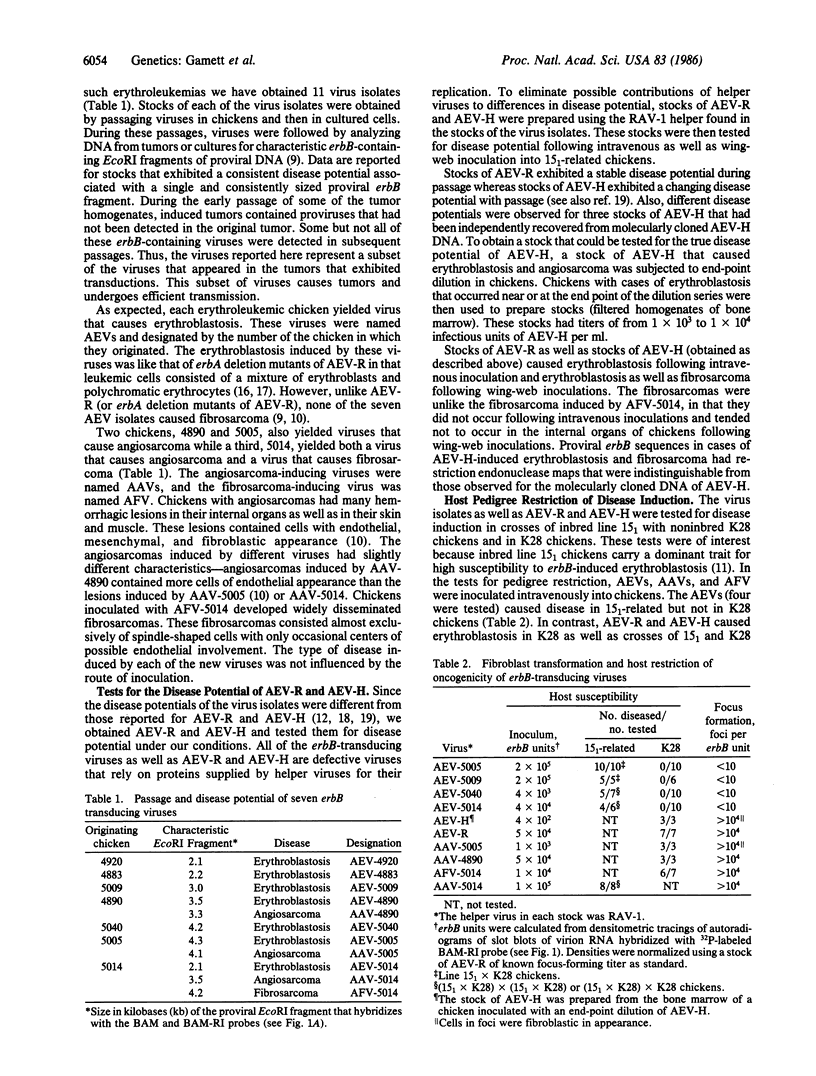
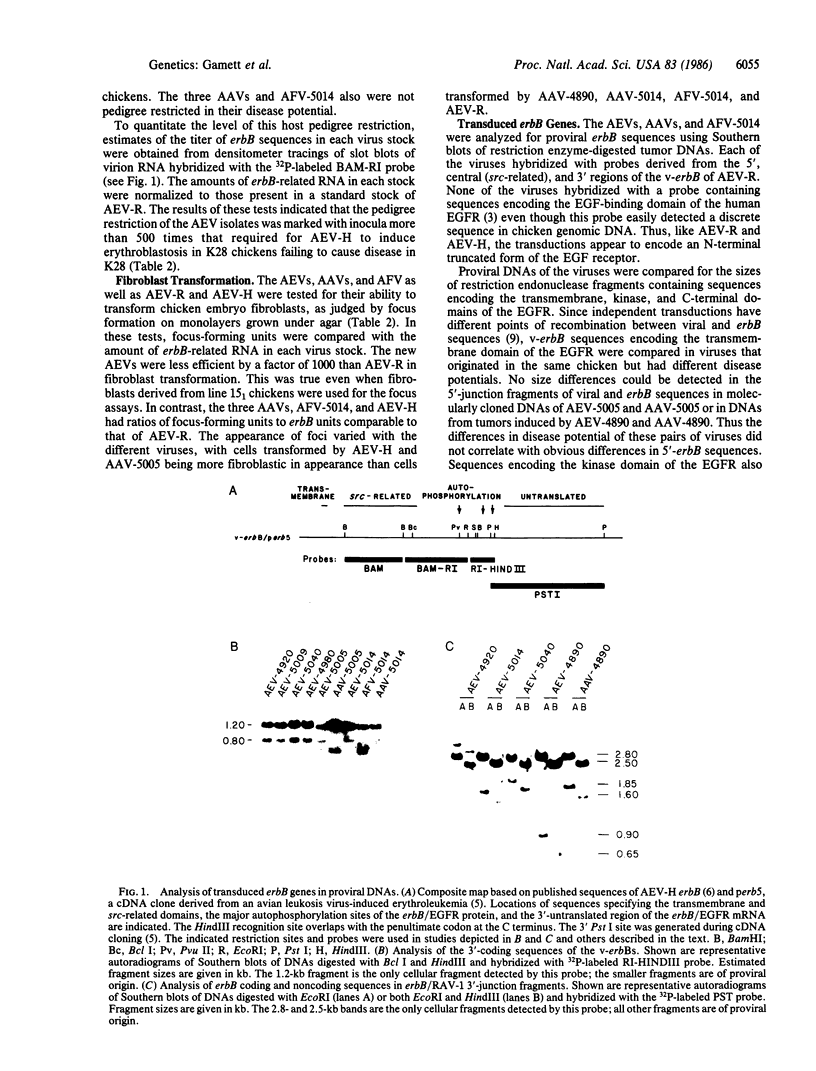
Abstract
Eleven recently isolated erbB-transducing viruses as well as avian erythroblastosis virus (AEV)-R (ES4) and AEV-H have been characterized for the type of disease they cause, their ability to transform fibroblasts in culture, their ability to cause disease in pedigrees of chicken that differ in susceptibility to erbB-induced erythroblastosis, and the structure of their erbB genes. Differences in each of the biological parameters correlated with differences in erbB sequences encoding the C-terminal domain of the epidermal growth factor receptor (EGFR). Seven viruses were strain restricted in their ability to induce erythroblastosis and did not transform fibroblasts. These seven viruses contained v-erbB genes encoding the complete C terminus of the EGFR. AEV-R and AEV-H were not pedigree restricted in their ability to induce erythroblastosis and could transform fibroblasts. These viruses contain v-erbB genes that lack codons for the immediate C terminus of the EGFR. Three viruses caused angiosarcoma and one caused fibrosarcoma. The angiosarcoma and fibrosarcoma-inducing viruses were not strain restricted and did not cause erythroblastosis. The v-erbB genes of each of these viruses contained extensive internal deletions or 3' truncations in sequences encoding the C-terminal domain of the EGFR.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Debuire B., Henry C., Bernissa M., Biserte G., Claverie J. M., Saule S., Martin P., Stehelin D. Sequencing the erbA gene of avian erythroblastosis virus reveals a new type of oncogene. Science. 1984 Jun 29;224(4656):1456–1459. doi: 10.1126/science.6328658. [DOI] [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Fink D., Beug H., Royer-Pokora B. Oncornavirus-induced sarcoma formation obscured by rapid development of lethal leukemia. Cancer Res. 1977 Jan;37(1):59–63. [PubMed] [Google Scholar]
- Hihara H., Yamamoto H., Shimohira H., Arai K., Shimizu T. Avian erythroblastosis virus isolated from chick erythroblastosis induced by lymphatic leukemia virus subgroup A. J Natl Cancer Inst. 1983 May;70(5):891–897. [PubMed] [Google Scholar]
- Hunter T. The epidermal growth factor receptor gene and its product. Nature. 1984 Oct 4;311(5985):414–416. doi: 10.1038/311414a0. [DOI] [PubMed] [Google Scholar]
- Miles B. D., Robinson H. L. High-frequency transduction of c-erbB in avian leukosis virus-induced erythroblastosis. J Virol. 1985 May;54(2):295–303. doi: 10.1128/jvi.54.2.295-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Nishida T., Sakamoto S., Yamamoto T., Hayman M., Kawai S., Toyoshima K. Comparison of genome structures among three different strains of avian erythroblastosis virus. Gan. 1984 Apr;75(4):325–333. [PubMed] [Google Scholar]
- Robinson H. L. Intracellular restriction on the growth of induced subgroup E avian type C viruses in chicken cells. J Virol. 1976 Jun;18(3):856–866. doi: 10.1128/jvi.18.3.856-866.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Jensen L., Coffin J. M. Sequences outside of the long terminal repeat determine the lymphomogenic potential of Rous-associated virus type 1. J Virol. 1985 Sep;55(3):752–759. doi: 10.1128/jvi.55.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Miles B. D., Catalano D. E., Briles W. E., Crittenden L. B. Susceptibility to erbB-induced erythroblastosis is a dominant trait of 151 chickens. J Virol. 1985 Sep;55(3):617–622. doi: 10.1128/jvi.55.3.617-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Privalsky M. L., Moscovici G., Moscovici C., Bishop J. M. Site-specific mutagenesis of avian erythroblastosis virus: erb-B is required for oncogenicity. Virology. 1983 Oct 15;130(1):155–178. doi: 10.1016/0042-6822(83)90125-3. [DOI] [PubMed] [Google Scholar]
- Tracy S. E., Woda B. A., Robinson H. L. Induction of angiosarcoma by a c-erbB transducing virus. J Virol. 1985 May;54(2):304–310. doi: 10.1128/jvi.54.2.304-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Yamamoto T., Hihara H., Nishida T., Kawai S., Toyoshima K. A new avian erythroblastosis virus, AEV-H, carries erbB gene responsible for the induction of both erythroblastosis and sarcomas. Cell. 1983 Aug;34(1):225–232. doi: 10.1016/0092-8674(83)90153-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]