Abstract
Purpose.
We described anatomic age-related changes in the human eye to determine potential areas of investigation that may lead to identifying eyes at risk for age-related disease.
Methods.
A descriptive review of anatomic changes in the eye related to aging was performed in the context of current areas of investigation. The review was performed specifically for differing anatomic ocular structures, including cornea, trabecular meshwork, lens, uveal tract, Bruch's membrane, retina, RPE, vitreous, sclera, and optic nerve.
Results.
Age-related changes occur in all ocular tissues. The cornea flattens and there is an attrition of endothelial cells. The shape of the trabecular meshwork changes and there is a loss of trabecular endothelium. The lens grows and becomes cataractous. The ciliary body becomes collagenized, there are choroidal vascular changes, and Bruch's membrane thickens. Retinal vessels become hyalinized and there is a loss of rods before cones in the macula. RPE morphometric changes occur with aging. The vitreous becomes liquefied and there is a loss of vitreous compartmentalization. The sclera becomes rigid and may become calcified. The optic nerve exhibits structural changes with age.
Conclusions.
There are numerous anatomic age-related changes in the human eye. Current areas of investigation related to these changes include adaptive optics scanning laser ophthalmoscopy imaging of the RPE mosaic in the context of aging, and drug delivery devices that overcome age-related alterations to retinal and macular perfusion.
Keywords: aging, anatomy, pathology
Introduction
Numerous anatomic changes occur in the eye with age. These changes generally include loss and attenuation of cells, such as the corneal endothelium and RPE; degenerative processes, such as vitreous liquefaction; and accumulations of materials, such as drusen. There are research opportunities to image the effects of aging, thus, predicting diseases that are characterized by abnormal or premature aging as well as understanding the effects of aging on therapeutic drug delivery to arrest ocular disease.
Cornea
As the eye ages, the cornea flattens.1 The thickness of Bowman's layer, 8 to 10 μm, remains constant throughout life. There is a tendency for calcific deposition at the periphery of Bowman's layer with aging. Arcus senilis, a deposition of lipid near the limbus, also may occur with aging. The stromal keratocyte density appears higher in children than adults. Arcus senilis appears in the peripheral cornea with aging in some patients.2 The thickness of Descemet's membrane increases with aging.3 There is a decrease in corneal endothelial cell density that occurs with aging.4 The most precipitous decrease in cell density occurs in the first five years of life. In general, the corneal endothelial cell density decreases from approximately 5000 cells/mm2 at birth to 3000 cells/mm2 in older age. There is a direct correlation between histologic corneal endothelial cell number and specular microscopic endothelial cell density.5 The corneal endothelial cell density is not uniform, with an increased endothelial cell density in the paracentral and peripheral regions of the cornea, which declines with age at a rate of between 0.2% and 0.6% per year.6 There is some evidence that endothelial stem cells are present in a regenerative zone near Schwalbe's line which give rise to a paracentral storage zone of cells.6,7
Trabecular Meshwork
With age, the trabecular meshwork changes histologically from a long, wedge shape to a shorter, more rhomboidal form.8 The trabeculae become progressively thickened and ultrastructural examination shows a change in the appearance of extracellular materials.8 The cellularity of the trabecular meshwork decreases with age.9,10 A progressive decrease in trabecular endothelial cellularity (58%) and absolute cell number (47%) was documented in newborns to persons aged 81 years.9 This change parallels the decrease in corneal endothelial cell density and corresponds with senescence of cultured trabecular meshwork cells.11 Aqueous outflow spaces in the trabecular meshwork also decreases with age, which may account for an increase in intraocular pressure.10 This is thought to be due to an accumulation of extracellular sulfated proteoglycans with accompanying changes in collagen/microfibril architecture.12–14 Additionally, there is an age-related reduction of giant vacuoles and intracellular pores in Schlemm's canal.15 All of these changes may result in a decrease in aqueous outflow facility.
Ciliary Body
With advancing age, the stroma of the ciliary processes becomes collagenized, the processes appear to become less vascularized, and they also appear shorter and more blunt.16 The cellularity of the ciliary body appears to diminish with age16 and the ciliary body smooth muscle bundles undergo age-related changes in morphology suggestive of an antero-inwards displacement of the muscle mass.17 Occasionally, age-related hyperplasia of the ciliary body nonpigmented epithelium occurs and forms a Fuchs adenoma. This is a benign lesion that may occur as an incidental finding in eye bank eyes, although occasionally it clinically mimics a malignant neoplasm.18
Lens
The shape of the crystalline lens in histologic sections changes with age. In infants, the lens assumes a reniform configuration, whereas in adults, it is more oval.16,19 The lens increases in weight from approximately 90 mg at birth to 150 mg at age 20 years, 190 mg at age 40 years, and 240 mg at age 80 years.20 Cataracts are associated with aging and are manifest with color changes in the lens due to oxidation of lens proteins. The most common age-related histologic cataractous changes are equatorial and posterior cortical degeneration.19 These changes result in inward turning of equatorial cortical fibers, and a difference in staining with hematoxylin and eosin between the lens nucleus and cortex. This may be accompanied by posterior migration of the lens epithelium.19 These cells may lie along the inner surface of the posterior capsule, and become balloon-like and swollen (Wedl cells).19 A proliferation of these cells along the posterior capsule results in posterior subcapsular cataract.21 Eosinophilic fluid may appear between lens cortical cells with age, and when the cells eventually break down with aging, the released protein from the cells results in Morgagnian globules and eventual liquefaction of the cortex, with the lens nucleus floating in a sea of liquefied cortex (Morgagnian cataract).19
Retina
With aging, there is diffuse thickening of the internal limiting membrane of the retina and diminution of neural elements with gliosis in the peripheral retina.22 These changes lead to disorganization in the area of the ora serrata, and the RPE may migrate into the sensory retina in this area. There may be a reduction of nuclei in the outer nuclear layer of the retina with age.23 Corpora amylacea may be observed in the peripapillary retinal nerve fiber layer, optic nerve head, and optic nerve as an aging process. These bodies appear to be accumulations of intracellular organelles, including neurotubules, mitochondria ,and dense bodies.24,25 Curcio et al. have demonstrated a progressive, age-related loss of rods before cones in the macula with an accompanying decline in scotopic sensitivity compared to photopic sensitivity.26 Typical peripheral cystoid degeneration, which is not seen in infant eyes, is present in virtually 100% of adults eyes.16 Retinal vessels exhibit changes associated with aging. These include widespread loss of cellularity in the peripheral capillaries of elderly persons with attachment of the inner limiting membrane to the peripheral vascular arcades.22,27,28 Additionally, there is a diminution in the number of capillaries around the fovea.29 Arteriosclerotic changes also can occur in retinal vessels with aging. These include thickening and hyalinization of the vessel wall.22 Other arteriosclerotic changes, including hyperplasia of the muscular layer and fibrinoid necrosis of the vessel wall, occur in the setting of hypertension.22 Peripheral retinal degenerations are associated with aging, including typical and reticular peripheral cystoid degeneration (TPCD), paving stone (cobblestone) degeneration, and lattice degeneration.22 TPCD, which appears as microscopic cystoid spaces in the inner to outer plexiform layers, is observed in approximately 87% of autopsy eyes of all age groups, and nearly 100% of eyes of older adults.30 These cystoid spaces are bridged by Müller cells, and when these bridges collapse, age-related retinoschisis may ensue. Reticular peripheral cystoid degeneration is similar to TPCD with the exception that the cystoid spaces are in the nerve fiber layer. Peripheral chorioretinal atrophy (paving stone degeneration, cobblestone degeneration) is seen in up to 27% individuals over the age of 20 years.31 This degeneration is thought to be due to choroidal vascular insufficiency and results in ovoid areas of RPE atrophy, with overlying outer retinal atrophy surrounded by RPE hypertrophy and hyperplasia.22 Lattice degeneration of the retina is found in approximately 11% of autopsy eyes. It is age-related, occurs in the mid-periphery, is caused by vitreoretinal traction, and is characterized by inner retinal thinning, glial proliferation around the edges of the lesion, overlying liquid vitreous, hyalinized vessels, and underlying hypertrophy and hyperplasia of the RPE.22 Lattice degeneration results in areas of retinal structural weakening and retinal holes may appear in areas of this degeneration.32
Retinal Pigment Epithelium and Bruch's Membrane
The RPE increases in density from birth to two years of age, when the adult density is achieved.33 With aging, the RPE becomes more pleomorphic, with the macular RPE becoming narrower with an increased height, and opposite occurring in the periphery.22,34 Peripheral RPE cells become broader, lower, vacuolated, and pleomorphic with aging.22 Lipofuscin accumulates in the cytoplasm with aging and the lipofuscin-associated A2E-epoxides may be toxic to RPE.35,36 There is clinical interest in RPE lipofuscin-related fundus autofluorescence patterns with regard to aging and age-related macular degeneration,37 although the histologic correlations of these findings are unclear.38 Autofluorescence imaging of the RPE with the adaptive optics scanning laser ophthalmoscope (AOSLO) or a two-photon tunable dye laser may prove to be tools for further evaluation aging changes of the RPE mosaic.39–41 Sub-RPE nodular drusen accumulate with age.22 These drusen are excrescences formed on the inner aspect of Bruch's membrane, and are composed of granular substance, lipid, protein, crystalline deposits of calcium, and residual bodies. There are several histopathologic types of drusen, including hard, soft, confluent, and large drusen.42 Hard drusen are nonspecific and age-related. Soft, confluent, and large drusen are associated with age-related macular degeneration.42 Eosinophilic, brush-like material that accumulates external to the basement membrane of the RPE with age is termed “basal laminar deposit.”43 This material contains granular material, noncoated and coated vesicles, and wide-spaced collagen.22 Although the presence of basal laminar deposit may be associated with aging, it becomes very thick in age-related macular degeneration.42–44 Basal linear deposit, which accumulates between the basement membrane and plasma membrane of the RPE, may be a specific ultrastructural marker for AMD.45 Bruch's membrane itself becomes thickened and may become calcified with aging. The thickening includes focal and diffuse thickening of the inner aspect of Bruch's membrane.22 Lipid, including cholesterol, accumulates in Bruch's membrane with aging.46,47 Curcio et al. have noted that Bruch's membrane ages like the arterial intima and other connective tissues for which lipoproteins are the source of extracellular cholesterol.47 Some of the lipid in Bruch's membrane appears to arise from esterified cholesterol-rich apolipoprotein B–containing lipoprotein particles produced by the RPE.48
Choroid
There are clinical data using optical coherence tomography (OCT) indicating an inverse relationship between age and choroidal volume.49 Histopathologic studies have shown a negative correlation between age and choriocapillaris density.50 Although there may be an early increase in choriocapillaris density in age-related macular degeneration,51 eventually there is a decrease that is more pronounced than in normal aging.50,51
Vitreous
With aging, vitreous attachments to the retina weaken, thus resulting in posterior vitreous detachment.52 The space between the detached vitreous and retina is filled with liquefied vitreous. In one study of 786 eyes examined postmortem, 16% of the eyes from patients aged 45 to 65 years and 41% of eyes from patients aged over 65 years had a posterior vitreous detachment.53 A subsequent study showed that posterior vitreous detachment was present in 63% of postmortem eyes from patients aged in their 70s.54 Posterior vitreous detachment may result in the formed vitreous contracting forward to the vitreous base, thus causing traction on the peripheral retina and occasionally a retinal tear.22,52 As the vitreous liquefies and the formed vitreous collapses with aging, vitreous channels and compartments collapse, thus potentially affecting intravitreal drug delivery to the posterior retina.55
Sclera
The sclera becomes more rigid as a person ages.56 There may be relative dehydration, especially anterior to the insertion of recti muscles, resulting in calcium salt deposition.56 The midportion of the involved sclera in this area contains a calcified plaque.57 A similar age-related process may occur posteriorly, thus resulting in posterior scleral calcification.
Optic Nerve
Connective tissue within the fibrovascular pial septae becomes more abundant with age.58 Such thickening may result in impairment of exchange of nutrients and other metabolites between the capillaries and nerve fibers.58 Cellular and extracellular material may accumulate in the meninges and optic nerve fiber bundles with age. These include arachnoid cell nests and corpora arenacea (psammoma bodies) in the meninges, and corpora amylacea, which were described previously in the retina section. Schnabel cavernous degeneration of the optic nerve has been determined to be an age-related phenomenon related to chronic vascular occlusive disease.59 Histopathologic findings include loss of nerve fiber bundles and proteoglycan accumulation within the optic nerve.
Future Directions for Research
The anatomic alterations in the eye associated with aging offer potential areas of research and therapeutic intervention. Studies may be directed toward decreasing corneal endothelial cell loss with age. Research regarding trabecular meshwork aging changes, including extracellular matrix modulation, may prevent the decrease in outflow facility that occurs with age. Ciliary body dynamics may be studied with regard to aging changes to lessen the severity of presbyopia. Pharmacologic intervention for the prevention of cataracts may be investigated. Aging changes occur in the retinal and choroidal vasculature. These changes are related to retinal vascular disease, such as branch retinal vein occlusion and hypertensive retinopathy, as well as outer retinal ischemia secondary to choroidal vascular insufficiency. Potential areas of research related to these findings include real-time retinal and choroidal blood flow studies, such as may be determined using laser Doppler, and therapeutic interventions that result in normalization of these blood flows.60 Neuroprotection may be investigated as a way to lessen age-related loss of photoreceptors and retinal ganglion cells. Another potential area of research based on anatomic considerations of aging is in vivo imaging aging changes in the retina and RPE over time for early prediction of disease and early therapeutic intervention. An example of this is AOSLO imaging of the RPE mosaic as a method to predict age-related macular degeneration before there are fundus and vision changes.39,44,61 The AOSLO has been developed and may image the RPE mosaic.39,41 There are early histologic signs of AMD involving the RPE/Bruch's membrane complex that do not result in visible fundus changes.44 Imaging the RPE with predictive modeling may result in early detection of AMD and allow for early pharmacologic intervention. Analysis of RPE morphometry has been useful in identifying murine phenotype and age (Jiang Y, Chrenek MA, Gardner C, Boatright JH, Grossniklaus HE, Nickerson JM, unpublished observations, 2013).62 Comparison of in vivo fundus images of the RPE mosaic with ex vivo flat mounts including the underlying anatomic changes (Fig. 1) may be used to model and predict AMD progression. Investigations may be directed toward maintenance of Bruch's membrane. Another area of research is drug delivery to the posterior ocular compartment. Vitreous changes with age result in a dynamic change in vitreous compartments, which may impact diffusion of intravitreally injected drugs to the macula (Fig. 2).55 There are areas of opportunity to study these vitreous compartmentalization changes with age and develop drugs or techniques of drug delivery that overcome barriers to drug perfusion to the macula. Finally, optic nerve neuroprotection and biomechanics may be investigated with regard to glaucoma damage and other optic neuropathies.
Figure 1.
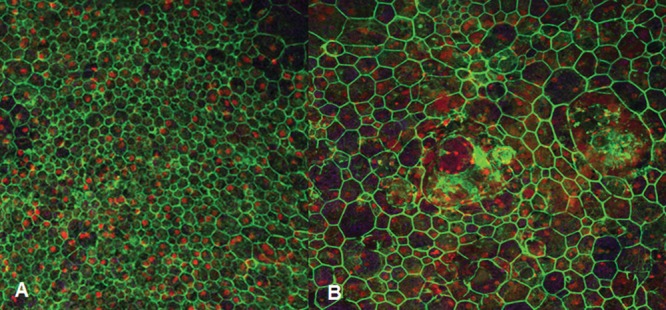
RPE sheet mosaic in the macula. (A) Flat mount from age-matched patient without age-related macular degeneration showing compact, hexagonal cell borders. (B) Flat mount from age-matched patient with age-related macular degeneration showing considerable cell border pleomorphism and multiple nuclei per cell (ex vivo human RPE flat mounts imaged stained with fluorescent antibody for ZO1, counterstained with propidium iodine, and imaged with 488 nm excitation and 510 emission filters).
Figure 2.
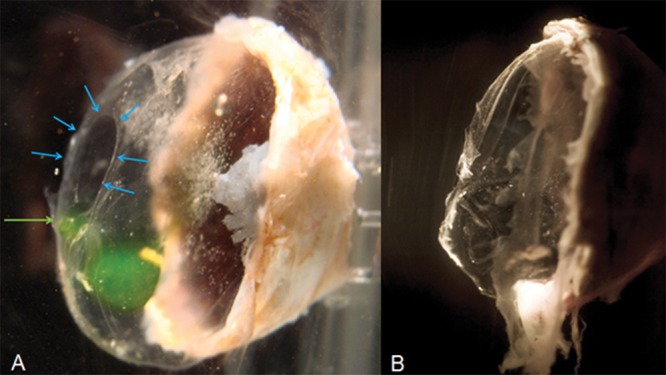
Age-related changes of vitreous anatomy. (A) The vitreous of a 65-year-old woman shows a premacular opening (blue arrows). Fluorescein dextran injected through the pars plana has migrated to the premacular opening (green arrow). (B) The vitreous of a 75-year-old man has collapsed, there is no visible premacular opening, and fluorescein dextran injected at the pars plana has not migrated posteriorly (ex vivo preparation of human eye bank eyes, frozen with posterior sclera dissected, and thawed).
Acknowledgments
Supported in part by NEI Grants P30 06360, R01EY016470, and R01021592, and an unrestricted departmental grant from Research to Prevent Blindness, Inc.
Disclosure: H.E. Grossniklaus, None; J.M. Nickerson, None; H.F. Edelhauser, None; L.A.M.K. Bergman, None; L. Berglin, None
References
- 1. Spencer WH. Chapter 3. Cornea. In: Spencer WH. ed Ophthalmic Pathology. An Atlas and Textbook. 4th ed. Philadelphia, PA: W. B. Saunders, Co.; 1996: 157–333 [Google Scholar]
- 2. Cogan DG, Kuwabara T. Arcus senilis: its pathology and histochemistry. Arch Ophthalmol. 1959; 61: 553–560 [PubMed] [Google Scholar]
- 3. Johnson DH, Bourne WM, Campbell RJ. The ultrastructure of Descemet's membrane. I. Change with age in normal corneas. Arch Ophthalmol. 1982; 100: 1942–1947 [DOI] [PubMed] [Google Scholar]
- 4. Edelhauser HF. The balance between corneal transparency and edema. The Proctor lecture. Invest Ophthalmol Vis Sci. 2006; 47: 1755–1767 [DOI] [PubMed] [Google Scholar]
- 5. Williams KK, Noe RL, Grossniklaus HE, Drews-Botsch C, Edelhauser HF. Correlation of histologic corneal endothelial cell counts with specular microscopic cell density. Arch Ophthalmol. 1992; 110: 1146–1149 [DOI] [PubMed] [Google Scholar]
- 6. Amann J, Holley GP, Lee SB, Edelhauser HF. Increased endothelial cell density in the paracentral and peripheral regions of the human cornea. Am J Ophthalmol. 2003; 135: 584–590 [DOI] [PubMed] [Google Scholar]
- 7. Whikehart DRA, Parikh CH, Vaughn A, Mishler K, Edelhauser HF. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis. 2005; 11: 816–824 [PubMed] [Google Scholar]
- 8. McMenamin PG, Lee WR, Aitken DA. Age-related changes in the human outflow apparatus. Ophthalmology. 1986; 93: 194–209 [DOI] [PubMed] [Google Scholar]
- 9. Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in the trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981; 21: 714–727 [PubMed] [Google Scholar]
- 10. Miyazaki M, Segawa K, Urakawa Y. Age-related changes in the trabecular meshwork of the normal human eye. Jpn J Ophthalmol. 1987; 31: 558–569 [PubMed] [Google Scholar]
- 11. Yamazaki Y, Matsunaga H, Nishikawa M, et al. Senescence in cultured trabecular meshwork cells. Br J Ophthalmol. 2007; 91: 808–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirano K, Kobayashi M, Kobayashi K, Hosino T, Awaya S. Age-related changes of microfibrils in the cornea and trabecular meshwork of the human eye. Jpn J Ophthalmol. 1991; 35: 166–174 [PubMed] [Google Scholar]
- 13. Gong H, Freddo TF, Johnson M. Age-related changes of sulfated proteoglycans in the normal human trabecular meshwork. Exp Eye Res. 1992; 55: 691–709 [DOI] [PubMed] [Google Scholar]
- 14. Machova L, Kubena K, Holubova M. Collagen architecture of sclerocorneal trabeculae in relation to age. Cesk Oftalmol. 1992; 48: 86–91 [PubMed] [Google Scholar]
- 15. Boldea RC, Roy S, Mermoud A. Ageing of Schlemm's canal in nonglaucomatous subjects. Int Ophthalmol. 2991; 24: 67–77 [DOI] [PubMed] [Google Scholar]
- 16. Folberg R. Chapter 1. The eye. In: Spencer WH. ed Ophthalmic Pathology. An Atlas and Textbook. 4th ed. Philadelphia, PA: W. B. Saunders, Co.; 1996: 1–37 [Google Scholar]
- 17. Sheppard AL, Davies LN. The effect of ageing on the in vivo human ciliary muscle morphology and contractility. Invest Ophthalmol Vis Sci. 2011; 52: 1809–1816 [DOI] [PubMed] [Google Scholar]
- 18. Shields JA, Shields CL, Eagle RC, Jr, , Firedman ES, Wheatley HM. Age-related hyperplasia of the nonpigmented ciliary body epithelium (Fuchs adenoma) simulating a ciliary body malignant neoplasm. Arch Ophthalmol. 2009; 127: 1224–1225 [DOI] [PubMed] [Google Scholar]
- 19. Eagle RC Jr, Spencer WH. Chapter 5. The lens. In: Spencer WH. ed Ophthalmic Pathology. An Atlas and Textbook. 4th ed. Philadelphia, PA: W. B. Saunders, Co.; 1996: 372–437 [Google Scholar]
- 20. Van Heynigen R. What happens to the human lens in cataract. Sci Am. 1976; 233: 70–81 [DOI] [PubMed] [Google Scholar]
- 21. Streeten BW, Eshaghian J. Human posterior subcapsular cataract: a gross and flat preparation study. Arch Ophthalmol. 1978; 96: 1653–1658 [DOI] [PubMed] [Google Scholar]
- 22. Green WR. Chapter 9. Retina. In: Spencer WH. ed Ophthalmic Pathology. An Atlas and Textbook. 4th ed. Philadelphia, PA: W. B. Saunders, Co.; 1996: 667–1331 [Google Scholar]
- 23. Garner S, Henkind P. Aging an degeneration of the human macula: I. Outer nuclear layer and photoreceptors. Br J Ophthalmol. 1981; 65: 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avendano J, Rodrigues MM, Hackett JJ, et al. Corpora amylacea of the optic nerve and retina: a form of neuronal degeneration. Invest Ophthalmol Vis Sci. 1980; 19: 550–555 [PubMed] [Google Scholar]
- 25. Woodford B, T'so MOM. An ultrastructure study of the corpora amylacea of the optic nerve head and retina. Am J Ophthalmol. 1980; 90: 492–502 [DOI] [PubMed] [Google Scholar]
- 26. Jackson GR, Owlsey C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002; 1: 381–396 [DOI] [PubMed] [Google Scholar]
- 27. Cogan DG. Development and senescence of the human retinal vasculature. Doyne memorial lecture. Trans Ophthalmol Soc U K. 1963; 83: 465–491 [PubMed] [Google Scholar]
- 28. Kuwabara T, Coagan DG. Retinal vascular patterns; VII. Acellular change. Invest Ophthalmol Vis Sci. 1965; 4: 1049–1064 [PubMed] [Google Scholar]
- 29. Kornszweig AL, Eliasoph I, Feldstein M. Retinal vasculature in the aged. Bull N Y Acad Med. 1964; 40: 116–129 [PMC free article] [PubMed] [Google Scholar]
- 30. O'Malley PF, Allen RA. Peripheral cystoid degeneration of the retina: incidence and distribution in 1,000 autopsy eyes. Arch Ophthalmol. 1967; 77: 769–776 [DOI] [PubMed] [Google Scholar]
- 31. O'Malley PF, Allen RA, Straatsma BR, et al. Paving stone degeneration of the retina. Arch Ophthalmol. 1965; 73: 169–182 [DOI] [PubMed] [Google Scholar]
- 32. Straatsma BR, Allen RA. Lattice degeneration of the retina. Trans Am Acad Ophthalmol Otolaryngol. 1962; 66: 600–613 [PubMed] [Google Scholar]
- 33. Streeten BW. Development of the human retinal pigment epithelium and the posterior segment. Arch Ophthalmol. 1969; 81: 383–394 [DOI] [PubMed] [Google Scholar]
- 34. Friedman E, T'so MOM. The retinal pigment epithelium II. Histologic changes associated with age. Arch Ophthalmol. 1968; 79: 315–320 [DOI] [PubMed] [Google Scholar]
- 35. Dorey CK, Wu G, Ebenstein D, et al. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989; 30: 1691–1699 [PubMed] [Google Scholar]
- 36. Sparrow JR, Vollmer-Snarr HR, Zhou J, et al. A2E-epoxides damage DNA in retinal pigment epithelial cells. J Biol Chem. 2003; 278: 18207–18213 [DOI] [PubMed] [Google Scholar]
- 37. Bindewald A, Bird AC, Dandekar SS, Dolar-Szcasny J, et al. Classification of fundus autofluorescence patterns in early age-related macular disease. Invest Ophthalmol Vis Sci. 2005; 46: 3309–3314 [DOI] [PubMed] [Google Scholar]
- 38. Rudolf M, Vogt SD, Curcio CA, et al. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology. 2013; 120: 821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roorda A, Zhang Y, Duncan JL. High-resolution in vivo imaging of the RPE mosaic in eyes with retinal disease. Invest Ophthalmol Vis Sci. 2007; 48: 2297–2303 [DOI] [PubMed] [Google Scholar]
- 40. Bindewald-Wittich A, Han M, Schmitz-Valckenberg S, et al. Two-photon-excited fluorescence imaging of human RPE cells with a femtosecond Ti: sapphire laser. Invest Ophthalmol Vis Sci. 2006; 57: 4553–4557 [DOI] [PubMed] [Google Scholar]
- 41. Rossi EA, Chung M, Dubra A, Hunter JJ, Merigan WH, Williams DR. Imaging retinal mosaics in the living eye. Eye. 2011; 25: 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spraul CW, Grossniklaus HE. Characterics of drusen and Bruch's membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol. 1997; 115: 267–273 [DOI] [PubMed] [Google Scholar]
- 43. Sarks SH. New vessel formation beneath the retinal pigment epithelium in senile eyes. Br J Ophthalmol. 1973; 57: 951–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007; 48: 968–977 [DOI] [PubMed] [Google Scholar]
- 45. Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999; 117: 329–339 [DOI] [PubMed] [Google Scholar]
- 46. Pauleikoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology. 1990; 97: 171–178 [PubMed] [Google Scholar]
- 47. Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001; 42: 265–274 [PubMed] [Google Scholar]
- 48. Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J Lipid Res. 2010; 51: 451–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barteselli G, Chhablani J, El-Emam S, et al. Choroidal volume variations with age, axial length and sex in health subjects: a three-dimensional analysis. Ophthalmology. 2012; 119: 2572–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramratten RS, van der Schaft TL, Mooy CM, et al. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994; 35: 2857–2864 [PubMed] [Google Scholar]
- 51. Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999; 44 (suppl): S10–S32 [DOI] [PubMed] [Google Scholar]
- 52. Spencer WH. Chapter 8. Vitreous. In: Spencer WH. ed Ophthalmic Pathology. An Atlas and Textbook. 4th ed. Philadelphia, PA: W. B. Saunders, Co.; 1996: 623–666 [Google Scholar]
- 53. Foos RY. Posterior vitreous detachment. Trans Am Acad Ophthalmol Otolaryngol. 1972; 76: 480–49 [PubMed] [Google Scholar]
- 54. Foos RY, Wheller NC. Vitreoretinal junction synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982; 89: 1502–1512 [DOI] [PubMed] [Google Scholar]
- 55. Edelhauser HF, Rowe-Randleman CL, Robinson MR, et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010; 51: 5403–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spencer WH. Chapter 4. Sclera. In: Spencer WH. ed Ophthalmic Pathology. An Atlas and Textbook. 4th ed. Philadelphia, PA: W. B. Saunders, Co.; 1996: 334–371 [Google Scholar]
- 57. Cogan DG, Kuwabara T. Focal senile translucency of the sclera. Arch Ophthalmol. 1959; 62: 604–610 [DOI] [PubMed] [Google Scholar]
- 58. Rao NA, Spencer WH. Chapter 7. Optic nerve. In: Spencer WH. ed Ophthalmic Pathology. An Atlas and Textbook. 4th ed. Philadelphia, PA: W. B. Saunders, Co.; 1996: 513–622 [Google Scholar]
- 59. Giarelli L, Falconieri G, Cameron JD, Pheley AM. Schnabel cavernous degeneration. A vascular change of the aging eye. Arch Pathol Lab Med. 2003; 127: 1314–1319 [DOI] [PubMed] [Google Scholar]
- 60. Kagemann L, Harris A. The clinical utility of colour Doppler imaging. Eye. 2007; 21: 1015–1016 [DOI] [PubMed] [Google Scholar]
- 61. Morgan JI, Hunter JJ, Masella B, et al. Light-induced retinal changes observed with high-resolution autofluorescence imaging of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2008; 49: 3715–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chrenek MA, Dalal N, Gardner C, et al. Analysis of the RPE sheet in the rd10 retinal degeneration model. Adv Exp Med Biol. 2012; 723: 641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]


