriched Notch1 LoF mutation in LGK974-responsive HNSCC cell lines. (A) Diagrams of potential LoF mutations of Notch1 in HNSCC cell lines. C, C terminus; LNR, Lin12-Notch repeat; N, N terminus; PEST, proline, glutamic acid, serine, threonine-rich; TMD, transmembrane domain. Frameshift (FS) and nonsense mutations (X), including E488fs, A495fs, K538fs, G192X, P460fs, and E216X, are highlighted in red. (B) The list of Notch1 FS and nonsense mutations in HNSCC cell lines. (C) Notch1 C478F showed complete reduction of activities compared with the WT in a Notch1 reporter gene assay with or without DLL1 stimulation.
To further characterize the functional consequence of the missense mutations, such as Notch1 C478F, which is identified in the HN30 cells, a Notch reporter gene assay was performed with overexpression of the WT or the C478F mutant (Fig. 5C and Fig. S4B). The C478F mutation is located in the extracellular domain of Notch1, which is presumably critical for Notch receptor and ligand interaction. Indeed, in the presence of the Notch ligand DLL1, the activity of mutant C478F Notch1 was abolished in this Notch reporter gene assay (Fig. 5C).
To determine if restoration of Notch signaling activity can inhibit cell growth in Notch1 mutant cells, HN30 cells were infected with lentivirus expressing either WT full-length Notch1 or constitutively active Notch intracellular domain (NICD). After 17 d of selection with G418, cells infected with Notch1 or NICD showed dramatically reduced total cell numbers compared with the control group (Fig. S5). These data suggest that Notch signaling functions as a tumor suppressor gene in Notch1 mutant cells.
Notch suppresses Wnt signaling in keratinocytes in multiple models (30, 31). Inhibition of Notch signaling through Notch1 KO or expression of dominant negative MAML1 in keratinocytes or skin tissues from genetically engineered mice correlated with Wnt activation (30, 31). Overexpression of the NICD or the Notch ligand, Jagged1, resulted in inhibition of Wnt3 and Wnt4 expression in mouse keratinocytes (32).
To test if loss of Notch signaling could lead to up-regulation of Wnt3/4, human primary neonatal keratinocytes were treated with a γ-secretase inhibitor (GSI) that inhibits Notch signaling. As shown in Fig. S6, inhibition of the Notch pathway by a GSI induced Wnt3/4 expression, consistent with the report using mouse primary keratinocytes and supporting the antagonism between Notch and Wnt in keratinocytes (32).
The underlying mechanism of action in the LGK974-responsive cell lines with WT Notch1 remains to be fully elucidated. Potentially, there could be multiple routes to dysregulate the Notch pathway. Indeed, a heterozygous nonsense mutation in Deltex 3-like (DTX3L; also called B lymphoma- and BAL-associated protein), an E3 ubiquitin ligase (33), was identified in the HNSCC cell line SNU1076. The cellular function of DTX3L in the mammalian Notch pathway is not clear, but its Drosophila homolog Deltex is a positive regulator of Notch signaling in Drosophila (34). In an SNU1076 xenograft model in mice, LGK974 at the dose of 5 mg/kg per day substantially inhibited the Wnt pathway, which was indicated by a 70% reduction of AXIN2 levels (Fig. S7A). Moreover, LGK974 significantly inhibited tumor growth (T/C: 25%) after 14 d of treatment (Fig. S7B). Loss of DTX3L may, therefore, provide an alternative mechanism to inactivate the Notch signaling pathway.
Discussion
Given the well-established role of dysregulated Wnt signaling in cancer, the potential exists for targeting this pathway therapeutically. Indeed, multiple attempts to develop antibodies against key Wnt coreceptors, including LRP6 and FZD, have been reported (11, 35, 36) with varying degrees of success. A PORCN inhibitor, IWP2, has been reported to show good potency and specificity in inhibiting Wnt signaling in vitro (37, 38). Furthermore, C59, a preclinical compound from our PORCN inhibitor optimization efforts, was found to have potent activity in a mechanistic Wnt-dependent mouse tumor model (22).
Wnt signaling is known to be involved in normal tissue homeostasis. In genetic mouse models, overexpression of DKK1 or loss of TCF4 induced severe intestinal toxicity (23, 24). Pharmacological Wnt inhibitors, such as Tankyrase inhibitors, also recapitulated the gut toxicity at high doses (37, 39). These results are consistent with our data using a very high dose of LGK974 at 20 mg/kg per day. However, in contrast to the tissue toxicity caused by Wnt inhibition through genetic means or Tankyrase inhibitors, LGK974 is well-tolerated at the efficacious doses. We showed that sustained Wnt pathway inhibition is not required to achieve tumor regression by LGK974, providing a therapeutic window for tumor efficacy while sparing the normal Wnt-dependent tissues.
Through exome sequencing, we found that LoF Notch1 mutations are highly enriched in LGK974-responsive HNSCC cell lines. LoF Notch1 mutation in HNSCC is consistent with the concept that Notch is a context-dependent oncogene or tumor suppressor gene (40). In genetically engineered mouse models, there is a strong link between loss of Notch, Wnt activation, and skin cancer formation. For example, in the skin-specific Notch1 KO or dominant negative MAML1 transgenic mice, spontaneous skin tumors were observed (30, 31, 41). At the molecular level, Notch suppresses Wnt signaling in keratinocytes and skin tissues (30, 31). We also confirmed the increased expression of Wnt3/4 in human keratinocytes on inhibition of Notch signaling using a GSI. In light of the antagonism between Notch and Wnt signaling in skin-related tissues, loss of Notch signaling and its subsequent activation of the Wnt pathway could provide an underlying mechanism of action for increased sensitivity to LGK974 in the Notch1 mutant-containing squamous cell carcinomas. In addition to HNSCCs, LoF Notch1 mutations have been reported in esophageal squamous cell carcinoma and cutaneous squamous cell carcinoma tumors (42, 43), supporting the evaluation of Wnt inhibition in those settings.
Not all HNSCC cell lines with LoF mutations of Notch1 are responsive to LGK974, such as UMSCC47. It is possible that there are other genetic or epigenetic changes leading to the disruption of Notch and Wnt cross-talk.
From our unbiased bioinformatics analysis, in addition to Notch1, LoF mutations of a few less-characterized proteins showed enrichment in LGK974-responsive cell lines, such as FAM58A (Table S3). This gene is mutated in an X-linked dominant genetic syndrome with syndactyly, telecanthus, and anogenital and renal malformations (44). Its cellular function is unknown but hypothesized to be related to MYCN because of the role of MYCN in Feingold syndrome with overlapping clinical symptoms (44). It will be of interest to explore the underlying mechanism between FAM58A mutations and LGK974 responsiveness.
From recent large-scale genomic sequencing efforts in human HNSCC patient samples and cell lines, genetic defects in addition to Notch may contribute to Wnt activation, such as LoF mutations of FAT1 (28, 45, 46). FAT1 is a protocadherin protein that is reported to bind to β-catenin and prevent its nuclear translocation (45). LoF mutants lose their tumor suppressor function and promote Wnt/β-catenin signaling (45). From our exome sequencing and cell line profiling efforts, we observed an enrichment of LGK974 responders in FAT1 mutant HNSCC cell lines (Fig. S8), which is consistent with the role of FAT1 in Wnt signaling. Furthermore, in mouse transgenic skin tumor models, activating mutation of HRAS substantially increased Wnt signaling, and β-catenin is required in HRAS-driven tumorigenesis (12, 47). Consistently, from our analysis, four of five cell lines with HRAS mutations responded to LGK974 (Fig. S9), supporting the notion that multiple mechanisms are involved in LGK974 responsiveness.
In this report, we described the successful discovery of a potent, selective, and orally bioavailable PORCN inhibitor, LGK974. We showed compelling evidence for inhibition of Wnt signaling in vivo, attenuation of tumor growth in human disease models at well-tolerated doses, and identification of potential genetic markers to enrich for tumors that are responsive to LGK974. These studies lay the foundation for the treatment of Wnt-driven tumors in the clinic.
Materials and Methods
All cell lines were cultured in a humidified incubator at 37 °C with 5% (vol/vol) CO2. All compounds were initially dissolved in 100% DMSO and then diluted to the indicated concentrations for studies in vitro. Detailed methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We gratefully thank Dr. F. Cong for the Wnt reporter gene construct and Wnts/Fzd cDNA expression constructs; T. Boulineau and J. Watson for histology studies; X. Cui, T. Tuntland, N. Englund, A. Schumacher, H. Gao, C. Fryer, D. Han, G. Zhang, J. Cheng, P. Gordon, W. Richmond, M. Warmuth, and M. Yao for assistance and discussions; and Vivarium staff for animal handling. This work was supported by the Novartis Research Foundation.
Footnotes
Conflict of interest statement: J. Liu, S.P., M.H.H., N.N., T.W., S.K., D.C., J. Li, C.T., A.P., A.S., C.K., Y.W., C.L., P.M., W.R.S., L.P., A.L.B., H.M.S., M.E.M., J. Che, G.V., and J.L.H. are employees of Novartis.
This article is a PNAS Direct Submission. M.d.l.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314239110/-/DCSupplemental.
References
- 1.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4(5):pii:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nusse R, Varmus H. Three decades of Wnts: A personal perspective on how a scientific field developed. EMBO J. 2012;31(12):2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Seshagiri S, et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488(7413):660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao HX, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485(7397):195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 6.Koo BK, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 7.Theodorou V, et al. MMTV insertional mutagenesis identifies genes, gene families and pathways involved in mammary cancer. Nat Genet. 2007;39(6):759–769. doi: 10.1038/ng2034. [DOI] [PubMed] [Google Scholar]
- 8.Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther. 2004;3(1):36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- 9.Geyer FC, et al. β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24(2):209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 10.Khramtsov AI, et al. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176(6):2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurney A, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 2012;109(29):11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malanchi I, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452(7187):650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 13.Heidel FH, et al. Genetic and pharmacologic inhibition of β-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell. 2012;10(4):412–424. doi: 10.1016/j.stem.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327(5973):1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herr P, Hausmann G, Basler K. WNT secretion and signalling in human disease. Trends Mol Med. 2012;18(8):483–493. doi: 10.1016/j.molmed.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11(6):791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. 2011;355(2):275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci USA. 2011;108(31):12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 20.Nusse R. Wnts and Hedgehogs: Lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development. 2003;130(22):5297–5305. doi: 10.1242/dev.00821. [DOI] [PubMed] [Google Scholar]
- 21.Cheng D, Zhang G, Han D, Gao W, Pan S. N-(hetero) aryl, 2-(hetero) aryl-substituted acetamides for use as Wnt signaling modulators. Patent Cooperation Treaty and US patent publication US 101849 (2010) 2010 [Google Scholar]
- 22.Proffitt KD, et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013;73(2):502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 23.Kuhnert F, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101(1):266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Es JH, et al. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol. 2012;32(10):1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker N, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105(4):533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal N, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopan R. Notch signaling. Cold Spring Harb Perspect Biol. 2012;4(10):pii:a011213. doi: 10.1101/cshperspect.a011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proweller A, et al. Impaired notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res. 2006;66(15):7438–7444. doi: 10.1158/0008-5472.CAN-06-0793. [DOI] [PubMed] [Google Scholar]
- 31.Nicolas M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33(3):416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 32.Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 2005;19(12):1485–1495. doi: 10.1101/gad.341405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeyama K, et al. The BAL-binding protein BBAP and related Deltex family members exhibit ubiquitin-protein isopeptide ligase activity. J Biol Chem. 2003;278(24):21930–21937. doi: 10.1074/jbc.M301157200. [DOI] [PubMed] [Google Scholar]
- 34.Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: Recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13(9):654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong Y, et al. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS One. 2010;5(9):e12682. doi: 10.1371/journal.pone.0012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ettenberg SA, et al. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci USA. 2010;107(35):15473–15478. doi: 10.1073/pnas.1007428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5(2):100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dodge ME, et al. Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine. J Biol Chem. 2012;287(27):23246–23254. doi: 10.1074/jbc.M112.372029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau T, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73(10):3132–3144. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- 40.Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: It’s NOTCH what you think. J Exp Med. 2011;208(10):1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009;16(1):55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal N, et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov. 2012;2(10):899–905. doi: 10.1158/2159-8290.CD-12-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang NJ, et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci USA. 2011;108(43):17761–17766. doi: 10.1073/pnas.1114669108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unger S, et al. Mutations in the cyclin family member FAM58A cause an X-linked dominant disorder characterized by syndactyly, telecanthus and anogenital and renal malformations. Nat Genet. 2008;40(3):287–289. doi: 10.1038/ng.86. [DOI] [PubMed] [Google Scholar]
- 45.Morris LG, et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat Genet. 2013;45(3):253–261. doi: 10.1038/ng.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickering CR, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3(7):770–781. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beronja S, et al. RNAi screens in mice identify physiological regulators of oncogenic growth. Nature. 2013;501(7466):185–190. doi: 10.1038/nature12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.