Abstract
The development of treatment protocols with reduced toxicity and equivalent or improved efficacy for Trypanosoma cruzi infection is a priority. We tested the effectiveness of benznidazole (BZ), nifurtimox (NFX), other prospective drugs in intermittent and combined treatment protocols to cure T. cruzi infection initiated with susceptible and drug-resistant parasite strains. A 40-day course of BZ, NFX, or the oxaborale AN4169 cured 100% of mice, whereas posaconazole (POS), and NTLA-1 (a nitro-triazole) cured approximately 90% and 20% of mice, respectively. Reducing the overall dosage of BZ or NFX by using an intermittent (once every 5 days) schedule or combining 5 daily doses of POS with 7 intermittent doses of BZ also provided approximately 100% cure. T. cruzi strains resistant to BZ were also found to be resistant to other drugs (POS), and extending the time of treatment or combining drugs did not increase cure rates with these isolates. Thus, dosing schedules for anti–T. cruzi compounds should be determined empirically, and compounds targeting different pathways may be combined to yield effective therapies with reduced toxicity. This work also suggests that standard treatment protocols using BZ and NFX may be significantly overdosing patients, perhaps contributing to the adverse events.
Keywords: Chagas disease, Trypanosoma cruzi, benznidazole, intermittent treatment, drug discovery
Chagas disease results from persistent infection with the protozoan parasite Trypanosoma cruzi. It is one of the world's most neglected tropical diseases [1, 2] and is the highest impact parasitic disease in Latin America. Chemotherapy of this infection remains an enormous challenge. Benznidazole (BZ) and nifurtimox (NFX), the available drugs for the treatment of T. cruzi infection, are usually recommended in the acute phase or short-term chronic phase of the infection. However, although both drugs have proven positive impact on chronic infection [3–7], they are not consistently used in part because of their substantial side effects and the difficulty of determining treatment outcomes in chronically infected subjects [8–10]. Thus, there is an urgent need to develop new compounds and treatment options as well as better assays to determine treatment outcomes and cure criteria.
Posaconazole (POS), a licensed antifungal triazole derivative [11], and E1224, a ravuconazole prodrug [12], both of which target ergosterol biosynthesis, are the only new drugs developed in the last 40 years that have moved into human clinical efficacy trials for T. cruzi infection. In addition to the development of new anti–T. cruzi compounds, there is also interest in improving the efficacy of existing or new drugs by using combination therapies. This strategy, which has been used in other infectious diseases, such as human immunodeficiency virus [13], tuberculosis [14, 15], and malaria [16], can not only strengthen the antipathogen effects of a particular compound but also decrease the likelihood of development of drug resistance [17]. Not all human infections are cured by BZ treatment [18, 19], and this variable outcome has been attributed to the relative resistance of some T. cruzi strains to BZ [20]. In addition to being more effective, combined drug treatment for T. cruzi infection might allow for a reduced dosing of compounds such as BZ, whose toxicity is thought to be cumulative, and the shortening of the treatment periods. Both toxicity and the long course of treatment are significant impediments to wider use of BZ in the treatment of chronic T. cruzi infection.
In the studies described here, we used an experimental mouse model of T. cruzi infection to test the effectiveness of several anti–T.cruzi compounds, as well as combined and intermittent treatment strategies, to cure T. cruzi infection. In addition, we studied and rigorously validated the use of immunological changes in the parasite-specific CD8+ T-cells compartment as biomarkers of treatment efficacy and cure in this infection.
METHODS
Mice, Parasites and Infections
C57BL/6 (Ly5.2+) mice were purchased from the National Cancer Institute and maintained in the University of Georgia animal facility under specific pathogen-free conditions. Tissue culture trypomastigotes of the CL, Brazil, Montalbania, or Colombiana strain of T. cruzi were obtained from passage through Vero cells. Mice were infected intraperitoneally with 1000 tissue culture trypomastigotes of T. cruzi and killed by carbon dioxide inhalation. This study was carried out in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and Association for Assessment and Accreditation of Laboratory Animal Care accreditation guidelines. The protocol was approved by the University of Georgia Institutional Animal Care and Use Committee.
Treatments
Infected mice were treated according to the indicated schedules. BZ was prepared by pulverization of tablets followed by suspension in distilled water. Mice received 100 mg/kg body weight orally by gavage. POS was dissolved in an aqueous solution of 2% methylcellulose and 0.5% Tween 80 and delivered orally at 20 mg/kg/day. NTLA-1 (a nitro-triazole derivative; gift of Maria Papadopulou, NorthShore University Health System) was suspended in phosphate-buffered saline and given intraperitoneally at 2 mg/kg/day. Allopurinol (gift of Susana Laucella, Instituto de Parasitologia Mario Fatala Chaben) was prepared by pulverization of tablets followed by suspension in distilled water and was given orally at 30 mg/kg/day. For the animals receiving a combination of drugs, BZ + allopurinol or BZ + POS, the 2 compounds were administered individually separated by 30 minutes. NFX (kindly provided by Metronomx, Houston, TX) was prepared by pulverization of tablets followed by suspension in distilled water. Mice received 100 mg/kg orally. AN1469 (kindly provided by Anacor Pharmaceuticals, Inc, Palo Alto, CA) was suspended in 1% of sodium carboxymethylcellulose with 0.1% Tween 80 and given orally at 20 mg/kg/day.
Assessment of Treatment Efficacy
Mice were immunosuppressed with cyclophosphamide (200 mg/kg/day) intraperitoneally at 2–3 day intervals for a total of 4 or 5 doses. After immunosuppression, parasitemias were quantified as described previously [21]. The DNA preparation, generation of polymerase chain reaction (PCR) standards, and detection of parasite tissue load by real-time PCR was carried out as described previously [21, 22]. Skeletal muscle tissues were collected at various time points and fixed in 10% buffered formalin for the histopathological analysis as described previously [21]. For the hemoculture experiments, blood from immunosuppressed mice was collected and cultured in liver infusion broth and tryptose medium [23], and the detection of T. cruzi was assessed every week for 2 months.
T-Cell Phenotyping
Mouse peripheral blood was obtained by retro-orbital venipuncture, collected in sodium citrate solution, washed, and stained as previously described [21]. Briefly, whole blood was incubated with the tetramer-PE and the following labeled antibodies: anti-CD62L APC, anti-CD8 EF450, and anti-CD127 PECy7. Cells were also stained with anti-CD4, anti-CD11b, and anti-B220 for use as an exclusion channel. At least 500 000 cells were acquired using a Cyan flow cytometer and analyzed with FlowJo software. The MHC I tetramer TSKB20 (ANYKFTLV/Kb) was synthesized at the Tetramer Core Facility at Emory University.
Statistical Analysis
Statistical analysis was performed by analysis of variance and unpaired t test using the GraphPad PRISM 5.0 software. Differences between 2 groups were considered significant at P < .05.
RESULTS
Immunophenotypic Markers as Surrogates for the Assessment of Drug Efficacy and Cure
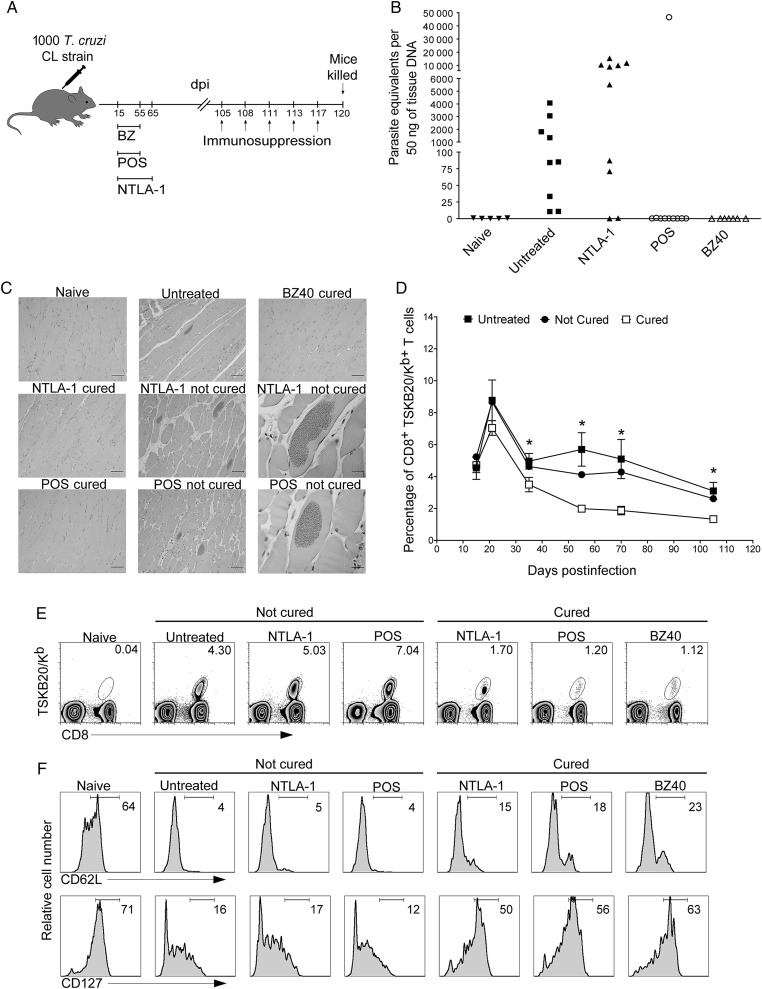
We have previously used immunosuppression with cyclophosphamide to demonstrate that treatment of T. cruzi–infected mice with the antifungal POS or the nitrotriazole NTLA-1 suppresses parasitemia but failed to provide parasitological cure in all treated mice [24]. Mice cured with BZ, NTLA-1, or POS exhibited undetectable levels of parasite DNA in skeletal muscle tissue and an absence of histological evidence of infection or disease, whereas drug failure was indicated by relatively high levels of parasite DNA in tissue, amastigote nests, and a significant inflammation in the skeletal muscle after immunosuppression (Figure 1A–C and data not shown). BZ-induced cure also results in a decrease in the frequency of T. cruzi–specific (TSKB20 epitope–specific) CD8+ T cells as well as an altered T memory phenotype [21], so we asked whether changes in the frequency or phenotype of T. cruzi–specific CD8+ T cells might be diagnostic of treatment outcomes. For all 3 drug treatments, the frequency of T. cruzi–specific TSKB20+ CD8+ T cells in cured mice was consistently 2–2.5 fold lower as compared with either untreated or treated, but not cured, mice (P < .05) (Figure 1D and 1E). Additionally, the population of TSKB20-specific CD8+ T cells in mice cured by BZ, POS, or NTLA-1 treatment were predominantly CD62Lhi and CD127hi, indicative of a change from a TEM to a TCM phenotype after antigen clearance (Figure 1F; P < .05), whereas TSKB20-specific CD8+ T cells in mice in which treatment with POS or NTLA-1 failed to cure the infection exhibited a TE/TEM memory phenotype (CD62Llo, CD127lo) similar to infected, untreated mice (Figure 1F). These results support the use of T-cell phenotypic markers on T. cruzi–specific T cells as surrogates for the assessment of drug efficacy and cure in this infection.
Figure 1.
A shift to a TCM phenotype in the Trypanosoma cruzi–specific CD8+ T cells is diagnostic of cure in T. cruzi infection. A, Schematic representation of infection, treatment, and immunosuppression. B, T. cruzi DNA isolated from skeletal muscle tissues of untreated or treated mice and suppressed with cyclophosphamide (15 days after suppression started) determined by quantitative real time polymerase chain reaction. C, Histological sections of the skeletal muscle at 120 days postinfection of naive, untreated, benznidazole 40-day–, posaconazole–, and NTLA-1–treated mice that were cyclophosphamide suppressed. Scale bar represents 200 µm in the left and middle columns and in the upper panel of the right column. In the right column middle and bottom row scale bar represents 20 µm. D and E, The MHC-peptide tetramer of the immunodominant TSKB20/Kb epitope was used to detect T. cruzi–specific CD8+ T cells in the blood of untreated (filled squares) and treated mice (cured: open square; not cured: filled circles) during the evolution of the infection (D) and at 105 days postinfection before the immunosuppression (E). Data in (D) are shown as mean ± standard error of the mean. *P < .05 between cured and not cured groups or cured and untreated groups. Numbers in (E) indicate the percentage of tetramer+ cells among the CD8+ T-cells population. F, Expression of classical memory (CD62L) and memory maintenance (CD127) markers in blood on the CD8+ T cells (naive) and on the CD8+ TSKB20-tetramer+ T cells from untreated and treated mice at 105 days postinfection. Data are representative of 2 independent experiments with 5–10 mice per group. Abbreviations: BZ, benznidazole; dpi, days postinfection; POS, posaconazole; T. cruzi, Trypanosoma cruzi.
Treatment Efficacy in Mice Infected With Drug-Resistant Strains
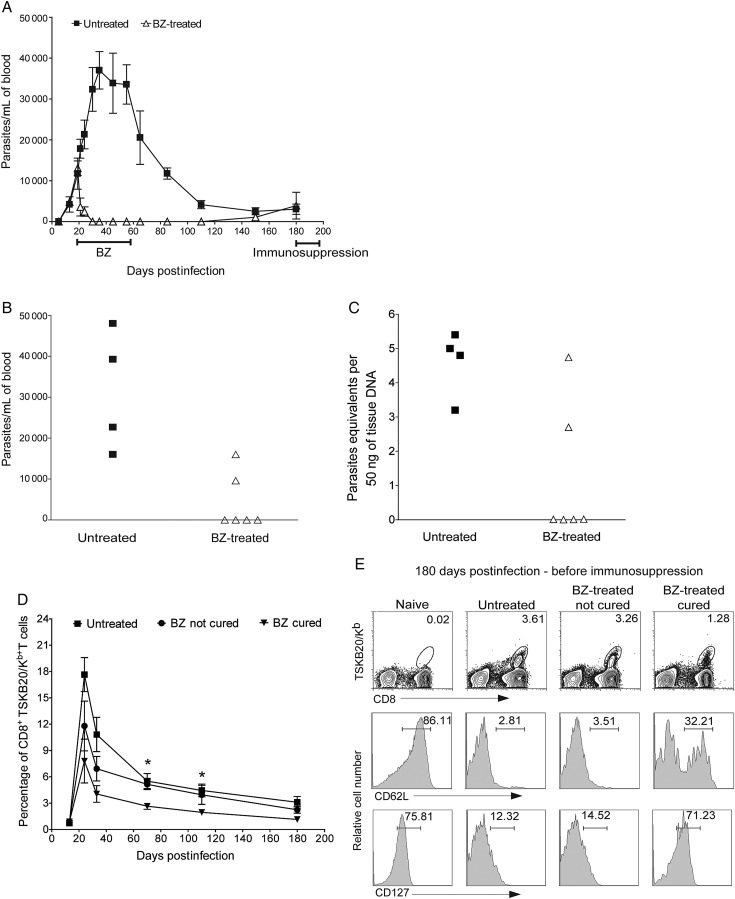
The apparent failure of BZ and NFX to consistently cure T. cruzi infection in humans has been associated with a relative resistance of some of the T. cruzi isolates to these therapies [20, 25, 26]. The Colombiana strain has been characterized as BZ resistant, based on a 20-day treatment course [20, 26] that we find to be ineffective in curing the majority of infections with BZ-sensitive T. cruzi strains [21, 24]. Therefore, we asked if a 40-day treatment regimen might cure infections with this “drug-resistant” strain. Initiation of treatment at 15 days postinfection results in a rapid reduction in parasites in the blood to undetectable levels. However, one-third of the BZ-treated mice showed a reoccurrence of parasites in blood at 150 days postinfection (Figure 2A) and a further exacerbation after cyclophosphamide immunosuppression, whereas the remaining treated mice appeared parasite-free after immunosuppression (Figure 2B and 2C). Moreover, mice cured of the Colombiana infection using BZ showed a reduced frequency of TSKB20-tetramer CD8+ T cells, as well as a predominant TCM memory phenotype (CD62Lhi and CD127hi) (Figure 2D and 2E; P < .05).
Figure 2.
Acute treatment with benznidazole (BZ) in mice infected with Trypanosoma cruzi Colombiana strain. A, Parasitemia profile in untreated (▪), or BZ-treated (Δ) mice (treatment was carried out during 40 days from 15–55 days postinfection) infected with the BZ-resistant Colombiana strain of T. cruzi. B, Parasitemias in untreated (▪) or treated (Δ) mice at 195 days postinfection, after administration of the immunosuppressant cyclophosphamide (days 180, 183, 186, 188, and 192). C, T. cruzi DNA isolated from skeletal muscle tissues of untreated or treated mice and suppressed with cyclophosphamide (15 days after suppression started) determined by quantitative real time polymerase chain reaction. Detection of T. cruzi–specific CD8+ T cells in the blood of untreated and treated mice using the TSKB20-tetramer during the course of infection (D) and at 180 days postinfection (before cyclophosphamide suppression) (E). Data in (D) are shown as mean ± standard error of the mean. *P < .05 between cured and not cured groups or cured and untreated groups. Numbers in (E) indicate the percentage of tetramer+ cells among the CD8+ T-cells population (upper row). Expression of the classical memory (CD62L: middle row) and memory maintenance (CD127: bottom row) markers in blood on the CD8+ T cells (naive) and on the CD8+ TSKB20-tetramer+ T cells from untreated and treated mice at 180 days postinfection. Data are representative of 2 independent experiments with 6–8 mice per group. Abbreviation: BZ, benznidazole.
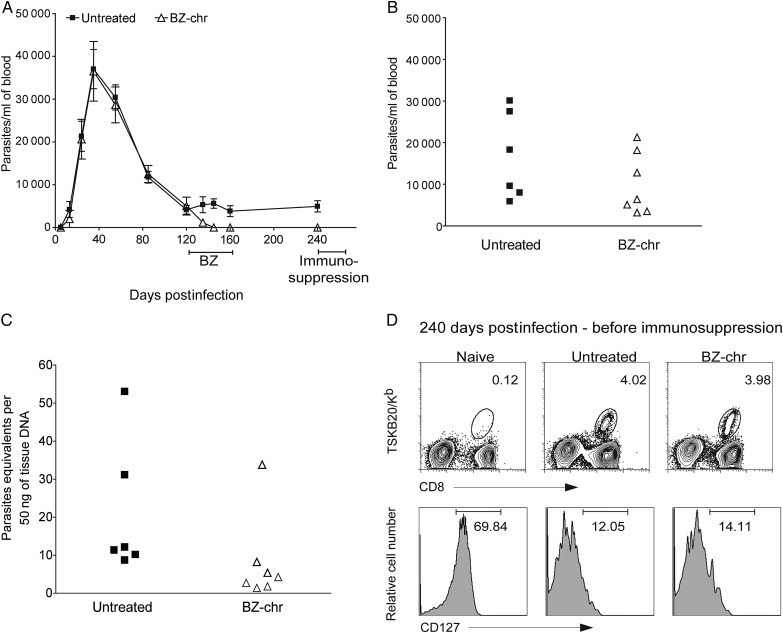
We also observed a relative resistance to BZ treatment in mice infected with the Montalbania strain of T. cruzi, with approximately two-thirds of BZ-treated mice showing lower numbers of TSKB20-tetramer+ CD8+ T cells as well as a higher expression of CD127 in the TSKB20-tetramer+ CD8+ T cells and no evidence of infection after the cyclophosphamide suppression (Supplementary Figure 1A–E and data not shown). Extending BZ treatment during Colombiana strain infection for an additional 20 days (a total of 60 consecutive days) resulted in a similar outcome as the 40-day treatment (Supplementary Figure 2A–E and Figure 2). Additionally, treatment of mice in the chronic phase of the Colombiana infection (120–160 days postinfection) suppressed parasitemias but failed to produce cure (Figure 3A–D).
Figure 3.
Chronic treatment with benznidazole (BZ) in mice infected with Trypanosoma cruzi Colombiana strain. A, Parasitemia profile in untreated (▪) or BZ-treated (Δ) mice (treatment was carried out during 40 days from 120–160 days postinfection) infected with the BZ-resistant Colombiana strain of T. cruzi. B, Parasitemias in untreated (▪) or treated (Δ) mice at 255 days postinfection, after administration of the immunosuppressant cyclophosphamide (days 240, 243, 246, 248). C, T. cruzi DNA isolated from skeletal muscle tissues of untreated or treated mice and suppressed with cyclophosphamide (15 days after suppression started) determined by quantitative real time polymerase chain reaction. D, Detection of T. cruzi–specific CD8+ T cells in the blood of untreated and treated mice using the TSKB20-tetramer at 240 days postinfection (before cyclophosphamide suppression). Numbers indicate the percentage of tetramer+ cells among the CD8+ T cells population (upper row). Expression of CD127 (bottom row) in blood on the CD8+ T cells (naive) and on the CD8+ TSKB20-tetramer+ T cells from untreated and treated mice at 240 days postinfection. Data are representative of 2 independent experiments each with 5–8 mice per group. Abbreviations: BZ, benznidazole; BZ-chr, chronically infected and treated with benznidazole.
Intermittent Treatment Protocols and Combination Therapies to Cure T. cruzi Infection
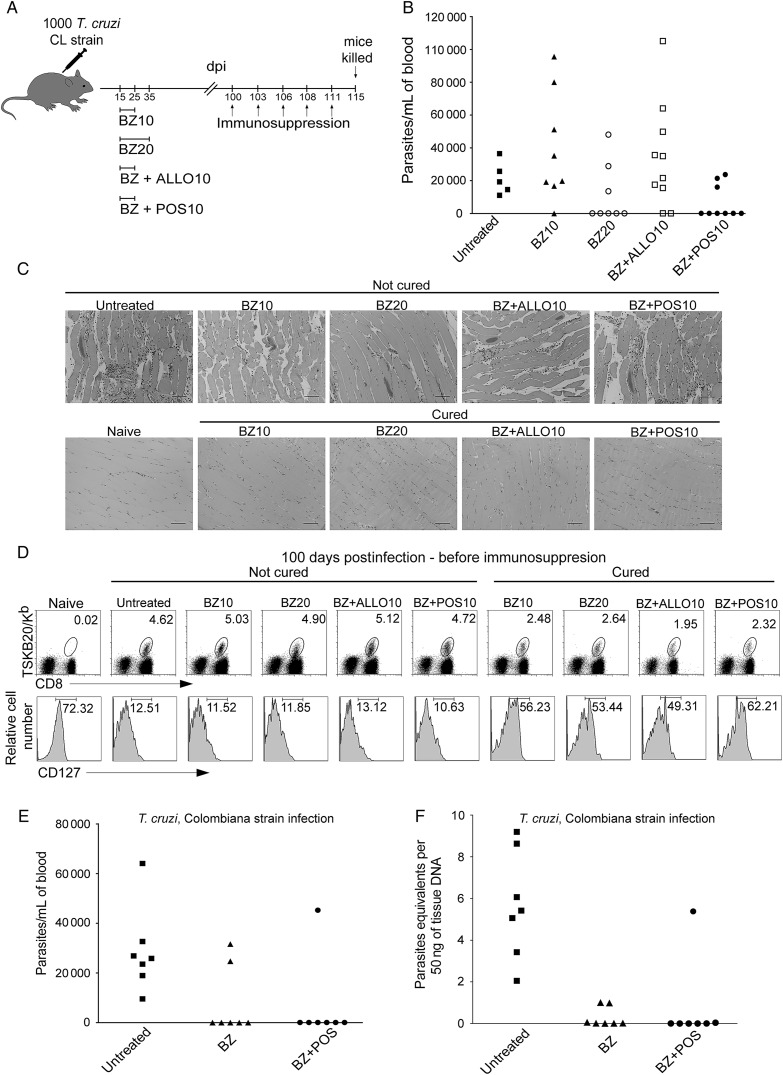
The cumulative toxicity of BZ and NFX prevents completion of treatment in a significant number of individuals [3]. We thus explored whether shorter treatment regimens and the use of a combination of anti–T. cruzi compounds could provide cure. Two-thirds of the mice infected with T. cruzi CL strain (BZ-sensitive) and treated for only 10 days with a combination of BZ and POS were cured (Figure 4A–C). This treatment protocol was as effective as a 20-day course of treatment with BZ alone and much better than BZ treatment alone for 10 days (approximately 12% of the mice cured) or the combination of BZ and allopurinol for 10 consecutive days (20% of the mice cured). Cured mice showed the expected lower frequency and TCM memory phenotype (CD127hi) for CD8+ TSKB20-tetramer+ T cells (Figure 4D; P < .05). However, combining BZ and POS treatment in mice infected with a BZ-resistant strain of T. cruzi was not significantly better in achieving cure than BZ alone (Figure 4E and 4F).
Figure 4.
The combination of anti–Trypanosoma cruzi compounds synergize to clear T. cruzi infection. A, Schematic representation of infection, treatment, and immunosuppression. B, Parasitemias in untreated and treated mice at 115 days postinfection, after administration of the immunosuppressant cyclophosphamide (days 100, 103, 106, 108, and 111). C, Histological sections of the skeletal muscle at 115 days postinfection of naive, untreated, and benznidazole (BZ)–treated mice that were cyclophosphamide suppressed. Scale bar represents 200 µm. D, Detection of T. cruzi–specific CD8+ T cells in the blood of untreated and treated mice using the TSKB20-tetramer at 100 days postinfection (before cyclophosphamide suppression) (upper row). Numbers indicate the percentage of tetramer+ cells among the CD8+ T-cells population. Expression of CD127 (bottom row) in blood on the CD8+ T cells (naive) and on the CD8+ TSKB20-tetramer+ T cells from untreated and treated mice at 100 days postinfection of naive, untreated, and BZ-treated mice that were cyclophosphamide suppressed. Data are representative of 2 independent experiments with 5–10 mice per group. E, Parasitemias in mice infected with T. cruzi. Colombiana strain, untreated, BZ-treated, and treated mice with a combination of BZ and posaconazole (treatment was carried out during 40 days from 15–55 days postinfection) at 105, after administration of the immunosuppressant cyclophosphamide (days 90, 93, 96, 98, and 101). F, T. cruzi DNA from untreated or treated mice in (E) suppressed with cyclophosphamide (15 days after suppression started) determined by quantitative real time polymerase chain reaction. Data are representative of 2 independent experiments with 3–7 mice per group. Abbreviations: ALLO, allopurinol; BZ, benznidazole; dpi, days postinfection; POS, posaconazole; T. cruzi, Trypanosoma cruzi.
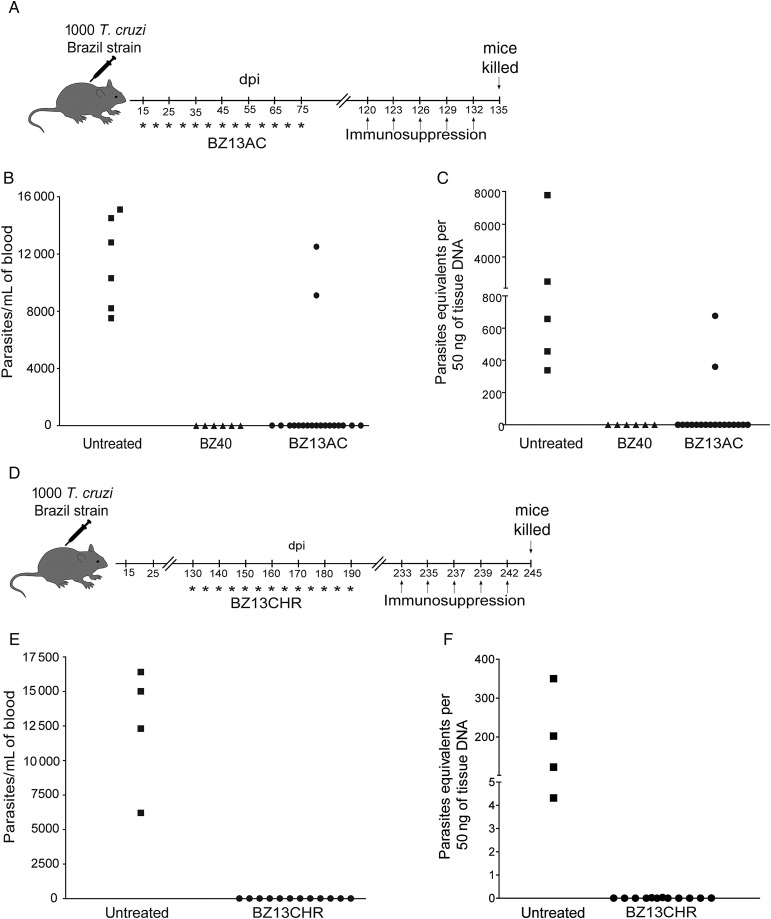
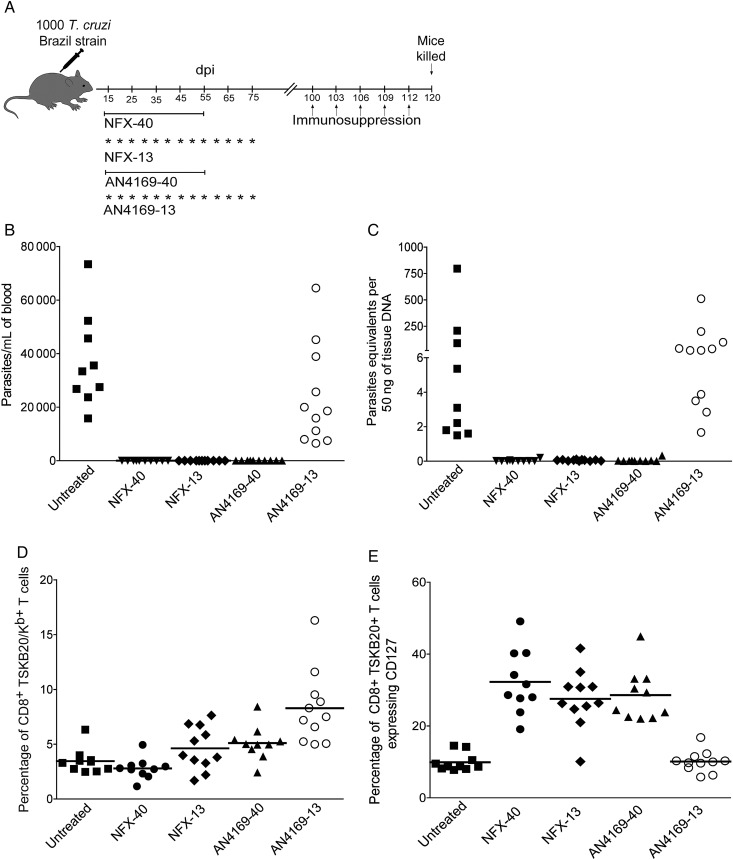
An alternative way to manage the cumulative toxicity of BZ and NFX is to treat less frequently than the standard once per day regimen. Mice infected with T. cruzi Brazil strain (a BZ-sensitive strain) and treated with 9 doses of BZ at 5-day intervals or a combination of initial daily treatments (5 or 10 days) followed by treatment at 5-day intervals for a total of 40 days (Supplementary Figure 3A–C) yielded between 45% and 75% cure rates. Extending the intermittent treatment period to 60 days (Figure 5) increased the cure rate to levels approaching that of daily 40-day treatment courses (95%–100%). Intermittent treatment with NFX was also curative (Figure 6). However this ability to achieve equivalent cure with daily and intermittent dosing does not apply to all potential antitrypanosomal compounds. AN4169, an oxaborole-containing compound develop by Anacor Pharmaceuticals and with activity in vitro against T. cruzi [27], cured 100% of the mice when administered for 40 consecutive days, but the intermittent dosing protocol with AN4169 resulted in no cures (Figure 6).
Figure 5.
Thirteen doses of benznidazole (BZ) over 60 days in the acute or chronic phase of the infection cures Trypanosoma cruzi–infected mice. A, Schematic representation of infection, acute treatment, and immunosuppression. B, Parasitemias in mice infected with T. cruzi, Brazil strain, untreated and treated mice with 13 doses of BZ over the course of 60 days (15 to 75 days postinfection) at 135 days postinfection, after administration of the immunosuppressant cyclophosphamide (days 120, 123, 126, 129, and 132). C, T. cruzi DNA isolated from skeletal muscle tissues of untreated or treated mice and suppressed with cyclophosphamide (17 days after suppression started) determined by quantitative real time polymerase chain reaction. D, Schematic representation of infection, chronic treatment, and immunosuppression. E, Parasitemias in mice infected with T. cruzi, Brazil strain, untreated and treated mice with 13 doses of BZ over the course of 60 days (130–190 days postinfection) at 245 days postinfection, after administration of the immunosuppressant cyclophosphamide (days 230, 233, 237, 239, and 242). F, T. cruzi DNA isolated from skeletal muscle tissues of untreated or treated mice and suppressed with cyclophosphamide (15 days after suppression started) determined by quantitative real time polymerase chain reaction. Data are representative of 10 mice per group. Abbreviations: AC, acute; BZ, benznidazole; CHR, chronically infected; dpi, days postinfection; POS, posaconazole; T. cruzi, Trypanosoma cruzi.
Figure 6.
Forty-day consecutive treatment with AN4169 and nifurtimox or intermittent dosing with nifutimox cures mice infected with Trypanosoma cruzi. A, Schematic representation of infection, treatment, and immunosuppression. B, Parasitemias in untreated and treated mice at 120 days postinfection, after administration of the immunosuppressant cyclophosphamide (days 100, 103, 106, 109, and 112). C, T. cruzi DNA isolated from skeletal muscle tissues of untreated or treated mice and suppressed with cyclophosphamide (20 days after suppression started) determined by quantitative reverse-transcription polymerase chain reaction. D, Detection of T. cruzi–specific CD8+ T cells in the blood of untreated and treated mice using the TSKB20-tetramer at 90 days postinfection (before cyclophosphamide suppression). E, Expression of CD127 in blood on the CD8+ T cells (naive) and on the CD8+ TSKB20-tetramer+ T cells from untreated and treated mice at 90 days postinfection. Data are representative of 10–11 mice per group. Abbreviations: dpi, days postinfection; NFX, nifurtimox; T. cruzi, Trypanosoma cruzi.
Combining Intermittent and Multiple Drug Treatment Protocols for Treatment of T. cruzi Infection
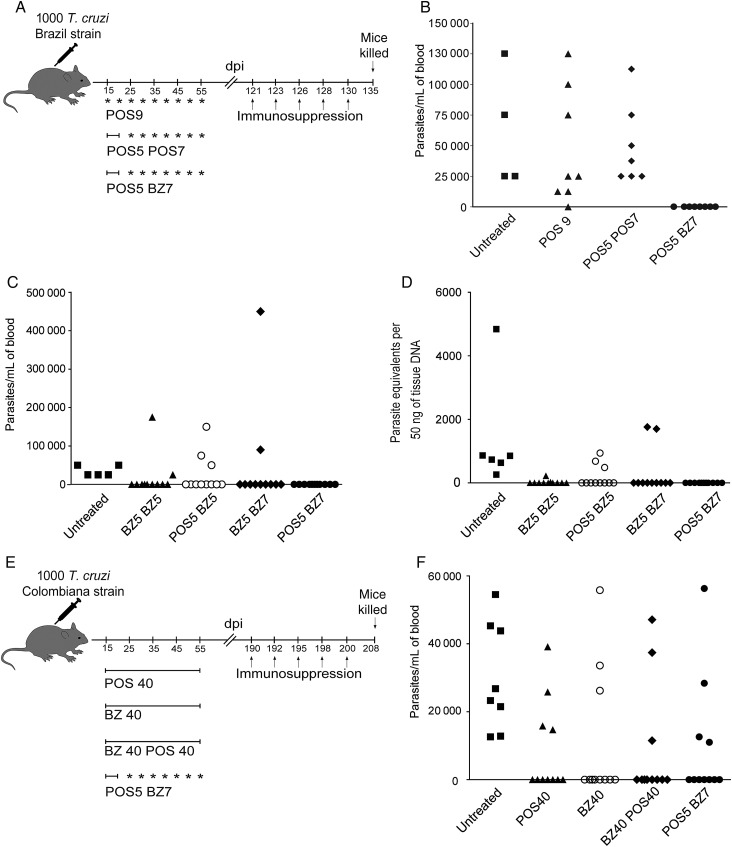
POS is thought to have fewer adverse effects relative to BZ, so we next asked if the cumulative dosage of BZ can be further reduced without loss of efficacy by preceding it with 5 daily doses of POS (Figure 7A). All the mice treated with the POS5/BZ7 protocol were cured based on the absence of parasites in blood after immunosuppression (Figure 7B). In contrast, intermittent treatment with POS alone provided only 1 cure among 15 treated mice (Figure 7A and 7B). Attempts to further reduce the length of the intermittent treatment after the initial 5 consecutive days of POS treatment revealed that although a high rate of cure (between 71%–81%) was achieved using 5 daily doses of BZ followed by 5 intermittent doses of BZ on a 5 day interval (BZ5/BZ5), 5 daily doses of POS followed by 5 intermittent doses of BZ on a 5 day interval (POS5/BZ5), and 5 daily doses of BZ followed by 7 intermittent doses of BZ on a 5 day interval (BZ5/BZ7) protocols, a 100% cure rate was only accomplished when the mice were treated with the 5 daily doses of posaconazole (POS) followed by 7 intermittent doses of benznidazole (BZ) on a 5 day interval (POS5/BZ7) protocol (Figure 7C and 7D). T. cruzi Colombiana strain–infected mice treated with the POS5/BZ7 protocol displayed a similar rate of cure as those treated with a 40-day regimen of either POS or BZ or with a 40-day regimen of daily doses of both POS and BZ (Figure 7E and 7F).
Figure 7.
Five consecutive doses of posaconazole (POS) followed by 7 intermittent doses of benznidazole (BZ) cures 100% of the mice infected with Trypanosoma cruzi. A, Schematic representation of infection, treatment, and immunosuppression. B, Parasitemias in mice infected with T. cruzi, Brazil strain, untreated and treated mice with 9 and 12 doses of POS and the combination of 5 doses of POS follow by 7 doses of BZ over the course of 40 days (15–55 days postinfection) at 135 days postinfection, after administration of the immunosuppressant cyclophosphamide (days 121, 123, 126, 128, and 130). C, Parasitemias in mice infected with T. cruzi, Brazil strain, untreated and treated mice with 5 consecutive doses of POS or BZ followed by 5 or 7 intermittent doses of BZ over the course of 40 days (15–55 days postinfection) at 127 days postinfection, after administration of cyclophosphamide (days 110, 112, 115, 117, and 120). D, T. cruzi DNA isolated from skeletal muscle tissues of untreated or treated mice and suppressed with cyclophosphamide (17 days after suppression started) determined by quantitative real time polymerase chain reaction. E, Schematic representation of infection, treatment, and immunosuppression in mice infected with T. cruzi Colombiana strain and treated with combination of BZ and POS on an intermittent and combined treatment regimen. F, Parasitemias in mice infected with T. cruzi, Colombiana strain, untreated and treated mice with a combination of 5 doses of POS follow by 7 doses of BZ over the course of 40 days (15–55 days postinfection) at 208 days postinfection, after administration of cyclophosphamide (days 190, 192, 1195, 198, and 200). Data are representative of 2 independent experiments with 8–10 mice per group. Abbreviations: BZ, benznidazole; dpi, days postinfection; POS, posaconazole; T. cruzi, Trypanosoma cruzi.
DISCUSSION
Treatment of T. cruzi infection relies on the use of 2 nitroderivative compounds, benznidazole and nifurtimox, developed >4 decades ago [10]. Despite showing effectiveness in curing both acute and chronic infections with T. cruzi [3–7], these drugs are underused because of their high rate of side effects, long courses of treatment, and unpredictable (and unmeasurable) treatment outcomes. The difficulty in determining treatment efficacy has been a long-standing problem [28] and has also greatly impacted the design of treatment protocols using BZ and NFX. The dosage level and daily frequency for administration of BZ are in keeping with the known pharmacokinetics in humans [29, 30], but the length of treatment is highly variable [10] and does not appear to be based upon rigorous evaluation of cure, particularly with respect to chronic infections.
We undertook this study to further validate accurate methods for determining treatment outcomes in T. cruzi infection and to use these methods to evaluate the reliability of protocols that are used in humans but that are not well supported experimentally. The most surprising and encouraging result of this study is the finding that T. cruzi infection can be cured in mice using a treatment regimen that greatly decreased the total dose of BZ or NFX. As few as 13 doses of BZ or NFX given at 5 day intervals, or 9 doses of BZ if preceded by a 5 day course of POS treatment performed equally as well as 40 daily doses of BZ or NFX. This result was unexpected based upon the documented half-life of BZ of 1–2 hours in mice [31, 32] and the presumption that the effectiveness of BZ depended on sustaining a concentration above the minimum inhibitory concentration [33]—hence the reason for giving BZ twice daily in humans where its half-life is approximately 12 hours [29]. This result is particularly significant because some of the side effects of BZ treatment can be eliminated with a reduction in the cumulative dose of BZ [10].
The effectiveness of the intermittent dosing regimen suggests that BZ acts via a maximum concentration mechanism. Drugs functioning through a maximum concentration mechanism have extended postantibiotic effects—impacting pathogen growth after complete removal of the compound [34].
It is noteworthy that POS and the oxaborale AN4169 failed to cure any mice when given only once every 5 days, indicating that the postantibiotic effects on T. cruzi are compound specific. Although as yet untested, it is possible that even less frequent dosing may be possible with BZ and NFX. Ongoing studies in nonhuman primates with naturally acquired, chronic T. cruzi infections will establish whether this reduced dosing regimen is likely to translate into improved treatment protocols for humans.
A more discouraging finding is the failure of various treatment regimens, including an extended course of treatment, alternative compounds, and drug combinations, to cure infections with naturally drug-resistant isolates of T. cruzi. The Colombiana strain is resistant not only to BZ but also to POS; the combination of BZ and POS and extending the length of daily treatments from 40 to 60 days did not improve the cure rate. Even more concerning was the complete failure of BZ treatment in mice with chronic Colombiana strain infections. Our previous studies in mice had failed to identify an infection length dependency on the efficacy of BZ in infections with the relatively drug-sensitive CL strain [21]. Other investigators have reported that BZ resistance occurs naturally in the absence of drug exposure [35] and also fluctuates depending on host and parasite passage conditions [37]. In our hands, the Colombiana strain is no more resistant to BZ in vitro [24, 38] than the Brazil strain, which is very susceptible to BZ-mediated cure in vivo. All of these observations suggest that naturally occurring resistance to BZ is not due to a genetic resistance to drug but is a phenotypic, in vivo resistance to multiple compounds that varies with the host species and becomes more evident with increasing length of infection. One possibility is that this phenotypic resistance is related to the tissue distribution of parasites and the inaccessibility to drug in certain cells or tissues. There is clearly a stochastic nature to the success of drug treatment in T. cruzi infection. Although a 40 day course of treatment of mice infected with the CL strain cures 100%, treatment for only 20 days cures >50% and the occasional animal cures with only 10 days of treatment. Likewise, >50% of mice infected with drug-resistance strains are cured if treated early in the infection, but few are cured once the infection is well established. Successful cure in humans is also variable and unpredictable. Alvarez and colleagues recently reported that BZ treatment aborted at approximately 10 days because of adverse effects achieved the criteria of cure in 20% of patients [3]. Clearly a better understanding is needed of the biology of T. cruzi in vivo, the variability of tissue distribution of different isolates, and the accessibility of these tissues to drugs.
POS not only failed on its own and in combination with BZ to clear infections with the BZ-resistant Colombiana strain, it also was modestly less effective in curing infections with BZ-sensitive strains. This observation is consistent with the unpublished reports of high failure rates of POS in humans and nonhuman primates with established T. cruzi infections (John Vandeberg, CDDC 2012 Meeting https://sites.google.com/site/chagasddc/ and Israel Molina, International Congress of Tropical Medicine http://ictmm2012.ioc.fiocruz.br/program_25_sept.html). Interestingly, the combination of POS and BZ did have a significant synergistic effect in the abbreviated 10-day treatment protocol, improving cure rates from 12.5% to 66.7%, and with POS as a pretreatment before a 7-dose intermittent treatment with BZ. Cencig et al have also recently reported synergism between BZ and POS in an abbreviated treatment protocol in T. cruzi–infected mice [39]. This enhancing effect of POS could be a result of the expected impact of POS on BZ metabolism. Moreira da Silva and colleagues reported that itraconazole increased the volume of distribution of BZ by 2.66-fold and prolonged its half-life in the plasma by >7-fold, to approximately 12 hours in mice [32]. Similar synergistic activity with BZ has been reported for the related compound ketoconazole [40] and would be expected of POS as well. Collectively, these results emphasize the quality of the cure assays that are available for testing anti–T. cruzi drugs, the multitude of variations in treatment protocols that have yet to be explored, and the need to fully use these models before proceeding to human trials with new compounds or protocols.
This study also presents the first demonstration of the ability of a new class of compounds, the oxaboroles, to provide sterile cure of T. cruzi infection. Oxaboroles are a unique boron-containing class of compounds with demonstrated antiparasitic activity. Oxaborole SCYX-7158 is currently in phase 1 trials for the treatment of human African trypanosomiasis, having exhibited efficacy against both the acute and chronic central nervous system stages of human African trypanosomiasis. The trypanocidal activity of oxaboroles demonstrated in T. brucei, excellent physicochemical and absorption, distribution, metabolism, and excretion profiles, including promising in vitro and in vivo pharmacokinetic properties, sufficient potency, and high permeability suggest that these are important leads for new and effective orally administered treatments for Chagas disease.
Lastly, this study provides additional support for the use of immunological biomarkers as accurate assessors of treatment efficacy in T. cruzi infection by showing that changes in the phenotype of T. cruzi–specific T cells correlate with cure, irrespective of the compound that is used, the treatment protocol, or the frequency of cures achieved with that compound. Unfortunately, inconsistent detection of T. cruzi–specific T cells [41, 42] limits the translation of such assays of immunological biomarkers into use for assessing treatment outcomes in humans. Other surrogate immunological markers have shown promise in assessing treatment outcomes [43] and are likely to be more widely employed because definitive evidence of parasitological cure is impractical because of the insensitivity of even the best assays. If we are to reliably assess in humans the effectiveness of new drugs and improved treatment protocols that appear promising in mice, then we are going to need to put much more effort into developing definitive tests of cure in humans and not just clearance of detectable parasites from blood. Such tests are likely to depend on immunological or other biomarkers.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Julie Nelson of the Center for Tropical and Emerging Global Diseases Flow Cytometry Facility at the University of Georgia for technical assistance, Courtney Boehlke and Gretchen Cooley for assistance with the parasites, Aaron Evan for assistance with the quantitative polymerase chain reaction, and the Tetramer Core Facility (Emory University) for synthesis of MHC I tetramers. We would like to thank Eric Eason and Yvonne Freund at Anacor Pharmaceuticals, Inc for providing AN4169 and Maria Papadopoulou at NorthShore University Health System for supplying NTLA-1.
Financial support. This work was supported by grants [P01 AI-44979 and R01 AI-089952 to R. L. T.] from the US National Institutes of Health.
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Control of Chagas disease. World Health Organ Tech Rep Ser. 2002;905:1–35. [PubMed] [Google Scholar]
- 2.Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–27. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez MG, Vigliano C, Lococo B, Petti M, Bertocchi G, Viotti R. Seronegative conversion after incomplete benznidazole treatment in chronic Chagas disease. Trans R Soc Trop Med Hyg. 2012;106:636–8. doi: 10.1016/j.trstmh.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Coura JR, Abreu LLD, Willcox HPF, Petana W. Estudo comparativo controlado com emprego de benznidazole, nifurtimox e placebo, na forma crônica da doença de Chagas, em uma área de campo com transmissão interrompida. I. Avaliação preliminar. Rev Soc Bras Med Trop. 1997;30:139–44. doi: 10.1590/s0037-86821997000200009. [DOI] [PubMed] [Google Scholar]
- 5.Coura JR, Borges-Pereira J. Chronic phase of Chagas disease: why should it be treated? A comprehensive review. Mem Inst Oswaldo Cruz. 2011;106:641–5. doi: 10.1590/s0074-02762011000600001. [DOI] [PubMed] [Google Scholar]
- 6.Viotti R, Vigliano C, Armenti H, Segura E. Treatment of chronic Chagas’ disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J. 1994;127:151–62. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 7.Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144:724–34. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 8.Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 9.Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Viotti R, Vigliano C, Lococo B, et al. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther. 2009;7:157–63. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- 11.Nagappan V, Deresinski S. Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007;45:1610–7. doi: 10.1086/523576. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante JM, Park HJ, Tarleton RL Consortium tCDD. Report of the 2nd Chagas Drug Discovery Consortium meeting, held on 3 November 2010; Atlanta GA, USA. Expert Opin Drug Discov. 2011;6:965–73. doi: 10.1517/17460441.2011.602063. [DOI] [PubMed] [Google Scholar]
- 13.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents: the panel on clinical practices for treatment of HIV*. Ann Intern Med. 2002;137:381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 14.Lienhardt C, Raviglione M, Spigelman M, et al. New drugs for the treatment of tuberculosis: needs, challenges, promise, and prospects for the future. J Infect Dis. 2012;205:S241–9. doi: 10.1093/infdis/jis034. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Treatment of Tuberculosis: Guidelines. 4th ed. Geneva, Switzerland: WHO; 2009. [PubMed] [Google Scholar]
- 16.World Health Organization. Geneva, Switzerland: WHO; 2001. Antimalarial Drug Combination Therapy: Report of a WHO Technical Consultation. [Google Scholar]
- 17.Benaim G, Sanders J, Garcia-Marchán Y, et al. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem. 2006;49:892–901. doi: 10.1021/jm050691f. [DOI] [PubMed] [Google Scholar]
- 18.Braga MS, Lauria-Pires L, ArgaÑAraz ER, Nascimento RJ, Teixeira ARL. Persistent infections in chronic Chagas’ disease patients treated with anti-Trypanosoma cruzi nitroderivatives. Rev Inst Med Trop Sao Paulo. 2000;42:157–61. doi: 10.1590/s0036-46652000000300009. [DOI] [PubMed] [Google Scholar]
- 19.CanÇAdo JR. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Rev Inst Med Trop Sao Paulo. 2002;44:29–37. [PubMed] [Google Scholar]
- 20.Filardi L, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg. 1986;81:755–64. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 21.Bustamante J, Bixby L, Tarleton R. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–92. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings KL, Tarleton RL. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol Biochem Parasitol. 2003;129:53–9. doi: 10.1016/s0166-6851(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 23.Rassi A, Amato Neto V, de Oliveira RL. Hemoculture in the LIT medium for Trypanosoma cruzi according to the Mourao-Mello method (1975) Rev Inst Med Trop Sao Paulo. 1981;23:57–60. [PubMed] [Google Scholar]
- 24.Canavaci A, Bustamante J, Padilla A, et al. In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLoS Negl Trop Dis. 2010;4:e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murta SM, Gazzinelli RT, Brener Z, Romanha AJ. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol Biochem Parasitol. 1998;93:203–14. doi: 10.1016/s0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]
- 26.Neal RA, van Bueren J. Comparative studies of drug susceptibility of five strains of Trypanosoma cruzi in vivo and in vitro. Trans R Soc Trop Med Hyg. 1988;82:709–14. doi: 10.1016/0035-9203(88)90208-8. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs RT, Plattner JJ, Keenan M. Boron-based drugs as antiprotozoals. Curr Opin Infect Dis. 2011;24:586–92. doi: 10.1097/QCO.0b013e32834c630e. [DOI] [PubMed] [Google Scholar]
- 28.Coura JR. Present situation and new strategies for Chagas disease chemotherapy: a proposal. Mem Inst Oswaldo Cruz. 2009;104:549–54. doi: 10.1590/s0074-02762009000400002. [DOI] [PubMed] [Google Scholar]
- 29.Raaflaub J, Ziegler WH. Single-dose pharmacokinetics of the trypanosomicide benznidazole in man. Arzneimittelforschung. 1979;29:1611–4. [PubMed] [Google Scholar]
- 30.Raaflaub J. Multiple-dose kinetics of the trypanosomicide benznidazole in man. Arzneimittelforschung. 1980;30:2192–4. [PubMed] [Google Scholar]
- 31.Workman P, White RA, Walton MI, Owen LN, Twentyman PR. Preclinical pharmacokinetics of benznidazole. Br J Cancer. 1984;50:291–303. doi: 10.1038/bjc.1984.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira da Silva R, Oliveira LT, Silva Barcellos NM, de Souza J, de Lana M. Preclinical monitoring of drug association in experimental chemotherapy of Chagas’ disease by a new HPLC-UV method. Antimicrob Agents Chemother. 2012;56:3344–8. doi: 10.1128/AAC.05785-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drusano GL. Antimicrobial pharmacodynamics: critical interactions of “bug and drug. Nat Rev Microbiol. 2004;2:289–300. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 34.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. quiz 11–12. [DOI] [PubMed] [Google Scholar]
- 35.Teston AP, Monteiro WM, Reis D, et al. In vivo susceptibility to benznidazole of Trypanosoma cruzi strains from the western Brazilian Amazon. Trop Med Int Health. 2013;18:85–95. doi: 10.1111/tmi.12014. [DOI] [PubMed] [Google Scholar]
- 36.Caldas S, Santos FM, de Lana M, et al. Trypanosoma cruzi: acute and long-term infection in the vertebrate host can modify the response to benznidazole. Exp Parasitol. 2008;118:315–23. doi: 10.1016/j.exppara.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Dos Santos FM, Caldas S, de Assis Cau SB, et al. Trypanosoma cruzi: induction of benznidazole resistance in vivo and its modulation by in vitro culturing and mice infection. Exp Parasitol. 2008;120:385–90. doi: 10.1016/j.exppara.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Bustamante JM, Tarleton RL. Methodological advances in drug discovery for Chagas disease. Expert Opin Drug Discov. 2011;6:653–61. doi: 10.1517/17460441.2011.573782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cencig S, Coltel N, Truyens C, Carlier Y. Evaluation of benznidazole treatment combined with nifurtimox, posaconazole or AmBisome(R) in mice infected with Trypanosoma cruzi strains. Int J Antimicrob Agents. 2012;40:527–32. doi: 10.1016/j.ijantimicag.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Araujo MS, Martins-Filho OA, Pereira ME, Brener Z. A combination of benznidazole and ketoconazole enhances efficacy of chemotherapy of experimental Chagas’ disease. J Antimicrob Chemother. 2000;45:819–24. doi: 10.1093/jac/45.6.819. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez MG, Postan M, Weatherly DB, et al. HLA class I-T cell epitopes from trans-sialidase proteins reveal functionally distinct subsets of CD8T cells in chronic chagas disease. PLoS Negl Trop Dis. 2008;2:e288. doi: 10.1371/journal.pntd.0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin DL, Weatherly DB, Laucella SA, et al. CD8+ T-Cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2006;2:e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laucella SA, Perez Mazliah D, Bertocchi G, et al. Changes in Trypanosoma cruzi-specific immune responses following treatment: surrogate markers of treatment efficacy. Clin Infect Dis. 2009;49:1675–84. doi: 10.1086/648072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.