Abstract
Reduced quality of life and financial burden due to visual impairment and blindness begin to increase dramatically when individuals reach the age of 40. The major causes of age-related vision loss can be traced to changes to the structure and function of the lens, one of the tissues responsible for focusing light on the retina. Age-related nuclear cataracts, which are caused by aggregation and condensation of proteins, diminish vision because they impede the transmission and focusing of light on the retina. In addition to the slow-developing age-related form, cataracts often develop rapidly as a complication of ocular surgery, such as following vitrectomy or as a consequence of vitreous gel degeneration. Posterior capsular opacification, which can develop following cataract removal, is caused by proliferation and inappropriate accumulation of lens epithelial cells on the surfaces of intraocular lenses and the posterior lens capsule. Presbyopia is a loss of accommodative amplitude and reduced ability to shift focus from far to near objects. Onset of presbyopia is associated with an increase in lens hardness and reduced ability of the lens to change shape in response to ciliary muscle contraction. Avenues of promising research that seek to delay or prevent these causes of low vision are discussed in light of our current understanding of disease pathogenesis and some challenges that must be met to achieve success.
Keywords: cataract, PCO, presbyopia, vitreous humor
Introduction
The lens is a remarkable tissue that is responsible for diffracting light so that it focuses precisely on the retina. Proper functioning of the lens requires it to be optically transparent, allowing light to travel unimpeded through the ocular media to reach its target. To meet the demands of near and far vision, the lens also must be pliable so that the surface curvature can change when needed to adjust the focal distance. These simple requirements are fulfilled for most people during their first 3 decades of life. But at later ages, clear vision begins to deteriorate. Corrective measures are then required to restore visual acuity to the level needed to live, work, and play effectively. Vision problems arise when the lens loses its transparency (cataract) or diminishes in its ability to change shape (presbyopia). Living with presbyopia requires a life style many would prefer to avoid. Corrective lenses (reading glasses, contact lenses) are nonsurgical answers to presbyopia, but they can be unacceptable in some occupational settings. We also pay a high price to restore vision degraded by cataracts. Although cataract surgery is safe and effective, it is not without complications. As a consequence of the sheer number of surgical procedures performed annually in the United States in recent years, cataracts were the most expensive eye disease, costing the Medicare budget more than any eye or vision condition with a medical diagnosis.1
The goal of the following pages is to briefly review what we know about mechanisms leading to the most common forms of cataract and lens defects, and to identify promising areas of research that could lead to more effective treatments than the ones currently available.
Cataract as a Global Burden
According to a 2012 World Health Organization report, it is estimated that 39 million people are blind worldwide. Cataract is responsible for the largest cause of blindness, affecting almost 18 million people.2 When one examines the global distribution of blindness due to cataracts, it is obvious that the burden disproportionately affects low- and middle-income countries. The incidence of cataract is known to increase with age, and no region of the world is immune to the age-related onset and development of vision-threatening cataract. However, the medical infrastructure and financial resources necessary to treat millions of cataracts each year is simply not available in many underdeveloped countries of the world. The scientific community is faced with a major challenge that must be met to effectively deal with the most common cause of blindness in the world. To do so, we must know more about the pathogenesis of cataracts. And we must determine key steps in cataract pathogenesis that can be targeted by new therapeutic strategies to delay or substantially prevent cataract blindness.
Cataract Mechanisms
Cataract is essentially an aggregation disease involving the crystallin proteins that accumulate to exceedingly high concentrations in fiber cells of the lens. The genes encoding α-, β-, and γ-crystallins are dramatically upregulated as lens cells differentiate from epithelium into elongated fiber cells.3 Due to the combined upregulation of gene transcription, robust levels of protein biosynthesis, and low levels of endogenous endoproteases, the protein content of human lens fiber cells rises to approximately 350 mg/mL. The high protein concentration is thought to subserve the refractive properties of the lens and is likely responsible for the refractive gradient necessary for proper optical performance of the lens.
Lens fiber cells lose most of their intracellular organelles during the differentiation process. Nuclei, mitochondria, and the protein biosynthetic capacity are lost in a process of organelle breakdown.4 Therefore, lens cells have an extremely limited capacity to restore crystallins that may become functionally damaged during the aging process. Similarly, breakdown of mitochondria limits capacity to generate ATP.
Cataract can be considered a “perfect storm” if one considers the biological context of the lens and the human eye. The fiber cells, which are the major cell type that constitutes the lens, are endowed with extremely high concentrations of proteins that are not replenished and that remain in situ for the lifetime of the tissue itself. Under normal conditions, lens cells will experience decades of exposure to stresses that are well known to destabilize proteins: ultraviolet light and chemical insult. Worse yet, abnormal conditions can place lens proteins at higher risk for destabilization. Chronic hyperglycemia induces posttranslational modifications of lens crystallins, such as formation of advanced glycation end products.5 Furthermore, the supply of enzymes that usually “search and destroy” damaged proteins is also limited, thus completing the “perfect storm.”
Extensive research has been carried out to better understand biochemical changes to lens crystallins that accumulate with aging and that may play a role in cataract formation. Posttranslational changes, including truncated forms of α- and β-crystallins, are commonly found at early ages in the noncataractous lens, but the abundance of these altered forms appears to be relatively small in comparison with the much larger pool of corresponding full-length proteins at early ages. Other structural changes seem to be associated with older lenses and cataract onset. For example, homo- and hetero-oligomeric protein complexes cross-linked through disulfide bridges accumulate to higher levels in cataracts. Formation of such abnormally cross-linked protein species are most likely a result of oxidative stress and loss of redox balance of glutathione, the major antioxidant in lens cells. The end result of these posttranslational changes is to reduce the stability of crystallin proteins and make it more likely that they will become structurally denatured and condense into aggregates containing other similarly destabilized proteins.6 Lens cytoplasm containing aggregates of proteins gives rise to refractive discontinuities because light must travel through protein-rich and protein-poor pockets as it traverses the lens. Such refractive discontinuities result in diffraction and back scatter of incident light rather than transmission along the optical axis to the neural retina.7 Light scattering induced by protein aggregates is responsible for the poor visual acuity, overall loss of light sensitivity, and white appearance of the lens in patients with cataract.
The major classes of crystallins expressed in the mammalian lens can be allocated on the basis of structural and functional properties into one of three groups, designated α-, β-, and γ-crystallins. The human α-crystallins are expressed from two related genes, αA- and αB-crystallin, which produce approximately 20-kDa protein subunits. αA and αB subunits associate in roughly a 3:1 ratio to form α-crystallin hetero-oligomeric complexes containing approximately 40 subunits in total. As the α-crystallins are evolutionarily related to the family of small heat shock proteins, they appear to be ideally suited as chaperone-like molecules capable of suppressing the aggregation of other proteins in the immediate cellular environment.8 Extensive studies have demonstrated the ability of both αA- and αB-crystallins to prevent the aggregation of client proteins that have been destabilized by a variety of insults. Structure-function studies point to a critical role for a highly conserved “α-crystallin domain” that is present among small heat shock proteins that express the chaperone-like ability to suppress protein aggregation. Indeed, synthetic peptide fragments containing amino acid sequences derived from the α-crystallin domain demonstrate chaperone-like activity when tested under a variety of conditions.9
Although α-crystallin in cytoplasm forms dynamic oligomeric complexes of approximately 100 nm in size, much larger complexes are formed after interaction with client proteins. Using dynamic light scattering to detect and size-estimate light-scattering elements, Datiles and coworkers10 reported an age-related loss of the smaller α-crystallin complexes in a human lens after development of a nuclear cataract. Perhaps this reflects a loss of chaperone-like capacity required to avoid aggregation of other lens proteins as they undergo transient unfolding induced by environmental and metabolic stresses over time. Because of the biosynthetic limits of lens cells to replenish native-size α-crystallin, it is possible that loss of native-sized α-crystallins therefore represents a risk factor but not necessarily a de facto cause of cataract development.11
What are the strategies that can be envisioned to offset the loss of native α-crystallin complexes and restore enough chaperone-like activity to prevent or substantially delay cataract formation?
Recent research has focused on introducing intact α-crystallin protein subunits or peptides containing the α-crystallin domain as a means to restore the protective effects in target cells. Peptides containing the α-crystallin domain (mini-chaperones), as well as full-length αB-crystallin have been shown to protect human fetal retinal pigment epithelial cells from the cell death and caspase activation induced by oxidative stress.12 Efficacy against the cataractogenic effects of oxidative and chemical stress has also been demonstrated with the use of α-crystallin–derived mini-chaperone peptides.13 As a replacement strategy against human cataract will likely require efficient delivery of therapeutic effectors to the lens cell cytoplasm, considerable effort is now being focused on strategies to enhance the penetration of therapeutic crystallins and associated peptides into target cells. Fusion of crystallin polypeptides to cell penetration peptide domains,14 as well as encapsulation of crystallins into biodegradable nano- and micro-particles, is being explored.12
Posterior Capsular Opacification
In a modern cataract procedure, the surgeon removes the cataractous lens by phacoemulsification. In this procedure, the hard lens nucleus is fragmented into small pieces using a narrow probe with a tip that vibrates at ultrasonic frequency. After aspiration of the resulting fragments of lens nucleus, as well as tissue from the outer cortex, an artificial IOL is placed into the “empty” capsular bag and the patient's vision restored after a recovery period of a few hours. Although cataract surgery is considered a safe and effective surgical procedure, it can be conservatively estimated that up to 20% of cataract cases develop posterior capsular opacification (PCO), which results in a loss of clear vision following a period of weeks to months after surgery.15
After the phacoemulsification procedure, a thin layer of lens epithelial cells (LECs) usually remain adherent to the inside of the capsular bag (Fig. 1). A portion of these residual LECs become stimulated to proliferate and migrate along the inner lining of the capsular bag until they reach the posterior aspect of the capsule. There the LECs continue to proliferate and become stacked into a disorganized mass of cells and induce wrinkling and contraction of the capsule. In addition, LECs can migrate over the surfaces of the IOL and proliferate to form masses of cells. PCO results when the site of LEC accumulation and/or wrinkling of the capsular bag falls in the optical axis. The disorganized cell masses and irregularities in the surface of the capsular bag cause scattering of light that would otherwise be focused on the retina, resulting in blurry vision and loss of visual acuity.
Figure 1.
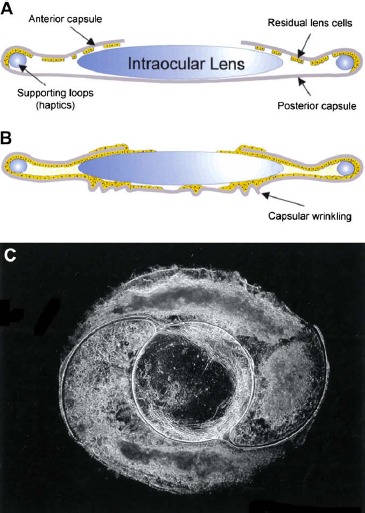
A schematic representation of (A) the postsurgical capsular bag containing an IOL and residual LEC at the anterior and equitorial capsule and (B) the extensive growth and capsular wrinkling that gives rise to posterior capsule opacification. (C) A dark-field micrograph of a capsular bag removed from a donor eye that had undergone cataract surgery before death that exhibits light-scattering regions beneath an intraocular lens. Reprinted from Wormstone IM, Wang L, Liu CSC. Posterior capsule opacification. Exp Eye Res. 2009;88:257–269. Copyright 2009, with permission from Elsevier.
Fortunately, there is an effective outpatient procedure to deal with PCO. To remove the light-scattering elements in the optical axis in the PCO eye, ophthalmologists can give patients a noninvasive clinical procedure involving a brief exposure of the eye to light emitted from a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser. The laser emits short pulses of light energy that create a hole in the posterior capsule (YAG capsulotomy). With cells and their substrate now removed from the optical axis, light can be appropriately diffracted to the retina to restore clear vision. Although the YAG procedure is noninvasive and fast, it is not without complications, which can include retinal detachments, retinal edema, and induction of spikes in IOP. Therefore, YAG capsulotomy may not be suitable for patients who have a history of retinal disease or glaucoma.
Given the possible sight-threatening complications and costs associated with treating PCO, there is a critical need to understand the cellular mechanism that leads to PCO and possible ways to target this complication with either pharmaceutical strategies or by the development of new technologies in IOL materials and design.
Surgical trauma to the eye, as in cataract extraction, induces the production of cytokines and growth factors as part of the wound response. In the context of PCO, TGFβ is one of the major growth factors involved in the response to cataract extraction.16 The TGFβ conveys its information by engaging cell surface receptor complexes, which then transmit their signal through the cell membrane to activate signaling pathways in the cytoplasm and nucleus. In LECs, TGFβ receptor binding causes activation of Smad proteins, which act by increasing the transcription of a battery of genes involved in cell proliferation.17 Thus, TGFβ leads to a shift in the behavior of LECs, transforming them from stationary, rarely dividing cells into cells that proliferate, adopt a fibroblast-like morphology, and express new protein markers, such as alpha smooth muscle actin (αSMA). This transformation from epithelial to a mesenchymal phenotype is known as EMT. By measuring cell proliferation and EMT markers in both human and nonhuman capsular bag models, a large number of laboratories have confirmed a central role for TGFβ.18 However, other factors are likely involved to some extent, as experimental models of PCO can be influenced by blockade of many other growth factors (epidermal growth factor, hepatocyte growth factor, FGF) or factors involved in the inflammatory response (IL-1, IL-6, aldo-keto reductases).18,19 Clearly, much work remains to be accomplished to work out a better understanding of the postsurgical response leading to PCO.
Use of optimized materials and design considerations in the manufacture and placement of IOLs may also diminish the risk of PCO development. It is now well recognized that IOLs made from hydrophobic materials are less favorable substrates for LEC adherence and migration. Manufacturers have adapted by innovating new materials and IOL surface coatings to optimize optical performance while minimizing the risk for PCO.
Mechanical factors relating to the placement of the IOL in the capsular bag also influence the risk of developing PCO. IOLs that have sharp edges are associated with significantly lower risk for PCO, presumably because this design facilitates formation of an efficient barrier to cell migration between the IOL and the inner surface of the lens capsule. IOL design enhancements are constantly being refined, with the next major goal being the production of an IOL that prevents PCO, functions as an accommodating lens, and performs without distracting visual defects such as halos and glare.
Presbyopia
Presbyopia is the condition whereby the ability to focus on near objects becomes diminished. The impact of presbyopia is nearly universal among people as they enter their middle-age years. Although near vision can be regained simply by the use of reading spectacles, for practical and professional reasons there is great demand for a solution that does not require corrective lenses.
The biological basis for presbyopia has received intense study over the years. To adjust for near vision, it is necessary for the lens to “round up” from its unaccommodated shape. This shape change is made possible when the ciliary muscles contract, drawing together the tissues that surround the lens in a sphincter-like motion (Fig. 2). In response, the body of the lens assumes a more rounded shape, altering the radius of curvature mainly at the anterior surfaces. The accommodation response reduces the focal length of the lens to allow clear focusing of near objects on the retina. Thus, the process of accommodation for near vision relies on a change in lens shape in response to contraction of the ciliary muscles.
Figure 2.
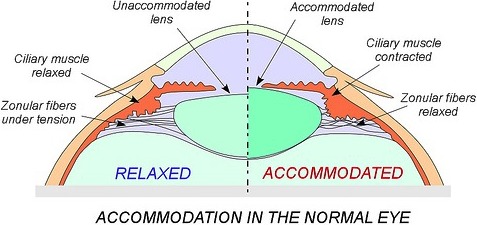
Accommodation in the normal eye. In the unaccommodated eye, the lens is held in a flattened shape by zonular fibers under tension. With contraction of the ciliary muscle, the zonular fibers are relaxed, which allows the lens to assume a more rounded shape with a steeper radius of curvature at the anterior surface. These changes allow clear vision at a closer distance. Reprinted with permission from Azar D, Gatinel D, Hoang-Xuan T, ed. Refractive Surgery. Philadelphia: Mosby-Elsevier; 2006:501–510.
Pliability is a critical factor that underlies the ability of the lens to change shape. Many investigators have shown that the human lens hardens over time, leading to the hypothesis that presbyopia occurs when the lens becomes too hard to change shape in response to ciliary muscle contraction. Studies by a variety of methods have demonstrated a marked increase in lens stiffness beginning in the third to fifth decade of life, which is roughly the age when loss of accommodation becomes noticeable in the early stages of presbyopia.20
The molecular basis for increased lens stiffness is not well understood. Fiber cells in the lens nucleus are held together by an array of intercellular adhesion molecules, gap junction complexes, and ball-and-socket–like interdigitations along their extensive lateral membranes. As the lens ages and more of the lens mass becomes internalized into the nuclear region, it is likely that cells are riveted together with these intercellular junctions, perhaps contributing to the diminished deformability of the tissue.
Metabolic changes in the aging lens could also contribute to a change in its mechanical properties. Recent evidence suggests that the nucleus becomes less accessible to glutathione as a result of the formation of a diffusion barrier in older lenses.21 Lack of sufficient levels of glutathione would result in a redox environment that would facilitate the disulfide-mediated crosslinking of proteins and structural elements in lens cells, which could lead to reduced diffusion of cytoplasmic protein complexes and associated loss of cellular pliability.
Other functional elements of the accommodation process also have been examined as factors in the pathogenesis of presbyopia. Given the key role of ciliary muscle contraction in lens accommodation, many have hypothesized that loss of muscle contractility could contribute to the disease mechanism. However, careful study of ciliary muscle function in the context of age-related effects has demonstrated that muscles retain a sufficient degree of contractility to support the accommodation process. Therefore, it is generally accepted that diminished ciliary muscle contractibility is not a likely cause of presbyopia. Similarly, increased stiffness of the capsule could explain a resistance to lens shape changes, but the available data do not support this as a likely explanation.
Given the decades of experience with cataract surgery, one reasonable approach to the treatment of presbyopia would be to replace the aged lens with one that has accommodation properties. However, despite innovations in the design and fabrication of IOLs, a simple accommodative device that is free from visual defects has yet to be developed. Many of the new accommodative IOL designs rely on the adjustable positioning of multiple optics or changes to flexible surface membranes to achieve the appropriate correction in focal length. Time will tell whether some of these designs will pass the challenges of long-term durability and optical performance in patients. In contrast to the IOL approach, some investigators are developing injectable materials that can be introduced as a liquid into the capsular bag following removal of the natural lens. A chemical or light-activated curing process is then used to induce transition of the material to a gel-like consistency.22 Although the resulting artificial lens is able to undergo shape changes in response to ciliary fiber contraction, further work is needed to create the refractive gradient index that will be required to achieve optical performance coming close to the natural lens.
Vitreous Degeneration
The vitreous body is a clear, gel-like substance that fills the cavity of the eye behind the lens and helps to stabilize the various retinal layers and retinal vasculature. The gel-like characteristics of the vitreous body result from a network of collagen fibrils that extend throughout the gel. Because the vitreous gel has no circulatory flow and does not mix, it effectively impedes the distribution of signaling molecules or nutrients that may be released from surrounding tissues.
Although the vitreous gel is remarkably stable, over time there is a gradual tendency for the gel to collapse, likely a result of degradation or alteration of the collagen fiber network. Patients with degenerate vitreous bodies are at increased risk for tractional forces to develop on the underlying retina, which predisposes them to retinal detachment and disruption of the delicate laminations that characterize the healthy retina. Apart from age-related vitreous liquefaction, other conditions can require removal of the vitreous gel, such as uncontrolled hemorrhaging from unstable retinal blood vessels, typical of patients with diabetic retinopathy. In such conditions, a vitrectomy procedure is used to remove the natural gel along with entrapped blood components followed by replacement with a balanced salt solution.
Although vitrectomy is a major therapeutic advance in the treatment of retinal disease, it carries with it a high risk for nuclear cataract development.23 Recent studies point to oxygen as a cataract-inducing by-product of the vitrectomy procedure.24 Most oxygen in the vitreous gel is thought to originate from the retinal vasculature. Because of its diffusion-limiting physical properties, an oxygen gradient exists in the native eye, with highest levels nearest the retina and comparatively lower levels farthest away near the posterior surface of the lens (Fig. 3). When the contents of the vitreous humor are removed by vitrectomy and replaced with a saline solution, the physical barrier to oxygen diffusion is destroyed. Because components in the vitreous replacement solution mix freely, small molecules released from retinal vessels are distributed throughout the vitreous cavity, thus exposing the posterior lens to abnormally high levels of oxygen. Compelling evidence implicating oxygen as a primary cause of nuclear cataracts after vitrectomy comes from the study of diabetic patients who have lower oxygen in their vitreous due to retinal ischemia. Such patients had less progression of nuclear opacification and nuclear cataracts in the year after vitrectomy. In contrast, more than half of nondiabetics and diabetics with no ischemic retinopathy required surgery for nuclear cataracts within 12 months after vitrectomy.25,26
Figure 3.
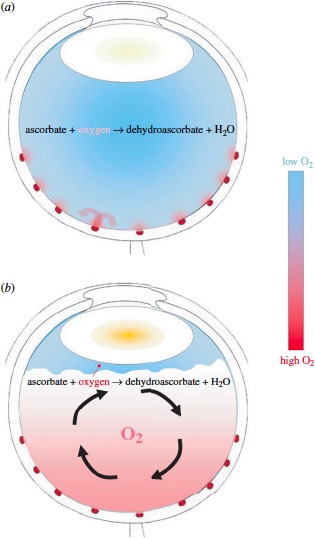
The distribution of oxygen (A) in the normal eye and (B) after degeneration or removal of the vitreous body. (A) In an eye with an intact vitreous body, oxygen diffuses into the vitreous gel from vessels near the surface of the retina. Much of this oxygen is consumed by retinal tissue farther from the vessel (curved red arrows). (B) After the vitreous gel is removed during vitrectomy or degenerates and detaches from the retinal surface, much of the vitreous cavity is filled with liquid. This fluid mixes readily (curved black arrows), carrying oxygen away from the retina and distributing it throughout the vitreous cavity. Mixing also delivers more oxygen to the posterior surface of the lens, where it diffuses into the lens, causing nuclear cataract. Reprinted with permission from Beebe DC, Holekamp NM, Siegfried C, Shui YB. Vitreoretinal influences on lens function and cataract. Philos Trans R Soc Lond B Biol Sci. 2011;366:1293–1300.
Mechanistic studies point to a diminished ability to fight off oxidative stress as an important factor in nuclear cataract formation. Even though the vitreous gel probably protects the aging lens from exposure to oxygen, the inexorable accumulation of oxidatively damaged proteins and lipids likely predisposes the lens to cataract development. Current evidence suggests that vitrectomy results in higher oxygen levels in the vitreous cavity, which would cause accelerated oxidative changes to proteins and lipids in the lens nucleus.27 Further study will be required to test the hypothesis linking increased nuclear sclerotic cataract to increased protein oxidation resulting from exposure to elevated oxygen levels in vitreous substitute solutions.
Needs and Opportunities in Lens Research
Advances in basic research suggest new strategies to delay or prevent vision loss due to age-related changes to the lens. Following is a list of unmet needs and challenges that must be met to achieve the goal of preventing age-related vision loss due to lens defects.
Research Needs and Opportunities in Age-Related Cataract
Confirm and extend the observation that nuclear cataract formation correlates with loss of native-size α-crystallin complexes in the living human lens. At present, we have no validated biomarkers in the precataractous lens that can be monitored noninvasively to monitor disease onset and progression. Recent measurements of α-crystallin changes by dynamic light scattering open the door to this possibility. Additional clinical studies need to be carried out to validate this observation and extend the approach to other patients at high risk for cataract formation, such as in diabetic patients or in patients after vitrectomy.
Loss of native α-crystallin complexes in the aging eye has been correlated with increased risk for nuclear cataract formation. This finding opens the possibility that restoration of α-crystallin, or functional equivalents in the form of small peptides, may delay the onset of cataract formation. Research needs to be conducted to develop effective inhibitors of protein aggregation and to identify the means of introducing these to the lens. Drug-delivery challenges must be overcome to ensure that inhibitors can reach the inner portions of the lens. More research is needed to better understand the structure and distribution of pores that connect lens fiber cells and that could facilitate the transit of therapeutic crystallins or aggregation inhibitors to the lens nucleus.
Drug targets need to be identified to block the development of PCO. The cataract surgery itself seems to provide a good opportunity for drug delivery, given that the site of disease pathogenesis is readily accessible at the time of surgery and the target cells of therapy are compartmentalized in the lens capsule.
Strategies need to be developed to delay protein oxidation and to eliminate damaged proteins as they accumulate in the aging lens.
Research Needs and Opportunities in Presbyopia
More research is needed to identify factors that contribute to the onset and development of presbyopia. Can they be modified?
Research is needed on the composition and delivery of materials that can be introduced to replace the natural lens after cataract surgery. Challenges must be met to have the replacement material restore accommodation and prevent PCO in the recovery period. In parallel, research is needed on the design and production of new IOLs that are free of visual defects (glare, halos) and that have design elements known to inhibit PCO.
Research Needs and Opportunities in Vitreous Degeneration and Substitution
More research is needed to better understand why vitrectomy is associated with significantly elevated risk for cataract. Current evidence suggests that oxygen in vitreous substitutes could contribute. Can this be validated? Are other factors involved?
Research is needed to develop and test the efficacy of vitreous substitutes with gel-like behavior. Do such materials protect the lens from oxygen released from the retinal vasculature and result in reduced risk for postvitrectomy cataract?
Almost 3 decades ago, in its national plan, the National Eye Institute reported that the need for surgery to correct cataract blindness would be reduced by 45% if a therapeutic strategy could be developed to delay the onset and progression of lens opacities by 10 years.28 Even though we have not yet achieved this ambitious goal, the problem has become only larger and more of a burden to the global health care enterprise. The 10-year goal remains a target worthy of the efforts of academic research and industry and deserves a renewed commitment.
Acknowledgments
The author thanks colleagues from his laboratory and department for their helpful suggestions during the planning and editing of this work. The author also thanks colleagues who permitted the re-use of previously published figures.
Supported by National Eye Institute Grants EY005856, EY020361, and EY021498.
Disclosure: J.M. Petrash, P
References
- 1. Salm M, Belsky D, Sloan FA. Trends in cost of major eye diseases to Medicare, 1991 to 2000. Am J Ophthalmol. 2006; 142: 976–982 [DOI] [PubMed] [Google Scholar]
- 2. Rao GN, Khanna R, Payal A. The global burden of cataract. Curr Opin Ophthalmol. 2011; 22: 4–9 [DOI] [PubMed] [Google Scholar]
- 3. Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: many roles for Pax-6. Bioessays. 1996; 18: 621–630 [DOI] [PubMed] [Google Scholar]
- 4. Bassnett S. Lens organelle degradation. Exp Eye Res. 2002; 74: 1–6 [DOI] [PubMed] [Google Scholar]
- 5. Nagaraj RH, Linetsky M, Stitt AW. The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 2012; 42: 1205–1220 [DOI] [PubMed] [Google Scholar]
- 6. Moreau KL, King JA. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med. 2012; 18: 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benedek GB. Cataract as a protein condensation disease: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1997; 38: 1911–1921 [PubMed] [Google Scholar]
- 8. Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992; 89: 10449–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhattacharyya J. Padmanabha Udupa EG, Wang J, Sharma KK. Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry. 2006; 45: 3069–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Datiles MB, III, , Ansari RR, Suh KI, et al. clinical detection of precataractous lens protein changes using dynamic light scattering. Arch Ophthalmol. 2008; 126: 1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McFall-Ngai MJ, Ding LL, Takemoto LJ, Horwitz J. Spatial and temporal mapping of the age-related changes in human lens crystallins. Exp Eye Res. 1985; 41: 745–758 [DOI] [PubMed] [Google Scholar]
- 12. Sreekumar PG, Chothe P, Sharma KK, et al. Antiapoptotic properties of alpha-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Invest Ophthalmol Vis Sci. 2013; 54: 2787–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nahomi RB, Wang B, Raghavan CT, et al. Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem. 2013; 288: 13022–13035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mueller NH, Ammar DA, Petrash JM. Cell penetration peptides for enhanced entry of alphaB-crystallin into lens cells. Invest Ophthalmol Vis Sci. 2013; 54: 2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wormstone IM. Posterior capsule opacification: a cell biological perspective. Exp Eye Res. 2002; 74: 337–347 [DOI] [PubMed] [Google Scholar]
- 16. Chong CC, Stump RJ, Lovicu FJ, McAvoy JW. TGFbeta promotes Wnt expression during cataract development. Exp Eye Res. 2009; 88: 307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saika S, Yamanaka O, Sumioka T, et al. Fibrotic disorders in the eye: targets of gene therapy. Prog Retin Eye Res. 2008; 27: 177–196 [DOI] [PubMed] [Google Scholar]
- 18. Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res. 2009; 88: 257–269 [DOI] [PubMed] [Google Scholar]
- 19. Yadav UC, Ighani-Hosseinabad F, van Kuijk FJ, Srivastava SK, Ramana KV. Prevention of posterior capsular opacification through aldose reductase inhibition. Invest Ophthalmol Vis Sci. 2009; 50: 752–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heys KR, Cram SL, Truscott RJ. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004; 10: 956–963 [PubMed] [Google Scholar]
- 21. Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005; 80: 709–725 [DOI] [PubMed] [Google Scholar]
- 22. Nishi Y, Mireskandari K, Khaw P, Findl O. Lens refilling to restore accommodation. J Cataract Refract Surg. 2009; 35: 374–382 [DOI] [PubMed] [Google Scholar]
- 23. Hsuan JD, Brown NA, Bron AJ, Patel CK, Rosen PH. Posterior subcapsular and nuclear cataract after vitrectomy. J Cataract Refract Surg. 2001; 27: 437–444 [DOI] [PubMed] [Google Scholar]
- 24. Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004; 45: 77–85 [DOI] [PubMed] [Google Scholar]
- 25. Holekamp NM, Shui YB, Beebe D. Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J Ophthalmol. 2006; 141: 1027–1032 [DOI] [PubMed] [Google Scholar]
- 26. Holekamp NM, Bai F, Shui YB, Almony A, Beebe DC. Ischemic diabetic retinopathy may protect against nuclear sclerotic cataract. Am J Ophthalmol. 2010; 150: 543–550.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beebe DC, Holekamp NM, Siegfried C, Shui YB. Vitreoretinal influences on lens function and cataract. Philos Trans R Soc Lond B Biol Sci. 2011; 366: 1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. National Advisory Eye Council Vision Research: A National Plan 1983-1987. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health; 1983. [Google Scholar]


