Abstract
The aging visual system is marked by a decline in some, but not all, key functions. Some of this decline is attributed to changes in the optics of the eye, but other aspects must have a neural basis. Across mammals, with aging there is remarkable persistence of central structures to which retinal ganglion cell (RGC) axons project with little or no loss of neurons. Similarly, RGC bodies in the retina are subject to variable age-related loss, with most mammals showing none over time. In contrast, the RGC axon itself is highly vulnerable. Across species, the rate of axon loss in the optic nerve is related inversely to the total number of axons at maturity and lifespan. The result of this scaling is approximately a 40% total decline in axon number. Evidence suggests that the consistent vulnerability of RGC axons to aging arises from their high metabolic demand combined with diminishing resources. Thus, therapeutic interventions that conserve bioenergetics may have potential to abate age-related decline in visual function.
Keywords: aging, retinal ganglion cell, optic nerve, lateral geniculate nucleus, superior colliculus, neurodegeneration, axonopathy
Aging and Vision
Age-related loss of sensory activity represents a costly and socially debilitating aspect of general senescence of the central nervous system (CNS). For human beings, this loss is felt most strongly through decline of the visual system, upon which we depend more than other sensory modalities. Accordingly, the visual areas represent the lion's share of cortical representation of sensory function. As we age, peripheral tissues of the eye (cornea, lens, vitreous humor, and so forth) undergo myriad structural changes that influence the efficiency of optical transmission.1 Certainly, age-related degradation in the optics of the eye contributes to some aspects of declining visual function.2,3 Decline in other key functions, such as spatial contrast sensitivity and motion sensitivity, must have a neural basis, since optic changes cannot account for the loss.4,5 Still, other functions remain remarkably intact, and this persistence may inform how well particular visual neural pathways age.6,7
Aging also influences the response of early visual structures to disease-relevant stressors. For glaucoma, the most common optic neuropathy, age is a prominent risk factor alongside IOP, central corneal thickness, and other ocular factors.8 Thus, age-related changes in the neural substrates for vision represent a backdrop for decline in some (but not all) normal functions and increased vulnerability to degenerative disease. The question at hand is whether age-related loss of function and increased susceptibility are related at a mechanistic level, and, if so, whether a common therapeutic intervention can influence both.
Survival of Central Structures
Structural studies indicate little or no age-related change in neuronal density or morphology in the primary visual cortex.9,10 This is not to say that the aging cortex is invulnerable to the ravages of time. Myriad transformations that affect physiological function occur at the cellular and subcellular levels.11 However, if age-related decline in visual function, even if only for a subset of perceptual modalities, reflects loss of tissue, more likely targets are structures that form the projection to the cortex.
The mammalian brain is characterized by a highly conserved projection of the retina into multiple central structures.12 At the optic chiasm, retinal ganglion cell (RGC) axons from the two eyes cross in forming the ipsilateral and contralateral optic tracts to the brain. In rodents, the contralateral projection dominates, while in other mammals RGC axons are parsed more evenly. In the human and nonhuman primate brains, the lateral geniculate nucleus (LGN) of the thalamus receives the primary RGC relay to the primary visual cortex, while in rodents this function is served by the more distal superior colliculus (SC) of the midbrain with a smaller LGN projection to the cortex. In late stages of glaucoma, relay neurons in the LGN of nonhuman primates diminish in number, as do those in postmortem tissue from human patients.13–15 This loss lags substantially behind degeneration of RGC axons in the optic nerve.14 Similarly, in the DBA2J mouse model of glaucoma, we found that SC volume begins to diminish in the oldest animals, but again well after RGC axonopathy in the optic nerve and tract has progressed significantly.16
The story is much different in normal aging. Magnetic resonance imaging of the human LGN shows approximately a 15% reduction in structural volume between 20 and 70 years of age.17 Histologic analysis of postmortem tissue indicates a more dramatic decline (30%).18 Interestingly, in nonhuman primates and rats, LGN volume increases slightly with age, which can lead to an apparent decline in neuronal density.19,20 Despite these changes in tissue volume, the number of neurons in the LGN—regardless of species—does not change.18–20 Similarly, there is remarkable constancy in the physiological response properties of individual LGN neurons in monkeys.5
Though the number and response qualities of RGC relay neurons do not appear to change with age, other aspects of the RGC projection do. The size and complexity of RGC axonal arbors in the mouse SC diminish with age.21 In the aging rabbit, the rate of slow axonal transport along RGC axons to central targets decreases.22 Similarly age-related shrinkage of the DBA2J SC follows deficits in RGC axonal transport after a period of persistence.16 Taken as whole, these observations suggest that even as aging has little influence on the structure of cortical projections from RGC central targets, the RGC axon itself may be more vulnerable.
Age-Related Decline in RGC Axons
The RGC axon is distinguished from the axons of other glutamatergic neurons in the retina by several important characteristics. Unlike photoreceptor and bipolar cell axons, which by necessity are completely unmyelinated, only the shortest segment of the RGC axon is unmyelinated. This corresponds to the length of axon that courses through the nerve fiber layer, through which light must pass to reach the photoreceptors. As the axon penetrates the laminar region of the optic nerve head it becomes myelinated, remaining so throughout the nerve proper and into the optic tract. The number of RGC axons in the optic nerve differs greatly between species, by a factor of 30 between mouse and human, as does the total length of the nerve.23 Even so, across mammals most RGC axons are very thin (0.2–0.7 μm in diameter), approximately half as thick the cone photoreceptor axon despite being 50-fold longer.24 While optimized for minimal firing rate and energy use,25,26 the small size of RGC axons has implications for susceptibility over time to light-induced damage, metabolic stress, disruption of Ca2+ homeostasis, and cytoskeletal degradation.12,27 This susceptibility may explain why axonal transport from the retina to the brain is affected early in glaucoma, and why decline in function is detected first at the distalmost projection sites.12,28 A small axon, especially one with a bioenergetically inefficient unmyelinated segment, also is at a disadvantage should available ATP diminish.29,30 Aging of the CNS includes as one of its many challenges an overall decline in available neuronal adenosine-5′-triphosphate (ATP) available for hydrolysis, which is necessary for the release of stored energy.11 This reduction is associated with axonal dysfunction in other systems and may explain increased RGC susceptibility in optic neuropathy.31–33 Available ATP diminishes in the optic nerve of the DBA2J mouse with age; this is exacerbated by the additional stress of elevated IOP.34 This stress-induced reduction may arise from focal shortages in ATP along the axon due to reduced axoplasmic flow.35
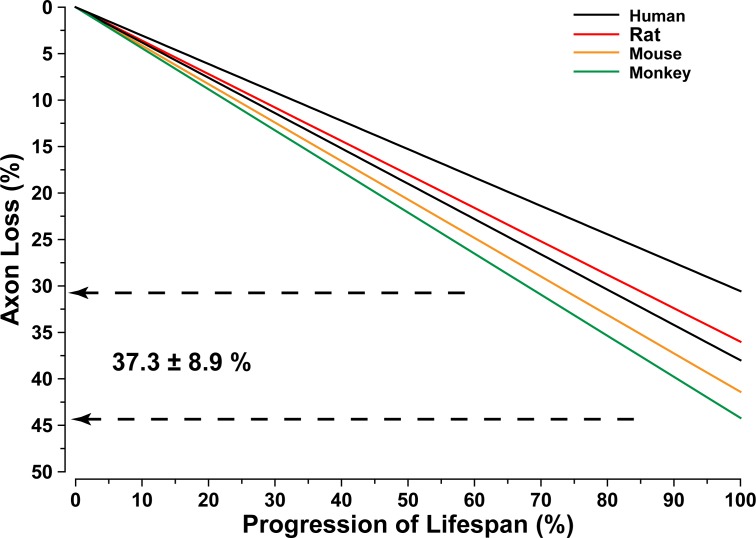
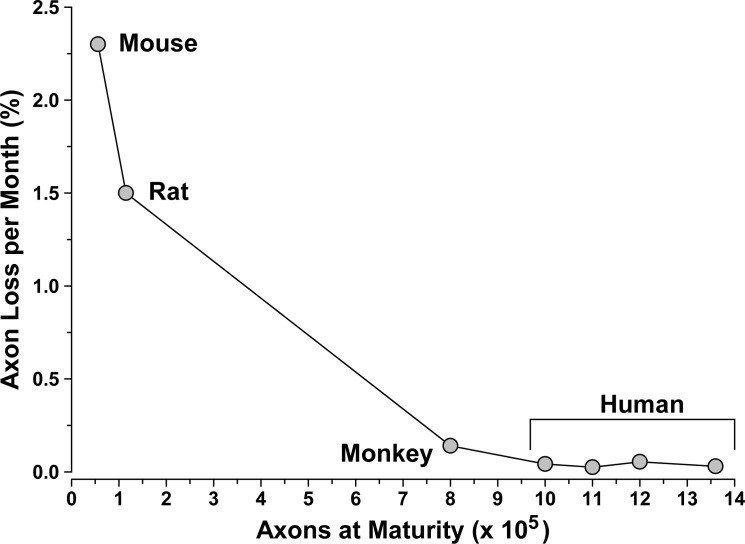
RGC axon loss in animal models of glaucoma is correlated strongly with accumulated exposure to elevated IOP, regardless of species or the nature of the insult.12 Similarly axon number in the optic nerve also declines regularly with age, at least for those studies documenting regular loss. This is an important caveat, for whether due to methodologic or strain differences, or large intrasample variability, some studies simply find little axon loss with age.36,37 Even so, most studies indicate a robust and regular age-related decline in axon number. For example, human and rhesus monkey nerves lose roughly 4500 axons annually.38–40 Other studies indicate an even higher rate of loss,41,42 but variability in the total number of axons at maturity is extremely high.36 This number is an important (and, as it turns out, interesting) determinant of rate of axon loss over lifespan. As the number of axons at peak increases, the rate of axon loss decreases proportionately (Fig. 1). Thus, from mouse (50,000 axons) to rat (100,000) to monkey (1 million) to human (1.5 million), there is a 57-fold decrease in axon loss per month. This scaling corresponds roughly to the fold-difference in lifespan in years, so that regardless of mammalian species the result is approximately a 40% loss in axons over the lifespan.43 It is important to note that the rodent data in Figure 1 arose from Fluoro-Gold (Santa Cruz Biotechnology, Inc., Dallas, TX) labeling of RGCs following retrograde axonal transport from the SC.43 We have shown previously that this assay better reflects RGC axonal integrity in the optic projection than cell body survival,12,44 and it is interpreted as such here.
Figure 1.
Axon loss in the aging optic nerve. Axon loss in the optic nerve as percent of total population at maturity across progression of lifespan. Rates of loss and lifespan from the report of Neufeld and Gachie43; second human set from the study of Jonas et al.40 Range (dashed lines) and average ± SD of total loss indicated. Regardless of species, decline in the number of axons over a lifetime is approximately 40%.
The calculation of total axon loss is made simple by assuming a constant rate over time, as in a linear process (Fig. 2). This seems a reasonable estimation based on the careful measurements for human nerves in the literature.40,41 The rate of loss in human postmortem nerves, roughly 0.5% annually, also is in accordance with in vivo optical coherence tomography measurements of changes in retinal nerve fiber layer thickness.45 This suggests that survival of the unmyelinated segment in the retina and nerve head is likely to be coupled tightly to survival of the distal axon segment in the nerve itself.
Figure 2.
Rate of axon loss depends on axon number. Rate of axon loss in the optic nerve as percent loss monthly as a function of total axons at maturity. Rates and axon number from various sources.38,40,41,43 Larger nerves have more axons and slower rate of decline, corresponding to a longer lifetime.
Persistence of RGC Cell Bodies With Aging
Certain neurons are highly susceptible to age-related loss. For example, the population of rod photoreceptors in the central human retina declines by 30% over a lifetime; the number of cones remains remarkably stable.46 One might assume prominent loss of RGC axons in the optic nerve as described across mammalian species must translate ipso facto to a corresponding decline in RGC bodies in the retina. This is not so. Whether RGC bodies in the retina also are susceptible to age-related loss is a far more equivocal question—at least when it comes to comparing human retina to that of other mammals.
In the rhesus monkey retina, over a near 30-year lifespan the number of RGC bodies does not change regardless of eccentricity from the fovea or retinal quadrant.47 The same is true of the marsupial wallaby retina, where RGC body number remains constant (approximately 200,000) even for those animals living the longest in captivity, up to 15 years.48 In C57BL/6 mouse retina, over a two-year lifespan the number of RGCs does not change.21 However, the dendritic arbors of at least some types of mouse RGC shrink by approximately 20% with a concomitant decrease in the density of inner plexiform layer synapses.21 Other reports that show age-related RGC body loss in C57 retina again used measurements based on retrograde axonal transport of Fluoro-Gold (Santa Cruz Biotechnology, Inc.) from the superior colliculus.49 It is not surprising the noted decrease was similar to axon loss, approximately 40%.49 Finally, the number of RGC bodies in the rat retina does not change with age (up to 30 months), though the retina itself enlarges.50 This enlargement can explain why cell density (cells per unit area), rather than total number, diminishes significantly.51 Thus, across several mammalian species RGC bodies persist over a lifetime.
Accurate cell counts in the human retina are difficult to obtain, due to varying health (and treatments) between subjects and differences in postmortem treatment of tissue. Immune-labeling of RGCs is difficult for a variety of technical reasons, so counts of ganglion cell layer neurons generally include RGC bodies and displaced amacrine cells. This practice is mitigated somewhat by focusing on changes in the central retina, where the fraction of displaced amacrine neurons is below 5%.52 Finally, variability even within tissues of the same age and health is quite high. Whether the RGC population is assessed by axon number in the optic nerve or cell body count in the retina, the possible range can differ 2-fold between normal donors.36,40,52
The human retina, like that of rodents,50 expands over a lifetime, increasing in area by approximately 15%.53 An early study found that RGC density in the human central retina, where displaced amacrine cells are fewest, decreases by a similar amount.54 If so, the result could be explained without actual RGC dropout. However, subsequent studies found a greater decline in central retina density ranging from 25% 53,55 to 43%.56 This decrease cannot be attributable entirely to expansion of retinal area, as total neuron number in the ganglion cell layer also declines by 30% to 45%.53,56 This decline is faster outside of the central retina, but a far greater fraction of displaced amacrine cells there makes estimating actual RGC loss more difficult.52,53 It is important to note that many postmortem samples from even the oldest donors demonstrate cell counts in the range for the youngest donors—and vice versa. Even so, it seems likely that the human retina, unlike that of nonhuman primates and other mammals, does in fact demonstrate some RGC body loss over a lifetime. This is a bit enigmatic, since the rate of RGC axon loss in other animals is far faster.
The Aging Visual Pathways: Key Needs and Opportunities
Could loss of visual function in normal aging bear mechanistic similarities to the most common age-related optic neuropathy, glaucoma? It seems likely. The critical question then is whether by abating age-related decline therapeutically we also can reduce susceptibility to age-related diseases, such as glaucoma. In glaucoma, progression is marked by early RGC axonal dysfunction followed by outright degeneration.12 Central targets in the brain and RGC bodies in the retina persist for a period following axonopathy that represents a window of therapeutic opportunity.57 Similarly, the broad survey presented here indicates a special vulnerability of the RGC axon to age-related decline, with a consistent loss of approximately 40% of the axons in the optic projection over a lifetime regardless of species. This decline occurs even as downstream neurons in central projection sights and in the primary visual cortex persist. Axon degeneration with aging is approximately proportional to the extent of expended lifespan. Thus, just as rate of progression in glaucoma depends on the degree of IOP-related stress,12 perhaps progression in normal aging depends on the actual extent of the lifetime. This could explain why only the human retina appears to progress to actual RGC body loss with age.
Most mechanistic models of aging invoke as a substrate for decline changes in metabolic resources due to diminished mitochondrial efficiency.11 A correlative result is reduced mitochondrial capacity to maintain homeostatic balance of intracellular Ca2+, decreased Na+/K+-ATPase activity, and increased oxidative injury.58 These changes in turn increase CNS vulnerability to stress or injury.11 Older mice with optic nerve crush demonstrate an accelerated loss of RGC bodies.26 Axons are particular vulnerable to the effects of reduced metabolic capacity, since the generation of action potentials is a severe strain on available ATP—more so than even synaptic transmission.59
It seems likely that the visual pathways, like the rest of the brain, are susceptible to diminished metabolic resources with aging. Thus, potential interventions to ameliorate age-related decline should include strategies either to boost bioenergetics or counter oxidative injury resulting from mitochondrial stress. Systemic administration of α-lipoic acid, a natural antioxidant, increased Na+/K+-ATPase activity and reduced neuronal lipofuscin in cortical and subcortical brain regions in aging rats.60 We found that dietary administration of α-lipoic acid reduced lipid peroxidation, protein nitrosylation, and DNA oxidation in retina of the aged DBA2J mouse, while improving overall axon function and survival in the optic nerve.61 Along these lines, systemic delivery of histone deacetylase (HDAC) inhibitor can reduce the transcriptional down-regulation that precedes outright RGC degeneration in the DBA2J.62 HDAC inhibitors also rescue axonal mitochondria, increase ATP levels and reduce excitotoxic levels of glutamate in metabolically challenged optic nerve.63 Thus, targeting oxidative and metabolic stress may be promising avenues for ameliorating the effects of aging on the visual pathways.
The mechanisms through which RGCs age intrinsically, individually and as a network, must inform the development of new neuro-enhancing therapies. Like glaucoma, which, after all, is age-related, normal aging involves a specific challenge to RGC axons in the optic projection. Part of this challenge doubtlessly is associated with axonal milieu in the optic nerve head and associated structures. However, the unmyelinated segment of the RGC axon, in the retina and optic nerve head, also forms close contacts with the processes of astrocyte glia. Since astrocytes are important mediators of neuronal health, any strategy to combat age-related decline must include a more systematic understanding of their contribution.
Acknowledgments
Supported by the Melza M. and Frank Theodore Barr Foundation through the Glaucoma Research Foundation, Research to Prevent Blindness, Inc., NIH EY017427, and the BrightFocus Foundation.
Disclosure: D.J. Calkins, None
References
- 1. Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of ‘real-world' targets. Br J Ophthalmol. 1987; 71: 791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurol Clin. 2003; 21: 709–728 [DOI] [PubMed] [Google Scholar]
- 3. Turner PL, Mainster MA. Circadian photoreception: ageing and the eye's important role in systemic health. Br J Ophthalmol. 2008; 92: 1439–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weale RA. Do years or quanta age the retina? Photochem Photobiol. 1989; 50: 429–438 [DOI] [PubMed] [Google Scholar]
- 5. Spear PD. Neural bases of visual deficits during aging. Vision Res. 1993; 33: 2589–2609 [DOI] [PubMed] [Google Scholar]
- 6. Enoch JM, Werner JS, Haegerstrom-Portnoy G, Lakshminarayanan V, Rynders M. Forever young: visual functions not affected or minimally affected by aging: a review. J Gerontol A Biol Sci Med Sci. 1999; 54: B336–B351 [DOI] [PubMed] [Google Scholar]
- 7. Elliott SL, Werner JS, Webster MA. Individual and age-related variation in chromatic contrast adaptation. J Vis. 2012; 12: 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008; 53 (suppl 1): S3–S10 [DOI] [PubMed] [Google Scholar]
- 9. Haug H, Kuhl S, Mecke E, Sass NL, Wasner K. The significance of morphometric procedures in the investigation of age changes in cytoarchitectonic structures of human brain. J Hirnforsch. 1984; 25: 353–374 [PubMed] [Google Scholar]
- 10. Vincent SL, Peters A, Tigges J. Effects of aging on the neurons within area 17 of rhesus monkey cerebral cortex. Anat Rec. 1989; 223: 329–341 [DOI] [PubMed] [Google Scholar]
- 11. Toescu EC. Normal brain ageing: models and mechanisms. Philos Trans R Soc Lond B Biol Sci. 2005; 360: 2347–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012; 31: 702–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yucel YH, Gupta N, Zhang Q, Mizisin AP, Kalichman MW, Weinreb RN. Memantine protects neurons from shrinkage in the lateral geniculate nucleus in experimental glaucoma. Arch Ophthalmol. 2006; 124: 217–225 [DOI] [PubMed] [Google Scholar]
- 14. Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003; 22: 465–481 [DOI] [PubMed] [Google Scholar]
- 15. Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yucel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006; 90: 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 2010; 107: 5196–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li M, He HG, Shi W, et al. Quantification of the human lateral geniculate nucleus in vivo using MR imaging based on morphometry: volume loss with age. AJNR Am J Neuroradiol. 2012; 33: 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Selemon LD, Begovic A. Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res. 2007; 151: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmad A, Spear PD. Effects of aging on the size, density, and number of rhesus monkey lateral geniculate neurons. J Comp Neurol. 1993; 334: 631–643 [DOI] [PubMed] [Google Scholar]
- 20. Diaz F, Villena A, Gonzalez P, Requena V, Rius F, Perez De Vargas I. Stereological age-related changes in neurons of the rat dorsal lateral geniculate nucleus. Anat Rec. 1999; 255: 396–400 [DOI] [PubMed] [Google Scholar]
- 21. Samuel MA, Zhang Y, Meister M, Sanes JR. Age-related alterations in neurons of the mouse retina. J Neurosci. 2011; 31: 16033–16044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karlsson JO, Inomata M, Kawashima S. Slow axonal transport of soluble proteins and calpain in retinal ganglion cells of aged rabbits. Neurosci Lett. 1992; 141: 127–129 [DOI] [PubMed] [Google Scholar]
- 23. Perge JA, Koch K, Miller R, Sterling P, Balasubramanian V. How the optic nerve allocates space, energy capacity, and information. J Neurosci. 2009; 29: 7917–7928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perge JA, Niven JE, Mugnaini E, Balasubramanian V, Sterling P. Why do axons differ in caliber? J Neurosci. 2012; 32: 626–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008; 211: 1792–1804 [DOI] [PubMed] [Google Scholar]
- 26. Wang AL, Yuan M, Neufeld AH. Age-related changes in neuronal susceptibility to damage: comparison of the retinal ganglion cells of young and old mice before and after optic nerve crush. Ann N Y Acad Sci. 2007; 1097: 64–66 [DOI] [PubMed] [Google Scholar]
- 27. Crish SD, Calkins DJ. Neurodegeneration in glaucoma: progression and calcium-dependent intracellular mechanisms. Neuroscience. 2011; 176: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crish SD, Dapper JD, Macnamee SE, et al. Failure of axonal transport induces a spatially coincident increase in astrocyte BDNF prior to synapse loss in a central target. Neuroscience. 2013; 229: 55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reeves TM, Smith TL, Williamson JC, Phillips LL. Unmyelinated axons show selective rostrocaudal pathology in the corpus callosum after traumatic brain injury. J Neuropathol Exp Neurol. 2012; 71: 198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stys PK. White matter injury mechanisms. Curr Mol Med. 2004; 4: 113–130 [DOI] [PubMed] [Google Scholar]
- 31. Chrysostomou V, Trounce IA, Crowston JG. Mechanisms of retinal ganglion cell injury in aging and glaucoma. Ophthalmic Res. 2010; 44: 173–178 [DOI] [PubMed] [Google Scholar]
- 32. Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer's-like axonal dystrophy. J Neurosci. 2011; 31: 7817–7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007; 292: C670–C686 [DOI] [PubMed] [Google Scholar]
- 34. Baltan S, Inman DM, Danilov CA, Morrison RS, Calkins DJ, Horner PJ. Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci. 2010; 30: 5644–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Band LR, Hall CL, Richardson G, Jensen OE, Siggers JH, Foss AJ. Intracellular flow in optic nerve axons: a mechanism for cell death in glaucoma. Invest Ophthalmol Vis Sci. 2009; 50: 3750–3758 [DOI] [PubMed] [Google Scholar]
- 36. Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989; 96: 26–32 [DOI] [PubMed] [Google Scholar]
- 37. Cepurna WO, Kayton RJ, Johnson EC, Morrison JC. Age related optic nerve axonal loss in adult Brown Norway rats. Exp Eye Res. 2005; 80: 877–884 [DOI] [PubMed] [Google Scholar]
- 38. Mikelberg FS, Drance SM, Schulzer M, Yidegiligne HM, Weis MM. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. 1989; 96: 1325–1328 [DOI] [PubMed] [Google Scholar]
- 39. Morrison JC, Cork LC, Dunkelberger GR, Brown A, Quigley HA. Aging changes of the rhesus monkey optic nerve. Invest Ophthalmol Vis Sci. 1990; 31: 1623–1627 [PubMed] [Google Scholar]
- 40. Jonas JB, Schmidt AM, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992; 33: 2012–2018 [PubMed] [Google Scholar]
- 41. Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000; 41: 741–748 [PubMed] [Google Scholar]
- 42. Sandell JH, Peters A. Effects of age on nerve fibers in the rhesus monkey optic nerve. J Comp Neurol. 2001; 429: 541–553 [DOI] [PubMed] [Google Scholar]
- 43. Neufeld AH, Gachie EN. The inherent, age-dependent loss of retinal ganglion cells is related to the lifespan of the species. Neurobiol Aging. 2003; 24: 167–172 [DOI] [PubMed] [Google Scholar]
- 44. Buckingham BP, Inman DM, Lambert W, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008; 28: 2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci. 2008; 49: 4437–4443 [DOI] [PubMed] [Google Scholar]
- 46. Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993; 34: 3278–3296 [PubMed] [Google Scholar]
- 47. Kim CB, Tom BW, Spear PD. Effects of aging on the densities, numbers, and sizes of retinal ganglion cells in rhesus monkey. Neurobiol Aging. 1996; 17: 431–438 [DOI] [PubMed] [Google Scholar]
- 48. Harman AM, Moore S. Number of neurons in the retinal ganglion cell layer of the quokka wallaby do not change throughout life. Anat Rec. 1999; 256: 78–83 [DOI] [PubMed] [Google Scholar]
- 49. Danias J, Lee KC, Zamora MF, et al. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest Ophthalmol Vis Sci. 2003; 44: 5151–5162 [DOI] [PubMed] [Google Scholar]
- 50. Harman AM, MacDonald A, Meyer P, Ahmat A. Numbers of neurons in the retinal ganglion cell layer of the rat do not change throughout life. Gerontology. 2003; 49: 350–355 [DOI] [PubMed] [Google Scholar]
- 51. Weisse I. Changes in the aging rat retina. Ophthalmic Res. 1995; 27 (suppl 1): 154–163 [DOI] [PubMed] [Google Scholar]
- 52. Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990; 300: 5–25 [DOI] [PubMed] [Google Scholar]
- 53. Harman A, Abrahams B, Moore S, Hoskins R. Neuronal density in the human retinal ganglion cell layer from 16–77 years. Anat Rec. 2000; 260: 124–131 [DOI] [PubMed] [Google Scholar]
- 54. Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992; 33: 1–17 [PubMed] [Google Scholar]
- 55. Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer's disease and aging. Ann Neurol. 1993; 33: 248–257 [DOI] [PubMed] [Google Scholar]
- 56. Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer's disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996; 17: 377–384 [DOI] [PubMed] [Google Scholar]
- 57. Lambert WS, Ruiz L, Crish SD, Wheeler LA, Calkins DJ. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol Neurodegener. 2011; 6: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Toescu EC, Verkhratsky A. Parameters of calcium homeostasis in normal neuronal ageing. J Anat. 2000; 197 (pt 4): 563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012; 32: 356–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arivazhagan P, Panneerselvam C. Alpha-lipoic acid increases Na+K+ATPase activity and reduces lipofuscin accumulation in discrete brain regions of aged rats. Ann N Y Acad Sci. 2004; 1019: 350–354 [DOI] [PubMed] [Google Scholar]
- 61. Inman DM, Lambert WS, Calkins DJ, Horner PJ. Alpha-lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS One. 2013; 8: e65389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pelzel HR, Schlamp CL, Waclawski M, Shaw MK, Nickells RW. Silencing of Fem1cR3 gene expression in the DBA/2J mouse precedes retinal ganglion cell death and is associated with histone deacetylase activity. Invest Ophthalmol Vis Sci. 2012; 53: 1428–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Baltan S, Murphy SP, Danilov CA, Bachleda A, Morrison RS. Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity. J Neurosci. 2011; 31: 3990–3999 [DOI] [PMC free article] [PubMed] [Google Scholar]