Abstract
When considering the burden of visual impairment on aging individuals and society at large, it is important to bear in mind that vision changes are a natural aspect of aging. In this article, we consider vision changes that are part of normal aging, the prevalence of abnormal vision changes caused by disorders of the visual system, and the anticipated incidence and impact of visual impairment as the US population ages. We then discuss the services available to reduce the impact of vision loss, and the extent to which those services can and should be improved, not only to be better prepared for the anticipated increase in low vision over the coming decades, but also to increase the awareness of interactions between visual impairment and comorbidities that are common among the elderly. Finally, we consider how to promote improved quality, availability, and acceptance of low vision care to lessen the impact of visual impairment on individuals, and its burden on society.
Keywords: visual impairment, normal aging, health care delivery
Introduction
Aging has a profound impact on human visual function. Not only does it affect the structures and function of the eye itself, as shown in articles throughout this special issue, but it also changes the functionality of many structures in the central nervous system that support visual perception and performance, visually-guided activities of daily living, and vision-related cognitive abilities. Thus, in aging individuals, the effects of pathologic changes in the eye and along the visual pathway are exacerbated by these “normal” age-related systemic and sensory changes, and by other comorbidities. Conversely, the self-care required to manage those changes and comorbidities may become too burdensome for an elderly person whose vision is affected by age or age-related disease. Here, we summarized the normal and pathologic changes in vision among the elderly population, and examined to what extent the impact of such changes can be mitigated at present, and what future improvements could be effected in this area.
Vision in aging populations has been reported in the context of major population-based longitudinal studies, some now spanning several decades of follow-up. The Beaver Dam,1 Blue Mountains,2 and Rotterdam3 eye studies sought to improve the knowledge of eye disease and visual impairment (VI) in a representative sample of the local population, as part of a broader epidemiologic inventory, while the Salisbury Eye Evaluation (SEE)4 and Smith Kettlewell Institute (SKI)5 studies sought to enroll the entire qualifying local population, and specifically targeted eye diseases. In the SKI study, with participants aged 58 to 102 at initial presentation, all available individuals in the oldest age cohorts, were enrolled and younger cohorts sampled proportionally to obtain a roughly flat distribution across ages. In the SEE study, all available individuals of African-American descent over age 65 (18% of this population segment) were recruited, with 61% and 56% random samples from the white population aged 75 and older, and 65 to 74, respectively. Both strategies resulted in maximizing statistical power by increased recruitment from otherwise underrepresented population segments.
While these population-based studies all collected information on important vision measures, such as refraction, cataract status, visual acuity (VA), and screening visual fields, the SKI and SEE studies made an effort to collect a broad range of additional measures related to vision, albeit with somewhat different emphases. The SKI study collected additional measures of contrast sensitivity, glare sensitivity, low contrast VA, stereopsis, color vision, and dark adaptation, all of which can be classified as visual function measures, whereas the SEE study concentrated on measured and self-reported performance in activities of daily living and, more recently, complex visually guided activities, such as driving.6
In the following sections we summarized the findings regarding vision changes in normal aging, followed by those due to prevalent eye disease, and their impact on quality of life and functional independence. We concluded this overview with potential improvements in intervention and care to maximize the use of patients' remaining vision.
Vision Changes in Normal Aging
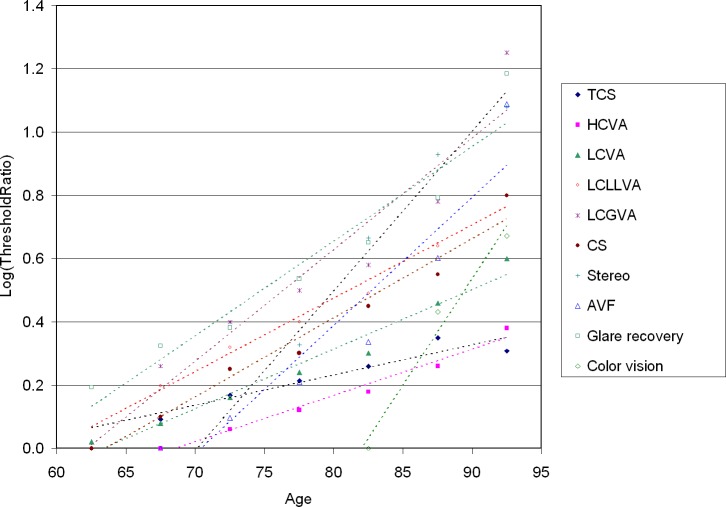
Vision changes in normal aging have been studied by a number of research groups; an excellent summary can be found in the 2011 review in Vision Research by Owsley.7 Normal aging brings about changes in the intraocular transmission and scatter of light, density of photoreceptors, efficacy of phototransduction and photopigment regeneration, and quality of synaptic transmission and signal processing in the retina and beyond. Most studies of vision and aging have examined a limited set of vision measures in small groups of older individuals, and compared these to normal adult values. The largest sample of very old individuals followed longitudinally can be found in the SKI study, and one of its most important findings is summarized in Figure 1, drafted using SKI study data kindly provided by G. Haegerstrom-Portnoy for this paper; VA and contrast sensitivity data from that study have been published previously.5 Figure 1 shows, on a logarithmic scale, how the thresholds for a variety of vision measures change with age, compared to the normal adult value. As indicated by these regression lines, thresholds increase proportionally from year to year, starting at the age where the line intersects the horizontal axis. The age of onset and the annual rate of change appear to vary markedly, depending on the measure of interest. The review by Owsley7 cites several studies that have hypothesized that the detection of second order visual stimuli (those thought to require the involvement of multiple detection mechanisms in visual cortex) are more severely affected by aging than simple stimuli, such as flicker detection (temporal contrast sensitivity [TCS] in Fig. 1), and this certainly could explain why TCS has the shallowest increase with age. Other measures in Figure 1, such as color vision and stereoacuity, may have artificially steep regression lines due to the poor discrimination abilities of the stereo cards and D-15 test used to measure them. What seems clear from this Figure is that the rise of high contrast VA starts close to a decade later than other measures, possibly because it is less affected by optical factors, such as yellowing of the lens and scatter in the intraocular media.
Figure 1.
Age dependence of the average threshold for 10 visual function measures, in log units relative to the normal adult value. Glare recovery was the time required to read 0.2 log units above threshold on the SKILL card, following a 1-minute glare exposure; color vision was the Adams desaturated D-15 score. For details see the report of Haegerstrom-Portnoy et al.5 HCVA, high contrast VA; LCVA, low contrast VA (low contrast Bailey-Lovie chart); LCLLVA, low contrast/low luminance VA (SKILL card); LCGVA, low contrast VA under glare conditions; CS, Pelli-Robson contrast sensitivity; Stereo, stereo plates (4 levels); AVF, Smith Kettlewell attentional visual field ratio.
A secondary effect of aging, not visible in Figure 1, but widely reported in studies of visual function in the elderly, is the increased range of values. While some elderly individuals appear to have the vision of a 30-year-old, others have markedly increased thresholds, even in the absence of overt pathology. In an analysis of psychophysical and electrophysiologic measures taken from a range of peer-reviewed papers, Johnson and Choy8 concluded that increasing variability with age may account for an important fraction of the overall average threshold increase seen in the population. They speculated that this may be due to latent pathology in many elderly individuals or to natural variability of the aging process.
One might expect a high degree of correlation between changes in different visual function measures within a single person, and, thus, hypothesize that elderly patients with good visual acuities would not show large changes in other measures. To test this hypothesis, Haegerstrom-Portnoy et al.5 performed a separate analysis limited to participants with best-corrected VA better than 20/40, that is, near normal, and determined the number of these near-normally sighted individuals showing a 10-fold worsening in other visual function measures increased rapidly with age, for almost every measure tested. This finding supports the notion that large changes in most of the vision measures shown in Figure 1 are part of normal aging rather than caused by undiagnosed eye pathology.
While the measures in Figure 1 all refer to basic psychophysical visual functions, changes with age in the performance of activities of daily living (ADL) have been studied in the SKI and SEE studies as well, in addition to smaller studies by other groups. As part of the SKI study, Lott et al.9 measured the distance, as a function of age, at which participants could recognize faces and/or facial expressions, and also asked the participants how often in daily life they had difficulty recognizing familiar faces from across a room or in dim light. They found a high correlation between the self reports and test data, and a steady decline of these abilities after age 65, with more pronounced losses after age 80.
Driving is another visual ADL that has been studied extensively in the last decade, both in simulators and on the open road, in the latter case with either a driving instructor in the vehicle, or with multiple camcorders set up to record the drivers actions, and the relation of the vehicle to other traffic, and to road markers and signals. Most vision studies find correlations between driving performance and driver age, but the role of vision is uncertain. In the Salisbury Eye Evaluation Driving Study (SEEDS), an increase in visual field defects was associated with increased wait times at stop signs in urban drivers10 and with self-restrictions in night driving,11 which also correlated with reduced contrast sensitivity; running red lights correlated with a reduction in attentional visual field.12 These and other aberrant driving behaviors, such as running stop signs or making unsafe lane changes, were correlated primarily with cognitive factors, suggesting that vision changes have a relatively minor role in accounting for age-related worsening in the performance of complex tasks, such as driving.
Considering these findings in normally sighted elderly populations, it becomes clear that the effects of vision loss due to eye disease in the elderly can be understood and addressed only in the context of the many changes associated with aging, including nonvisual disabilities and comorbidities that affect many elderly individuals
Vision Changes in Low Vision
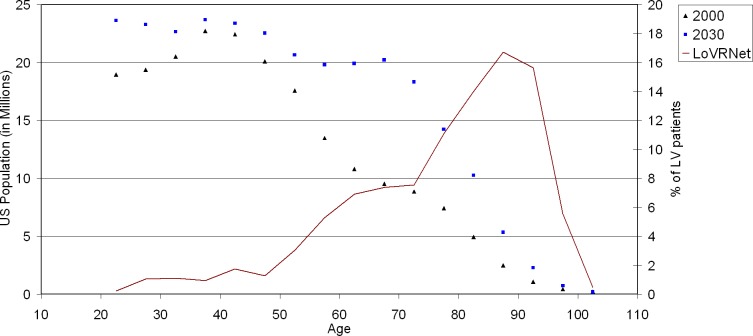
The condition of VI, defined as best-corrected VA 20/40 or worse in the better-seeing eye, affects millions of Americans. The actual prevalence of VI is unknown, but numbers ranging from 3 to 6 million are cited commonly. Based on the 1999 to 2002 National Health and Nutrition Examination Survey (NHANES) data, Vitale et al.13 estimated that, of the approximately 11 million Americans over age 12 with uncorrected VA worse than 20/40, fewer than 3 million could not be corrected to better than 20/40 in the better eye, and only these should be considered to be visually impaired. That still is a respectable number, and it is bound to increase as the population ages. The reason for this is seen easily in Figure 2, in which data from the report of Goldstein et al.,14 showing the age distribution of 764 patients presenting for low vision management at 28 US centers participating in the Low Vision Rehabilitation Network (LOVRNet) study, are plotted along with the age distribution of the US population in 2000 and 2030. Assuming that the causes of vision loss will remain constant, the near-doubling of the age cohorts over 60 inevitably will lead to a similarly increased demand for low vision services in all but the youngest age groups.
Figure 2.
Drawn line graph: age distribution of the patients enrolled for low vision rehabilitation at 28 centers participating in the Low LoVRNet study.13 Symbols: age distribution of the US population over age 20, in the years 2000 (Census data) and 2030 (projected), illustrating the expected doubling of demand for low vision services by 2030, compared to the beginning of the century.
For the purpose of third party coverage and demographics, the terms VI and “low vision” often are used interchangeably, referring in the United States to best-corrected VA in the range from 20/40 to 20/200 in the better eye; the World Health Organization places the boundaries for low vision at 20/60 and 20/400. Low vision care providers, on the other hand, tend to define the term low vision functionally as “a condition in which an individual is not able to perform customary visual tasks without tools beyond refractive correction, and without assistance,” acknowledging that high contrast VA is not necessarily the determining factor.
The condition of VI is strongly age-dependent, with 73% of the patients enrolled in the LoVRNet study over age 65.14 In that study, female sex also was overrepresented (66%), which may in part be due to greater willingness among women than men to seek care. Age and sex are not the only factors affecting VI prevalence in population-based studies. Entering VAs of participants in the SEE study showed the prevalence of VI among blacks being almost twice that among whites (10.4% vs. 5.6%).15 This matched previous reports in the Baltimore Eye Survey, where it was attributed to differences in racial prevalence of diabetic retinopathy and glaucoma, which are found predominantly in blacks, and of age-related macular degeneration (AMD), predominantly found in whites.16 A substantial percentage of VI in the Baltimore Eye Survey was found to be correctible through surgery (e.g., 36% was due to cataract) and other treatments. Similar percentages have been found in other population-based studies, an indication that lack of access to eye care may be an important cause of VI, even in the developed world.
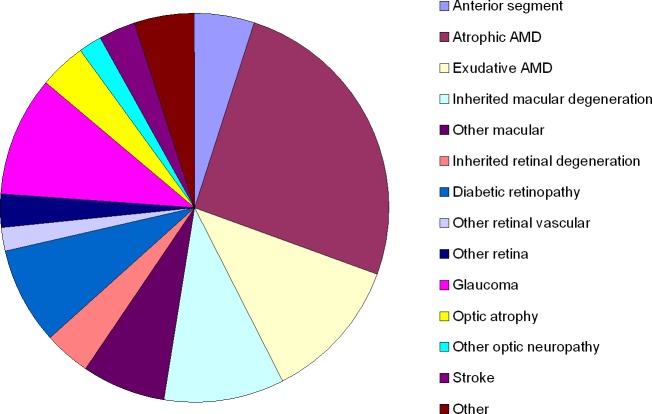
Data regarding cause-specific VI based on VA alone have been published for most of the population-based studies, but such data are harder to find for other types of VI, such as loss of contrast sensitivity, peripheral visual field, or patches of vision close to fixation (paracentral scotomas), dark adaptation, and glare recovery. Studies of low vision populations have been more diligent collecting such information, but even for those studies the information is incomplete. In the LoVRNet study, over half of the individuals seeking care suffered from conditions affecting the macula, and another 20% had other retinal conditions limiting their vision, as shown in Figure 3.14 As would be expected with this high prevalence of macular pathology, reduced VA was the primary cause of VI among these patients: using their habitual correction, 25% had VA < 20/200, and another 38% had VA of 20/70. Of the remaining 37%, just over one fifth (22%) had moderate or severe contrast sensitivity loss.
Figure 3.
Causes of VI among 675 participants in the LoVRNet study (based on Table 1 in the study of Vitale et al.14), ordered according to the affected stage along the visual pathway.
The effects of different types of VI on daily life can vary widely, and low vision rehabilitation programs will need to address the specific type of impairment, tailoring the intervention to each patient's unique situation. Loss of VA may cause difficulties with many activities of daily living that require seeing fine detail, including reading, completing a form, setting the dial of a thermostat, or using small tools. Loss of contrast sensitivity is more likely to affect activities that require the distinction of hue or gray scale, such as face recognition, seeing curbs and other drop-offs, and matching clothes and accessories. Loss of peripheral field leads to difficulties detecting obstacles, avoiding collisions while walking or driving, and orienting oneself relative to others, while paracentral scotomas in macular disease lead to distortions, metamorphopsias, and objects or text simply “disappearing.” Dark adaptation problems not only affect the ability to see at night, but also the ability to find a seat when entering a dimly lit restaurant or a dark movie theater, and the temporary vision loss one experiences on a bright day when driving into or out of a tunnel. Glare disability is the loss of vision one experiences when a bright light sources illuminates the eye while viewing a much dimmer target, such as a traffic light with the sun low in the sky, or road signs and pavement markings against oncoming headlights at night.
Role of Low Vision Rehabilitation
In the medical model of low vision care, patients with an indication of low vision are evaluated for possible deficits in their use of vision during the performance of daily activities. If confirmed, a treatment plan is formulated that then is implemented by one or more rehabilitation specialists. The goal of low vision rehabilitation is to reduce the impact of VI and minimize disability, through one or more concurrent approaches: prescription of assistive devices and training in their use, adaptations to the environment to reduce visual demand, and instruction as needed, and referral for the management of comorbidities that interact with VI.
Assistive devices have been revolutionized by improved optics, development and miniaturization of optoelectronics, the advent of digital technology, and the development of bioengineering applications. As recently as a generation ago, magnifying glasses and optical telescopes were the only assistive tools available to patients with vision loss. In the 1980s and 1990s, closed circuit television (CCTV) readers and a few head-mounted video magnifiers offered the potential of variable magnification and contrast enhancement/inversion, at substantial cost. Besides these devices, which were limited to making visual information more visible, nonoptical aids, such as signature guides, tactile markings on stove dials, and telephone news reading services, were developed to allow access for those whose visual deficit could not be remedied by optical means. The advent of text-to-speech conversion, mobile technology from small electronic magnifiers to cell phone cameras, and, most recently, a wide variety of mobile applications (apps) have greatly magnified the power and versatility, and reduced the cost of assistive technology. Most recently, an implantable telescope, electronic retinal implants, and advanced sensory substitution devices, such as the Brainport,17 have added new technologies to the field of low vision rehabilitation. The addition of such technological innovations has moved, and in a sense almost removed, the boundary between low vision rehab and blind rehab, since several of these technologies now are shared by the partially sighted and the functionally blind.
Adaptation of the environment has traditionally been limited to improving lighting, adding tactile bumps and audible signals at crosswalks, increasing visibility of obstacles and drop-offs, and substituting high- for low-contrast items. In recent years, this field also has been enhanced by testing the use of radio frequency identification (RFID) tags and other “smart elements” in the environment. These developments are likely to become more prominent as new and more affordable tools are being developed, especially if such technology is “dual use,” that is, can be grafted upon platforms that are used widely by consumers and, therefore, is affordable, such as the smart phone, and has utility to able-bodied users, such as speech output from a GPS route planner.
As these assistive devices and environmental adaptations become more sophisticated, the role of low vision rehabilitation experts, that is, teachers of the visually impaired, occupational therapists with training in low vision rehab, and orientation and mobility (O&M) trainers, will become increasingly important and demanding. This is true particularly if most of their clients will be in their 70s and beyond. Even the most ergonomic and user-friendly hi-tech device will require a carefully tailored instruction and practice program if it is to be accepted by this population. For the rehab experts themselves, the availability of continuing education courses covering the newly developed devices and their optimal use already has become crucial, and this trend is only expected to accelerate.
Role of Comorbidities
The presence of other disorders and health limitations among low vision patients is an important factor in planning their rehabilitation process, and the prevalence of such conditions should not be underestimated. Among 764 participants entering the LoVRNet study,14 only one-third qualified their general health as fair or poor, yet on closer questioning many of the remaining individuals considering themselves in good or even excellent health reported pain, high blood pressure, and falls in the last 2 years. When asked about their emotional state, 88% of respondents classified themselves as well-adjusted, yet with 42% reporting being frustrated, 23% anxious, and 22% depressed, among others, it appears that the qualification “well adjusted” does not tell the complete story. On detailed questioning, a wide range of physical and mental health problems was found in this study population. Successful low vision rehabilitation can happen only if the presence of these comorbid conditions is taken into account. One of the most important tasks of the low vision rehab specialist is to understand how to optimize the client's self care of these conditions.
Having low vision has an immediate impact on the ability to manage medication use, avoid falls, and maintain independence. For this reason, it is clear that a low vision rehabilitation plan will have to take into account the presence of comorbidities, and the tools required to allow the individuals to maintain or even improve their health status. Conversely, comorbid conditions, such as limited grip strength, movement limitations, memory problems, and emotional distress, inevitably will have a negative impact on the progress and success of a low vision rehabilitation plan. Here again, the low vision rehab specialist must understand how the rehab plan can be adjusted to minimize this impact and maximize progress toward functional independence.
Gaps in Low Vision Care Delivery
The appreciation of the need for low vision rehabilitation, and its availability and quality in the United States have made important progress over the last quarter century, especially since the acceptance of low vision care as a reimbursable form of assessment and rehabilitation under Medicare in the late 1990s. That's the good news. The not-so-good news is that there still are large areas of the country where eye care providers do not have a sufficient appreciation of the complexities associated with chronic VI, where thorough low vision evaluations are not performed, and where no qualified low vision rehabilitation therapist or O&M specialist is available. Moreover, most third party payers limit the amount of therapy by imposing an annual cap on the number of physical and/or occupational therapy units a beneficiary can receive, and this cap encompasses low vision rehabilitation; for patients with physical comorbidities, this can form an important barrier to obtaining adequate care in a timely fashion.
In addition to the availability of accessible high-quality care, patient motivation and support are important conditions for successful initiation and completion of the low vision rehab process. Two groups can have a critical role in creating the conditions that will foster success: caregivers (including relatives and friends) and the community at large. Awareness of VI has greatly improved over the last decades, through broadcast public service announcements, public education websites, and community-based initiatives for improved accessibility. Yet, although awareness of the condition may have improved, the public at large is not well informed about the possibilities of rehabilitation. Public service organizations, such as the Lions Clubs, are playing an important role, both in bringing the availability of low vision rehabilitation to the attention of members of their communities, and by providing transportation and other support to visually impaired community members.
The single most important gap in low vision care delivery in the United States is the lack of insurance coverage for assistive devices. Low vision patients who are employed or participate in vocational training, and who depend on certain devices for gainful employment are entitled to coverage of assistive devices through the mandates of the Americans with Disabilities Act and, state services for the blind and visually impaired, respectively. Similarly, the cost of assistive devices is covered for individuals with vision disabilities who qualify for Veterans Administration benefits. Unfortunately, these conditions do not apply to the great majority of visually impaired elderly individuals. The only cases in which Medicare or other third party payers have been compelled to cover assistive devices, such as a CCTV reader, through court action were those where the plaintiff successfully made the case that the assistive device functions as a prosthesis rather than the equivalent of a pair of glasses (which would exclude it from coverage under the Medicare statute). Ironically, the tendency for consumer products, such as smart phones, to be adapted as tools for the visually impaired has made it harder to claim reimbursement for them as prosthetic devices.
In a compelling study of the likely impact of a change in Medicare policy toward allowing coverage for assistive devices in cases of significant VI with good rehabilitation potential, Morse et al.18 estimated that the utilization cost of such a benefit would be on the order of $800 per person for approximately 40,000 Medicare beneficiaries per year, if a consistent set of qualification criteria was drafted. In other words, the cost to the Medicare program would be approximately 1% of the $3.4 billion it spends annually on cataract-related expenses.
Toward a Reduction of the Low Vision Burden
As is clear from the population distributions in Figure 2, a significant increase in the prevalence of low vision is expected, unless its incidence can be reduced along with that of the underlying disorders, through a combination of research and public health education as advocated by other investigators in this special issue. Even if the increase can be mitigated, it is likely that a substantial burden of low vision will persist well into the future, and, therefore, we need to look at the three ways we can address this burden.
Improving the Quality of Low Vision Care
There are many excellent low vision care providers, and a substantial effort is under way to collect additional evidence and further improve the standards of successful low vision rehabilitation. Further support from the National Eye Institute, and other sponsoring agencies and foundations will be required to obtain more detailed outcomes data and develop better care delivery models. This research effort will have to be translated into training programs, fellowships, and certification standards for low vision physicians and therapists to become experts in a wide range of rehabilitative options, and to maintain their expertise as these options increase. An important part of this training will have to address the understanding of comorbidities that are prevalent among older low vision patients, and the need for comanagement of these conditions with geriatricians and other care providers.
Increasing the Availability of Low Vision Care
It is unrealistic to expect that every low vision care provider will be trained in handling the most complex cases, and that every eye care provider seeing elderly patients will need this level of expertise. Low level certification standards should be established for most eye care providers, so they are competent to handle patients with mild VI and to recognize which patients should be referred to specialized secondary or tertiary centers, due either to their degree of impairment or to the complexity of their comorbidities. Such a tiered system also allows secondary centers to provide consultation services and initial rehabilitation, but then refer patients back to their community health centers for follow-up care. This system has been in use for many years in Sweden, where it has led to greatly improved access to low vision care. While such strictly organized multitiered care is unlikely to become the norm in the United States, it certainly can be promoted through a system of continuing education courses and certificates for primary level low vision care providers.
Increasing the Awareness of Low Vision Care
Even with the many options for low vision rehabilitation available today, too many patients with vision loss do not visit eye care providers, or are if they do, are not referred to a low vision care provider. Improving this situation will require education of eye care professionals as well as the public at large. It is encouraging that the National Eye Institute, Lions Clubs, and many patient advocacy organizations are fully behind these education efforts, and there is reason for optimism that the awareness of low vision care will not be the rate-limiting step in reducing the burden of low vision.
Reducing the Economic Burden of VI
Finally, there needs to be a wider recognition of the economic impact of low vision loss, and of the cost effectiveness of low vision care. This not only could help make funds available for training and certification programs for low vision care providers, it also may be the best hope for low vision patients that Medicare and other third party payers will consider coverage for low vision assistive devices. It is unlikely that such a change will be adopted any time soon, but a few carefully chosen demonstration projects could address the question whether coverage of devices would lead to better outcomes and a lower overall financial burden, through greater independence of the patient and reduced cost due to medical complications.
In summary, addressing the consequences of vision loss in the aging population should be an important aspect of the research agenda for visual health in the coming years.
Acknowledgments
Presented at the 2013 Ocular Research Symposia Foundation meeting on The Aging Eye, June 14–16, Rancho Palos Verdes, California.
Supported by National Institutes of Health Grants R01 EY012843 and R01 EY021220 (GD). The author alone is responsible for the content and writing of the paper.
Disclosure: G. Dagnelie, None
References
- 1. Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006; 142: 539–549 [DOI] [PubMed] [Google Scholar]
- 2. Taylor HR, Keeffe JE, Vu HT, et al. Vision loss in Australia. Med J Aust. 2005; 182: 565–568 [DOI] [PubMed] [Google Scholar]
- 3. Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001; 42: 2237–2241 [PubMed] [Google Scholar]
- 4. Rubin GS, West SK, Munoz B, et al. A comprehensive assessment of visual impairment in a population of older Americans. The SEE Study. Salisbury Eye Evaluation Project. Invest Ophthalmol Vis Sci. 1997; 38: 557–568 [PubMed] [Google Scholar]
- 5. Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Seeing into old age: vision function beyond acuity. Optom Vis Sci. 1999; 76: 141–158 [DOI] [PubMed] [Google Scholar]
- 6. Rao P, Munoz B, Turano K, Munro C, West SK. The decline in attentional visual fields over time among older participants in the Salisbury Eye Evaluation Driving Study. Invest Ophthalmol Vis Sci. 2013; 54: 1839–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Owsley C. Aging and vision. Vision Res. 2011; 51: 1610–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson MA, Choy D. On the definition of age-related norms for visual function testing. Appl Opt. 1987; 26: 1449–1454 [DOI] [PubMed] [Google Scholar]
- 9. Lott LA, Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Face recognition in the elderly. Optom Vis Sci. 2005; 82: 874–881 [DOI] [PubMed] [Google Scholar]
- 10. Keay L, Jasti S, Munoz B, et al. Urban and rural differences in older drivers' failure to stop at stop signs. Accid Anal Prev. 2009; 41: 995–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaleem MA, Munoz BE, Munro CA, Gower EW, West SK. Visual characteristics of elderly night drivers in the Salisbury Eye Evaluation Driving Study. Invest Ophthalmol Vis Sci. 2012; 53: 5161–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. West SK, Hahn DV, Baldwin KC, et al. Older drivers and failure to stop at red lights. J Gerontol A Biol Sci Med Sci. 2010; 65: 179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. Jama. 2006; 295: 2158–2163 [DOI] [PubMed] [Google Scholar]
- 14. Goldstein JE, Massof RW, Deremeik JT, et al. Baseline traits of low vision patients served by private outpatient clinical centers in the United States. Arch Ophthalmol. 2012; 130: 1028–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. West SK, Munoz B, Rubin GS, et al. Function and visual impairment in a population-based study of older adults. The SEE project. Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1997; 38: 72–82 [PubMed] [Google Scholar]
- 16. Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology. 1996; 103: 1721–1726 [DOI] [PubMed] [Google Scholar]
- 17. Nau A, Hertle RW, Yang D. Effect of tongue stimulation on nystagmus eye movements in blind patients. Brain Struct Funct. 2012; 217: 761–765 [DOI] [PubMed] [Google Scholar]
- 18. Morse AR, Massof RW, Cole RG, et al. Medicare coverage for vision assistive equipment. Arch Ophthalmol. 2010; 128: 1350–1357 [DOI] [PubMed] [Google Scholar]