Abstract
Background. The decreased immune response among elderly individuals results in reduced influenza vaccine efficacy. Strategies to improve vaccine efficacy in elderly individuals are needed. The goal of this study was to determine whether a cationic lipid/DNA complex (CLDC) can improve the efficacy of the trivalent inactivated influenza vaccine Fluzone in elderly nonhuman primates.
Methods. Elderly (age, >18 years) rhesus macaques were vaccinated with Fluzone, with or without CLDC, and challenged with a human seasonal influenza virus isolate, A/Memphis/7/2001(H1N1).
Results. We found that elderly macaques have significantly lower levels of circulating naive CD4+ T cells, naive CD8+ T cells, and B cells as compared to juvenile monkeys. Furthermore, on the day of challenge, recipients of Fluzone/CLDC had significantly higher plasma anti–influenza virus immunoglobulin G (P < .001) and immunoglobulin A (P < .001) titers than recipients of Fluzone alone. After virus challenge, only the Fluzone/CLDC-vaccinated animals had a significantly lower level of virus replication (P < .01) relative to the unvaccinated control animals.
Conclusions. These results demonstrate that CLDC can enhance the immunogenicity and efficacy of a licensed TIV in immunosenescent elderly monkeys.
Keywords: inactivated vaccine, elderly, macaques, immunosenescence, mucosal, antibody titers, CD8+ T cells
(See the editorial commentary by Kent on pages 4–5.)
Seasonal influenza A virus infection is a highly contagious, acute respiratory tract disease of humans that causes substantial morbidity and mortality, particularly among those who are young, old, and/or immunocompromised [1]. Trivalent inactivated influenza vaccines (TIVs) are a primary tool for reducing morbidity associated with influenza virus infection, and when TIV is correctly matched to the circulating epidemic strains, the vaccine can protect 59% of adults 18–65 years of age from laboratory-confirmed influenza [2]. However, evidence for a consistent level of vaccine-induced influenza protection in those aged ≥65 years remains elusive.
Weak immune responses to vaccination and increased susceptibility to infections are characteristic of immune senescence [3, 4]. Age-related immunological changes include decreased macrophage and dendritic cell phagocytosis, reduced natural killer cell cytotoxicity, loss of naive T cells, decreased lymphocyte receptor repertoire diversity, and dysregulation of cytokine/chemokine production [5, 6]. One approach to improve influenza vaccine efficacy is to use adjuvants to improve immune responses. CLDC is an adjuvant composed of 1:1 molar ratio of cationic DOTIM/cholesterol liposomes and noncoding plasmid DNA [7]. In mice and young adult macaques, the addition of CLDC to influenza vaccines enhances influenza virus–specific CD4+ and CD8+ T-cell responses and antibody responses [8–10]. The goal of this study was to determine whether the CLDC adjuvant could improve the efficacy of a licensed TIV in elderly rhesus macaques.
MATERIALS AND METHODS
Animals
Elderly rhesus macaques (Macaca mulatta; age, 18–22 years) used in the present study were housed at the California National Primate Research Center in accordance with the regulations of the Association for Assessment and Accreditation of Laboratory Animal Care International Standards. The Institutional Animal Use and Care Committee of the University of California, Davis, approved these experiments. Animal vital signs were regularly monitored. For blood collection, animals were anesthetized with 10 mg/kg of ketamine hydrochloride (Parke-Davis) injected intramuscularly. For virus inoculation and sample collection, animals were additionally anesthetized with 15–30 µg/kg of medetomadine HCl (Orion Pharma) injected intramuscularly, and anesthesia was reversed with 0.07–0.15 mg/kg of atipamezole HCl (Pfizer) injected intramuscularly.
Virus Strains and CLDC Adjuvant
A human influenza virus A/Memphis/7/2001(H1N1) 106.5 50% tissue culture infectious dose (TCID50) stock used for all animal inoculations in this study has been previously described [11, 12]. By using Vector NTI Advance 11.0 (Invitrogen), the amino acid sequences of all 8 genome segments of the A/New Caledonia/20/99(H1N1)-like component (NCBI taxon identification 381 512) of the 2006–2007 pediatric TIV (Fluzone; Sanofi-Pasteur) used in this study have >98% homology to the A/Memphis/7/2001 challenge strain (NCBI taxon identification 416 736), with the highest homology between the hemagglutinin (HA) sequences (99.6%). The CLDC adjuvant used in this study has been previously described [9]. CLDC was reconstituted in sterile water to 294 µg/mL and diluted in 5% dextrose in water (D5W) to 200 µg/mL. Each intramuscular vaccination consisted of 22.5 µg of HA per 0.25 mL of Fluzone added to either 0.25 mL of D5W (negative control for adjuvant) or 0.25 mL of the 200 µg/mL CLDC mixture.
Animal Vaccination, Inoculation, and Sample Collection
Twenty-seven elderly monkeys were assigned to one of 3 experimental groups (A, B, and C), with 9 animals assigned per group to achieve an even distribution of age and sex across all groups (Table 1). At weeks 0 and 2, group A was vaccinated with Fluzone alone, group B was vaccinated with Fluzone mixed with CLDC, and group C remained unvaccinated. Blood samples were collected 1 week before vaccination, on the day of but just before vaccination, and 1, 2, 3, 4, 5, and 6 weeks after vaccination. At week 6 after vaccination (day 0 after challenge), animals were challenged with virus (1 mL instilled intratracheally, 1 mL dripped intranasally, and a drop onto each conjunctiva) [12]. Nasopharyngeal, tracheal secretions, and blood samples were collected 7 and 4 days before challenge and 1, 2, 3, 7, and 14 days after challenge from all animals by previously described methods [12]. Blood samples were also collected from 29 elderly monkeys and 69 juvenile monkeys (3–9 years old) to assess age-related differences in circulating lymphocyte populations.
Table 1.
Changes in Peripheral Blood Lymphocyte Populations, by Age and Vaccine Formulation Received, Among Rhesus Macaques
| Variable | Age |
Formulation |
||||
|---|---|---|---|---|---|---|
| Juvenile Macaques (n = 69) | P b | Elderly Macaques (n = 56)a | Fluzone (n = 9)a | P b | Fluzone/CLDC (n = 9)a | |
| Age, y, mean ± SEM | 5.4 ± 0.2 | 20.2 ± 0.3 | 19.8 ± 0.5 | 19.6 ± 0.6 | ||
| Lymphocytes | 3062 ± 157 | <.001 | 1943 ± 147 | 2261 ± 467 | NS | 2535 ± 198 |
| CD4+ T cellsc | ||||||
| Overall | 1084 ± 59 | <.001 | 714 ± 55 | 730 ± 70 | NS | 1051 ± 90 |
| Naive | 366 ± 25 | <.001 | 117 ± 13 | 130 ± 27 | NS | 202 ± 32 |
| Central memory | 466 ± 39 | NS | 381 ± 26 | 417 ± 41 | NS | 485 ± 30 |
| Effector memory | 142 ± 14 | NS | 189 ± 26 | 139 ± 26 | <.05 | 306 ± 85 |
| Integrin β7+ | 546 ± 34 | <.01 | 375 ± 25 | 330 ± 34 | NS | 425 ± 47 |
| CD8+ T cellsd | ||||||
| Overall | 707 ± 45 | NS | 605 ± 68 | 447 ± 67 | NS | 889 ± 105 |
| Naive | 300 ± 23 | <.001 | 80 ± 10 | 89 ± 14 | NS | 96 ± 24 |
| Central memory | 114 ± 7 | NS | 111 ± 9 | 117 ± 14 | NS | 146 ± 19 |
| Effector memory | 78 ± 8 | NS | 92 ± 15 | 49 ± 13 | NS | 133 ± 32 |
| Terminal effector memory | 215 ± 21 | <.02 | 336 ± 49 | 223 ± 39 | <.05 | 513 ± 97 |
| Integrin β7+ | 425 ± 28 | NS | 455 ± 56 | 250 ± 46 | NS | 447 ± 59 |
| CD20+ B cellse | 850 ± 61 | <.001 | 450 ± 65 | 388 ± 74 | NS | 414 ± 68 |
Data are mean no. of cells/µL ± SEM, unless otherwise indicated.
Abbreviations: CLDC, cationic lipid/DNA complex; NS, not significant; SEM, standard error of the mean.
a Elderly macaques vaccinated with Fluzone (n = 18) represent a subset of the total elderly animals used to assess age-related differences.
b P values were calculated by a 2-tailed t test and compare the 2 absolute lymphocyte populations.
c Naive cells were defined as CD95−CD45RA+, central memory cells were defined as CD95+CD45RA−, and effector memory cells were defined as CD95+CD45RA+.
d Naive cells were defined as CD28+CD45RA+, central memory cells were defined as CD28+CD45RA−, effector memory cells were defined as CD28−CD45RA−, and terminal effector memory cells were defined as CD28−CD45RA+.
e CD3−CD20+ B lymphocytes.
Viral Replication Quantitation
As previously described [12], infectious viral titers in respiratory secretions were determined by a TCID50/mL end point dilution culture assay, and the log10 concentration of viral RNA copies in respiratory secretions was quantified by reverse-transcription polymerase chain reaction.
Influenza Virus Antibody Enzyme-Linked Immunosorbent Assay
Antibody titers to detergent-disrupted A/New Caledonia/20/99 influenza A (Biodesign International) in plasma and respiratory secretions were determined by a 2-step screening and titration method previously described [12]. Although the assay is designed to assess immune responses to the H1N1 component of the TIV used in these studies, it would detect all vaccine-induced antibodies to H3N2 that bind conserved antigens in the H1N1 component of the vaccine.
Hemagglutination Inhibition (HAI) Assay
H1 subtype HA-specific antibody titers were estimated using the revised World Health Organization HAI test as previously described [12, 13]. The viral antigen used in the HAI assay was the A/Memphis/7/01 stock grown in 10-day-old embryonated chicken eggs (Charles River).
Determination of Peripheral Lymphocyte Concentration by Flow Cytometry
Complete blood counts were determined by use of a HORIBA Pentra 60+ electronic cell counter (Horiba Diagnostics), and a 100-cell differential white blood cell count was determined manually using slides stained with Wright-Giemsa. Lymphocyte phenotype was determined by modification of a previously described cell-surface-staining method [14]. Whole blood collected in ethylenediaminetetraacetic acid–containing tubes was directly labeled per the manufacturer's instructions (BD Biosciences) with anti-CD8-FITC or -APC (clone SK1), anti-CD4-APC (clone M-L200), anti-CD3-PerCP (clone SP34), anti-CD20-APC (clone L27), anti-CD28-PE (clone L293), anti-CD45RA-FITC or -PE (clone 5H9), anti-HLADR-FITC or -PE (clone G46.6), anti-CD25-PE (clone 2A3), and anti-β7-PE (clone FIB504). The absolute lymphocyte number in a lymphocyte subset was calculated by multiplying the percentage of each lymphocyte subset obtained by flow cytometry with the absolute number of total lymphocytes obtained from the manual differential count.
H1N1-Specific T-Cell Responses
For intracellular staining to detect influenza virus–specific T cells in peripheral blood mononuclear cells (PBMCs), previously reported methods were used [12, 15].
Statistical Analysis
Data are reported as the mean and the standard error of the mean for each animal group, using Prism 5.0a software (GraphPad). Two groups were compared with a 2-tailed t test, and 3 groups were compared with a 1-way analysis of variance with the Tukey-Kramer post hoc test. Area under the curve (AUC) was calculated by Prism, using the trapezoid rule ΔX*(Y1 + Y2)/2, in which the area of a trapezoid under the curve is repeatedly calculated for a series of XY points with equally spaced X values.
RESULTS
Age-Related Reduction in the Naive Lymphocyte Population
To determine whether aged primates have blood lymphocyte populations that are similar to those of aged humans, we characterized the absolute number and relative frequency of total circulating lymphocytes, T cells, and B cells in 54 elderly and 69 juvenile macaques (Table 1). The mean total number of lymphocytes in elderly monkeys was 1.6-fold lower than that in juveniles (P < .001). Although the mean number of CD8+ T cells in elderly monkeys was similar to that in juveniles, elderly monkeys had a 1.5-fold lower mean number of CD4+ T cells (P < .001) and a 1.9-fold lower mean number of B cells (P < .001; Table 1).
The elderly and juvenile monkeys had a similar mean number of CD4+ central memory T cells (CD4+ TCM) and CD4+ effector memory T cells (CD4+ TEM). However, elderly monkeys had a 3.1-fold lower mean number of CD4+ naive T cells (CD4+ TN), compared with juvenile monkeys (P < .001). The relative frequency of peripheral CD4+ T cells was similar in both age groups, but compared with juveniles, elderly monkeys had a 20% decrease (P < .001) in the relative frequency of CD4+ TN and a 16% increase (P < .001) and 10% increase (P < .001) in the relative frequency of CD4+ TCM and CD4+ TEM, respectively (data not shown). The relative frequency of peripheral CD8+ T cells was 5% higher in elderly monkeys, compared with that in juveniles (P < .002). Although the mean numbers of CD8+ central memory T cells (CD8+ TCM) and CD8+ effector memory T cells (CD8+ TEM) were similar in elderly and juvenile monkeys, elderly monkeys had a 3.8-fold lower mean number of CD8+ naive T cells (CD8+ TN) and a 1.6-fold higher mean number of CD8+ late effector T cells (CD8+ TEMRA; P < .02). Thus, as in aged humans [6, 16], there were significant age-related changes in circulating lymphocytes of elderly monkeys, the most obvious being the reduced number of TN and CD20+ B cells.
To determine whether the Fluzone and Fluzone/CLDC recipients were immunologically similar, lymphocyte subsets were compared before vaccination (Table 1). Although the numbers of effector CD4+ T cells and terminal effector CD8+ T cells were significantly higher in the blood of Fluzone/CLDC recipients, compared with those in the blood of Fluzone recipients, the numbers of total lymphocytes, B cells, and naive and central memory T cells were similar in the 2 groups (Table 1).
Age-Related Reduction in α4β7+ Lymphocytes
To assess mucosal trafficking capability of T cells in elderly and juvenile monkeys, expression of β7-integrin on CD4+ and CD8+ lymphocytes were compared (Table 1). Although lymphocyte homing to pulmonary tissues is poorly characterized [17], high expression of α4β7-integrin on CD4+ and CD8+ TEM mediates migration into the gastrointestinal tract lamina propria by binding MADCAM on endothelial cells of the postcapillary venules [18, 19], and an anti-α4β7 monoclonal antibody can block trafficking of α4β7+ cells to the gut of rhesus macaques [20]. In elderly monkeys, the mean number and relative frequency of CD4+ TEM cells was larger than that in juveniles. However, in elderly animals, the absolute number of β7+CD4+ T cells was 1.5-fold lower (P < .01) and 13% less frequent (P < .001), compared with juveniles (Table 1). As with CD4+ TEM cells, the higher mean number and relative frequency of CD8+ TEM and TEMRA cells in elderly as compared to juvenile monkeys did not correlate with the number of β7+ T cells (Table 1). The reduced frequency and absolute number of β7+ T cells suggests that there is reduced capability for T-cell recirculation between immune inductive sites and immune effector sites in elderly macaques.
CLDC Enhances Anti–Influenza Virus H1N1 Antibody Responses in Elderly Monkeys After Fluzone Vaccination
To determine the effect of the CLDC adjuvant on Fluzone-induced A/New Caledonia/20/99(H1N1) antibody levels in elderly monkeys, we compared mean anti-A/New Caledonia/20/99(H1N1) IgG and IgA titers and HAI antibody titers in plasma at weeks 2, 4, and 6 after vaccination, as well as mean AUC values 0–6 weeks after vaccination for each group (Figure 1). In addition, mean anti–influenza virus antibody titers in elderly monkeys were compared to previously published anti–influenza virus antibody titers of juvenile monkeys vaccinated with the same Fluzone vaccine [11]. There were no local, hematologic, or systemic signs that might indicate an adverse reaction to vaccination in either the Fluzone or Fluzone/CLDC monkeys (data not shown). As we have previously reported for juvenile monkeys [11], all of the elderly animals in the present studies had plasma IgG antibodies to A/New Caledonia/20/99(H1N1) [11] before vaccination (Figure 1A).
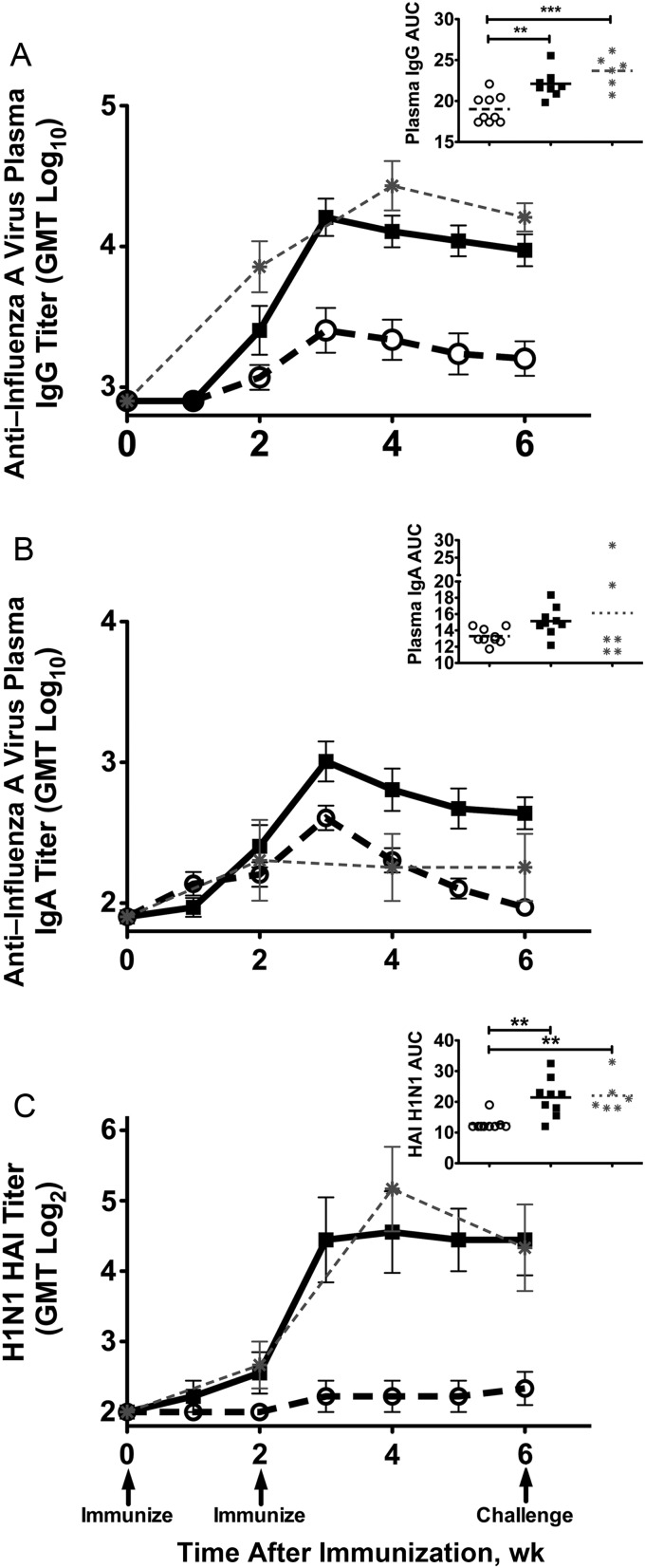
Figure 1.
Plasma anti–influenza virus antibody responses to Fluzone vaccination. A and B, Mean plasma whole A/New Caledonia/20/99 influenza virus–specific immunoglobulin G (IgG) antibody (A) and immunoglobulin A (IgA) antibody (B) titers detected by enzyme-linked immunosorbent assay. C, Mean plasma hemagglutinin inhibition (HAI) antibody titers against egg-grown A/Memphis/7/01. Arrows below the x-axis indicate vaccinations at weeks 0 and 2 after vaccination and on the day of challenge with A/New Caledonia/20/99-like A/Memphis/7/01 at week 6 after vaccination. P values were generated using an analysis of variance and Tukey-Kramer post hoc test; *P < .05, **P < .01, ***P < .001. Open circles, elderly Fluzone-only recipients (n = 9); squares, elderly Fluzone/cationic lipid/DNA complex (CLDC) recipients (n = 9); asterisks, juvenile Fluzone-only recipients (n = 6; data were previously published [11] and are inserted for comparison purposes). Abbreviations: AUC, area under the curve; GMT, geometric mean titer.
After 2 vaccinations, anti–influenza virus IgG responses were detectable in all 9 Fluzone/CLDC recipients but in only 4 of 9 Fluzone-only recipients, and the responses were transient in 2 of the monkeys in the latter group (Figure 1A). In contrast, anti–influenza virus IgG responses are detected in every juvenile macaque after 1 Fluzone vaccination [11]; thus, some elderly macaques are poor responders to Fluzone, but all macaques respond with addition of CLDC. The mean anti–influenza virus IgG plasma antibody AUC in the elderly animals vaccinated with Fluzone/CLDC was 1.2-log10 higher, compared with that in the elderly recipients of Fluzone only (P < .001; Figure 1A). The addition of CLDC to Fluzone immunization increased the mean anti–influenza virus IgG antibody titer strength in a higher proportion of elderly monkeys at week 4 after vaccination (1.1 log10; 78% vs 33%) and at week 6 after vaccination (1.2 log10; 100% vs 44%; P < .001). Similarly, the mean HAI antibody titer AUC was 1.7-log2 higher after Fluzone/CLDC vaccination, compared with Fluzone-only vaccination (P < .002; Figure 1C). Finally, the HAI antibody titers in Fluzone/CLDC-vaccinated elderly monkeys were similar to the HAI antibody titers in juvenile monkeys vaccinated with unadjuvanted Fluzone [11].
While all vaccinated animals had detectable anti–influenza virus IgA titers 1 week after boost, antibody titers in Fluzone-only recipients steadily declined until only 2 had detectable levels on the day of challenge (Figure 1B). As in juvenile monkeys [11], anti–influenza virus IgA responses in elderly monkeys vaccinated with Fluzone alone were weak and transient. In contrast, anti–influenza virus plasma IgA responses persisted in all 9 Fluzone/CLDC-vaccinated animals. Mean plasma IgA titers were higher in Fluzone/CLDC-vaccinated animals than in Fluzone-alone animals at week 4 (1.2 log10; P < .01) and week 6 after vaccination, the day of challenge (1.3 log10; P < .001). The plasma anti–influenza virus IgA mean AUC among the Fluzone/CLDC-vaccinated elderly animals was 1.1-log10 times that among the elderly Fluzone-only recipients (P < .01; Figure 1B).
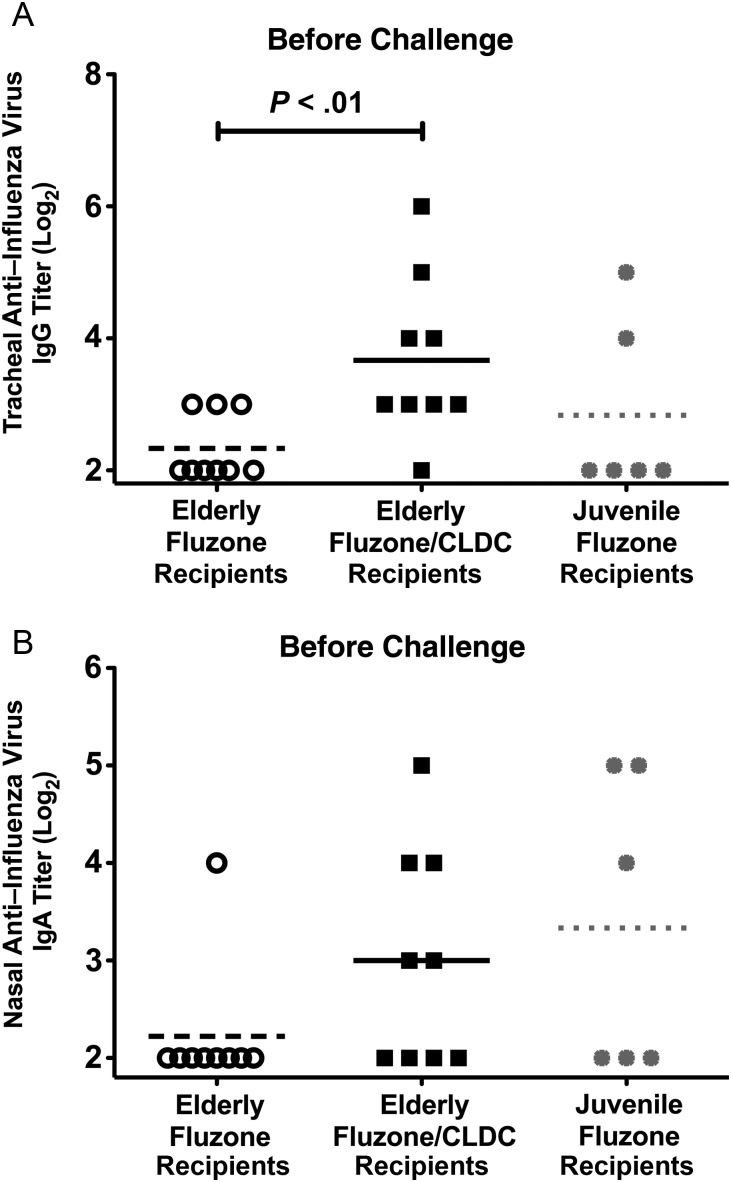
Eight of 9 Fluzone CLDC-vaccinated monkeys had detectable IgG in tracheal samples, compared with only 3 of 9 Fluzone-only recipients (Figure 2A). In Fluzone/CLDC-vaccinated monkeys with detectable anti–influenza virus IgG antibody in tracheal secretions, the mean titer was 1.6-log2 times that for Fluzone-only recipients (P < .01; Figure 2A). Furthermore, a greater proportion of elderly Fluzone/CLDC recipients had anti–influenza virus IgA antibodies in nasal secretions, compared with elderly Fluzone recipients (56% vs 11%; Figure 2B). A larger proportion of elderly Fluzone/CLDC recipients generated higher anti–influenza virus tracheal IgG and nasal IgA antibody titers than those produced by juveniles vaccinated with Fluzone only [11].
Figure 2.
Anti–influenza virus antibody responses in mucosal secretions following Fluzone vaccination. Immunoglobulin G (IgG) antibody titers in tracheal secretions (A) and immunoglobulin A (IgA) antibody titers in nasal secretions (B) detected by enzyme-linked immunosorbent assay 4 days before A/Memphis/7/01 challenge. P values were generated using a 2-tailed t test. Data for juvenile Fluzone-only recipients were previously published [11] and are inserted for comparison purposes. Abbreviation: CLDC, cationic lipid/DNA complex.
CLDC Limits Influenza Virus–Specific Interferon γ (IFN-γ) Secretion by Peripheral Blood CD4+ and CD8+ T cells
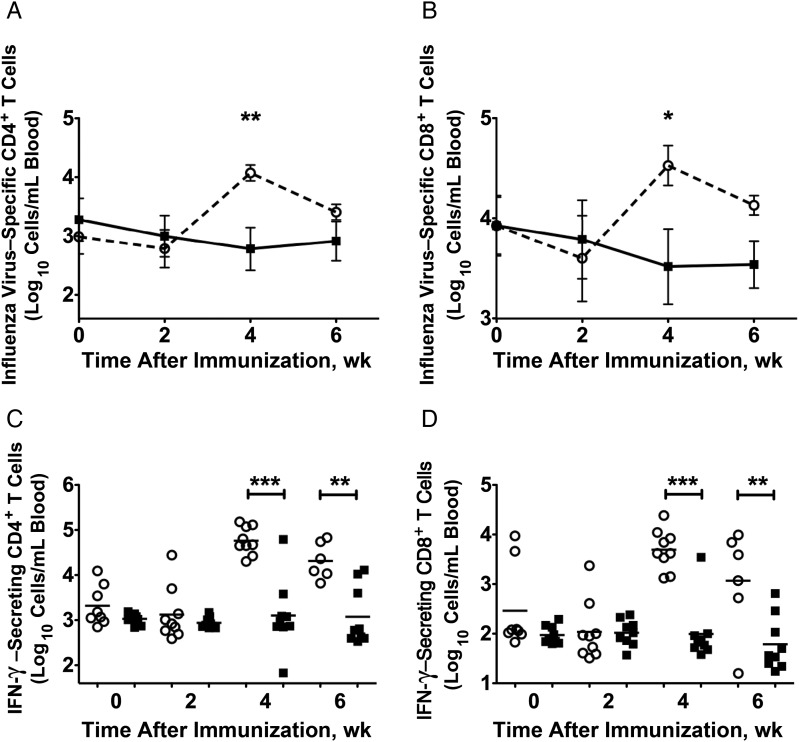
The effect of CLDC on H1N1-specific CD4+ and CD8+ T-cell responses after Fluzone vaccination were determined by intracellular staining of H1N1-stimulated PBMCs. Before vaccination, preexisting H1N1-specific CD4+ and CD8+ T-cell responses were detected in a similar proportion of animals in the vaccinated groups (Figure 3A and 3B). At week 4 after vaccination (2 weeks after boost), elderly Fluzone-only recipients had 1.3-log10 more H1N1-specific CD4+ T cells (P < .01) and 1.0 log10 more H1N1-specific CD8+ T cells (P < .05) than elderly Fluzone/CLDC recipients (Figure 3A and 3B). The increased H1N1-specific CD4+ and CD8+ T cells in the blood at week 4 after immunization in the Fluzone-only recipients was due to 1.6-log10 more IFN-γ–secreting CD4+ T cells (P < .01) and 1.2-log10 more IFN-γ–secreting CD8+ T cells (P < .01) in this group than in Fluzone/CLDC recipients (Figure 3C and 3D). Thus, in elderly monkeys, the addition of CLDC to Fluzone blunted the influenza virus–specific T-helper cell type 1 (Th1)–like T-cell responses by limiting the number of IFN-γ–secreting CD4+ and CD8+ T cells. The effect of CLDC on T-helper cell 2 (Th2)–like responses is unknown because we did not evaluate the number of T cells that secrete Th2 cytokines (interleukin 4, interleukin 6, or interleukin 10) in response to H1N1 stimulation.
Figure 3.
Influenza virus–specific CD4+ and CD8+ T-cell responses after vaccination. The mean absolute number of H1N1-specific CD4+ T-cell responses (A and C) and CD8+ T cell responses (B and D) are shown. A and B, The mean of the sum of H1N1-specific CD4+ (A) or CD8+ (B) T cells responding to whole inactivated H1N1 influenza virus (A/New Caledonia/20/99) antigen with expression of one of the 15 possible combinations of 4 immune stimulatory molecules that were assessed (interferon γ [IFN-γ], CD107a, perforin, and granzyme). C and D, Scatterplots indicate the total number of H1N1-specific IFN-γ–secreting T cells/mL of blood 0, 2, 4, and 6 weeks after infection. P values were generated using an analysis of variance and Tukey-Kramer post hoc test. *P < .05, **P < .01, ***P < .001. Open circles, elderly Fluzone-only recipients (n = 9); squares, elderly Fluzone/ cationic lipid/DNA complex (CLDC) recipients (n = 9).
Fluzone Vaccination does not reduce Influenza Virus Replication in Elderly Monkeys Without Addition of the CLDC Adjuvant
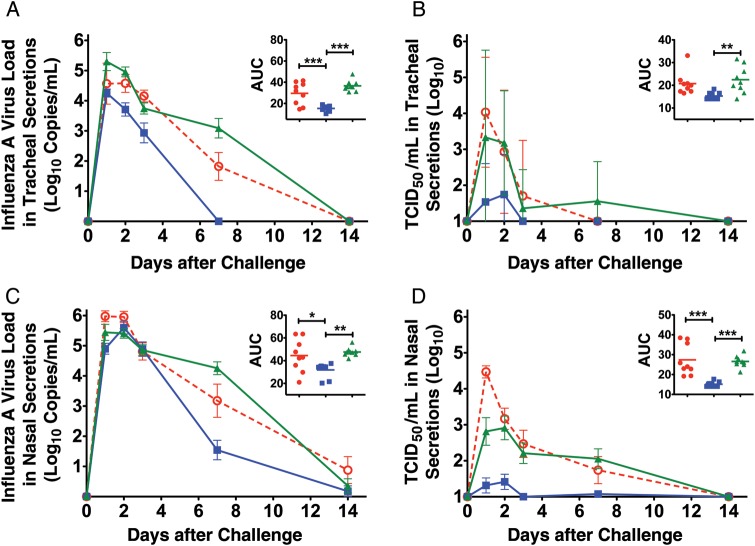
To determine the effect of CLDC on Fluzone efficacy, vaccinated elderly monkeys were challenged with A/Memphis/7/2001(H1N1), and levels of virus in respiratory secretions were determined. The mean peak viral RNA, mean viral RNA AUC, mean peak TCID50/mL, and mean TCID50/mL AUC values in the tracheal secretions of Fluzone recipients and unvaccinated groups were not significantly different (Figure 4A and 4B and Table 2). However, the peak viral RNA level was 1.2-log10 lower (P < .05) and the mean viral RNA AUC was 2.0-log10 lower (P < .001) in tracheal secretions of Fluzone/CLDC-vaccinated animals, compared with either Fluzone-only recipients or naive control animals (Figure 4A). Similarly, the mean peak TCID50/mL was 2.1-log10 lower (P < .01) and the mean TCID50/mL AUC (P < .01) was 1.5-log10 lower in tracheal secretions of Fluzone/CLDC recipients, compared with unvaccinated monkeys (Figure 4B and Table 2). Of note, infectious virus was isolated from the tracheal secretions of all Fluzone-vaccinated and unvaccinated animals after challenge, but infectious virus could only be isolated from tracheal secretions of 4 of 9 Fluzone/CLDC recipients (Figure 4B and Table 2).
Figure 4.
Virus shedding from the respiratory tract after influenza A virus challenge. A, Mean viral RNA copy number in tracheal lavage specimens. The inset shows the viral RNA area under the curve (AUC). B, Mean 50% tissue culture infectious dose (TCID50)/milliliter in tracheal lavage specimens. The inset shows the TCID50/milliliter AUC. C, Mean viral RNA copy number in nasal lavage specimens. The inset shows the viral RNA AUC. (D) Mean TCID50/milliliter in nasal lavage specimens. The inset shows the TCID50/milliliter AUC. The total level of virus shedding was estimated by converting the longitudinal data from each animal into AUCs. P values generated using an analysis of variance and Tukey-Kramer post hoc test. *P < .05, **P < .01, ***P < .001. Circles, animals vaccinated with Fluzone (n = 9); squares, animals vaccinated with Fluzone/ cationic lipid/DNA complex (CLDC) (n = 9); triangles, nonvaccinated control animals (n = 9).
Table 2.
Effect of Cationic Lipid/DNA Complex (CLDC) on Hemagglutination Inhibition (HAI) Titers and Influenza A Virus Replication in the Upper and Lower Respiratory Tract
| Animal, by Vaccine Formulation | Age, y | Sex | HAI Titer After Vaccination |
Tracheal Titera |
Nasal Titera |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 2 | Week 6b | Fold Increasec | Peak log10 TCID50/mL | Peak Day | Peak Log10 TCID50/mL | Peak Day | |||
| Fluzoned | ||||||||||
| 23 891 | 21.90 | F | 4 | 4 | 4 | 1 | 3.5 | 1 | 4.5 | 1 |
| 24 127 | 21.20 | F | 4 | 4 | 4 | 1 | 3.0 | 1 | 3.8 | 1 |
| 24 860 | 20.20 | F | 4 | 4 | 16 | 4 | 3.5 | 1 | 4.7 | 1 |
| 25 034 | 20.00 | M | 4 | 4 | 4 | 1 | 4.7 | 1 | 4.7 | 1 |
| 25 650 | 19.10 | F | 4 | 4 | 4 | 1 | 6.2 | 1 | 4.7 | 1 |
| 25 933 | 18.30 | F | 4 | 4 | 4 | 1 | 5.5 | 3 | 3.7 | 1 |
| 26 009 | 18.20 | F | 4 | 4 | 4 | 1 | 5.6 | 1 | 4.2 | 1 |
| 26 079 | 18.20 | M | 4 | 4 | 8 | 2 | 4.3 | 1 | 4.7 | 1 |
| 34 147 | 21.20 | M | 4 | 4 | 4 | 1 | 4.3 | 2 | 5.3 | 1 |
| Mean | 19.81 | … | 4.0 | 4.0 | 5.8e | 1.4 | 4.5f | 1.3 | 4.5f | 1.0 |
| Fluzone/CLDCd | ||||||||||
| 23 680 | 22.20 | M | 4 | 4 | 4 | 1 | 3.5 | 2 | 2.5 | 3 |
| 24 162 | 21.20 | M | 4 | 4 | 8 | 2 | 3.5 | 1 | 2.5 | 2 |
| 24 206 | 21.20 | F | 4 | 16 | 128 | 32 | 3.2 | 2 | 2.2 | 3 |
| 24 737 | 20.20 | F | 4 | 4 | 64 | 16 | 1.0 | 1 | 1.0 | 1 |
| 25 525 | 19.20 | F | 4 | 4 | 32 | 8 | 1.0 | 1 | 1.0 | 1 |
| 25 686 | 19.00 | M | 4 | 16 | 16 | 4 | 3.3 | 1 | 2.3 | 1 |
| 25 914 | 18.30 | M | 4 | 4 | 16 | 4 | 1.0 | 1 | 1.0 | 1 |
| 26 066 | 18.20 | F | 4 | 4 | 16 | 4 | 1.0 | 1 | 1.0 | 1 |
| 26 421 | 18.11 | M | 4 | 8 | 32 | 8 | 1.0 | 1 | 1.7 | 7 |
| Mean | 19.73 | 4.0 | 7.1 | 35.1e | 8.8 | 2.1f | 1.2 | 1.7f | 2.2 | |
| Unvaccinated | ||||||||||
| 24 223 | 21.20 | M | … | … | 4 | … | 5.0 | 2 | 3.7 | 1 |
| 24 244 | 21.10 | F | … | … | 4 | … | 6.0 | 1 | 3.5 | 2 |
| 24 375 | 21.00 | F | … | … | 4 | … | 3.2 | 1 | 4.0 | 2 |
| 25 424 | 19.20 | F | … | … | 4 | … | 6.0 | 1 | 3.5 | 1 |
| 25 630 | 19.10 | M | … | … | 4 | … | 6.5 | 1 | 4.0 | 1 |
| 25 696 | 19.00 | F | … | … | 4 | … | 4.3 | 1 | 3.5 | 7 |
| 25 710 | 19.00 | M | … | … | 4 | … | 3.7 | 2 | 3.7 | 1 |
| 26 170 | 18.10 | M | … | … | 4 | … | 1.0 | 1 | 2.5 | 7 |
| 26 244 | 18.10 | M | … | … | 4 | … | 3.5 | 2 | 3.2 | 3 |
| Mean | 19.53 | … | … | … | 4 | … | 4.4f | 1.3 | 3.5f | 2.8 |
a Peak 50% tissue culture infectious dose (TCID50)/mL inversely correlate with HAI titers at week 6 after vaccination (P < .006, by Pearson correlation).
b Day of A/Memphis/7/2001(H1N1) challenge.
c Titer at week 6 after vaccination (day of challenge) relative to that at week 0 after vaccination.
d Vaccinated twice with 22.5 µg HA from 2006–2007 pediatric Fluzone with or without 50 µg CLDC.
e Mean values for the Fluzone/CLDC group are 6-fold higher than that for the Fluzone-only group (P < .05, by the 2-tailed t test).
f Mean values for the Fluzone/CLDC group are significantly lower than those for the Fluzone-only and unvaccinated groups (P < .01, by 1-way analysis of variance with the Tukey post hoc test).
The viral RNA AUC level in the nasal secretions of Fluzone/CLDC recipients was at least 1.4-log10 lower than that in either Fluzone-vaccinated or naive control animals (P < .01; Figure 4C). Furthermore, in nasal secretions, mean peak TCID50/mL was 2.1-log10 lower (P < .001) and the mean TCID50/mL AUC was 1.8-log10 lower (P < .001) in Fluzone/CLDC recipients, compared with either Fluzone-only recipients or unvaccinated animals (Figure 4D and Table 2). Thus, Fluzone alone did not reduce H1N1 virus replication in elderly monkeys. In comparison, juveniles vaccinated with Fluzone alone had a 1.3-fold reduction in mean viral RNA AUC in tracheal secretions (P < .01), compared with unvaccinated juveniles ([11] and Carroll et al, unpublished data).
Correlates of Protection in Fluzone-Vaccinated Elderly Monkeys
Pearson's correlation analyses were used to determine the relationship between immune responses present on the day of challenge and the total viral RNA load and infectious virus peak level (data not shown) and the AUC in respiratory secretions. Plasma anti–influenza virus IgG and HAI antibody titers were inversely correlated with peak TCID50/mL, TCID50/mL AUC, and viral RNA AUC in both tracheal and nasal secretions (P < .03 at all time points; Table 3). Plasma anti–influenza virus IgA antibody titers were inversely correlated with peak TCID50/mL, peak viral RNA load, and TCID50/mL AUC in both tracheal and nasal secretions (P < .03 at all time points; Table 3). Furthermore, anti–influenza virus IgG in tracheal secretions were inversely correlated with peak viral RNA load and viral RNA AUC in tracheal secretions (P < .04; Table 3). In contrast to the negative correlation between viral RNA levels and influenza virus–specific antibody titers before challenge, there was a modest positive correlation between viral RNA AUC in tracheal secretions and the number of influenza virus–specific CD8+ IFN-γ–secreting T cells in the blood (P < .05; Table 3).
Table 3.
Pearson Correlations Between Influenza A Virus H1N1–Specific Systemic and Mucosal Immune Responses and Total or Infectious H1N1 Virus Replication Throughout the Respiratory Tract
| H1N1-Specific Responsesa | Viral RNA AUCb |
TCID50 AUCb |
||
|---|---|---|---|---|
| rc | P | rc | P | |
| Influenza virus levels in Nasal secretions | ||||
| Plasma IgG | −0.58 | .012 | −0.77 | <.001 |
| Plasma IgA | −0.43 | .072 | −0.68 | .002 |
| Plasma HAI | −0.51 | .032 | −0.62 | .006 |
| Nasal secretion IgG | −0.07 | .739 | −0.18 | .473 |
| Nasal secretion IgA | −0.03 | .893 | −0.14 | .574 |
| Blood CD8+ IFN-γ+–secreting T cells | 0.07 | .814 | 0.21 | .462 |
| Blood CD4+ IFN-γ+–secreting T cells | 0.08 | .788 | 0.47 | .075 |
| Influenza virus levels in Tracheal secretions | ||||
| Plasma IgG | −0.59 | .010 | −0.65 | .004 |
| Plasma IgA | −0.59 | .009 | −0.51 | .031 |
| Plasma HAI | −0.53 | .024 | −0.55 | .019 |
| Tracheal secretion IgG | −0.49 | .037 | −0.44 | .071 |
| Tracheal secretion IgA | −0.43 | .078 | −0.16 | .525 |
| Blood CD8+ IFN-γ+–secreting T cells | 0.52 | .049 | 0.38 | .158 |
| Blood CD4+ IFN-γ+–secreting T cells | 0.47 | .075 | 0.47 | .075 |
Abbreviations: HAI, hemagglutination inhibition; IFN-γ, interferon γ; IgA, immunoglobulin A; IgG, immunoglobulin G.
a H1N1-specific antibody responses and IFN-γ–secreting peripheral blood mononuclear cells were stimulated with disrupted A/New Caledonia/20/99(H1N1), a virus that is homologous to the A/Memphis/7/01(H1N1) challenge virus.
b Area under the curve (AUC) for total H1N1 virus or infectious virus (TCID50/mL) detected in nasal or tracheal respiratory secretions.
c r, the correlation coefficient, has a range of −1 to 1, with 1 denoting perfect correlation and −1 denoting perfect inverse correlation.
DISCUSSION
The aged macaques used in this study had clear evidence of immune senescence. Levels of circulating lymphocyte populations, especially TN and CD20+ B cells, in the aged monkeys were reduced, compared with those in juveniles. Similar reductions in numbers of TN helper cells and B cells in elderly humans [6] may impair the generation of TIV-induced CD4+ T-helper cell–dependent antibody responses that correlate with protection from disease [21]. Healthy young adult humans [2, 22] and juvenile macaques generate moderate antibody responses to Fluzone and have modest protection [2, 11]. However, we show that, similar to immunosenescent humans [23], elderly macaques generate poor antibody responses to Fluzone and are not protected from challenge.
The addition of CLDC to Fluzone markedly enhanced vaccine immunogenicity and efficacy in elderly macaques. The CLDC adjuvant increased the strength and duration of all Fluzone-elicited anti–influenza virus antibody responses in a higher proportion of elderly monkeys. In fact, this vaccine produced plasma influenza virus–specific antibody titers in elderly monkeys that were similar to those in juvenile monkeys vaccinated with Fluzone alone [11]. The Fluzone/CLDC-induced immune responses protected elderly animals from viral challenge, as infectious virus in tracheal secretions was undetectable in most elderly animals, and viral RNA levels in tracheal secretions were very low [12]. As in Fluzone-vaccinated juvenile monkeys [11], viral RNA load and infectious titers in respiratory secretions of elderly monkeys were inversely correlated with prechallenge anti–influenza virus IgG, IgA, and HAI titers in the plasma and with anti–influenza virus tracheal IgG titers.
The immunogenicity of influenza vaccines in clinical trials is judged on the basis of a rise (>4-fold) in either HAI or serum antibody titers after vaccination [24]. However, very few elderly humans attain a 4-fold rise in antibody titer [25, 26] without a substantial increase of the HA dose in the vaccine [27]. Optimal virus-specific antibody production largely depends on both functional antigen-presenting cells and CD4+ T-helper cells. However, human and nonhuman primate antigen-presenting cells demonstrate age-related dysregulated expression of pathogen-sensing receptors (Toll-like receptor and RIG-I), and the engagement of these receptors results in reduced ability to secrete cytokines (IFN-α, tumor necrosis factor, interleukin 6, and interleukin 12) [28, 29]. Increased frequency of CD8+CD28− TEMRA cells correlate with defective antibody responses to TIV [26]. These TEMRA cells produce IFN-γ but not interleukin 5 [30] and induce antigen-presenting cell tolerance to CD4+ T-helper cells through suppression of antigen-presenting cell costimulatory molecules [31]. Furthermore, low-to-no antibody responses following TIV receipt are associated with limited expansion of antigen-specific B cells because of reduced follicular T-helper cell activation [32, 33]. The mechanism of CLDC-enhanced immunogenicity remains unknown but may be related to enhanced antigen presentation, a dampened IFN-γ–driven Th1-type response to Fluzone, and a shifted immune response toward greater B-cell propagation and antibody production.
Notes
Acknowledgments. We thank the Primate Services Unit and the CNPRC for excellent technical assistance.
Financial support. This work was supported by the National Institutes of Health (Public Health Service grants P51RR00169 and U01AI074512.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields virology. 5th ed. Vol. 2. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2007. pp. 1691–740. [Google Scholar]
- 2.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 5.Haberthur K, Engelman F, Barron A, Messaoudi I. Immune senescence in aged nonhuman primates. Exp Gerontol. 2010;45:655–61. doi: 10.1016/j.exger.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009;333:413–29. doi: 10.1007/978-3-540-92165-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gowen BB, Fairman J, Smee DF, et al. Protective immunity against acute phleboviral infection elicited through immunostimulatory cationic liposome-DNA complexes. Antiviral Res. 2006;69:165–72. doi: 10.1016/j.antiviral.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Hong DK, Chang S, Botham CM, Giffon TD, Fairman J, Lewis DB. Cationic lipid/DNA complex-adjuvanted influenza A virus vaccination induces robust cross-protective immunity. J Virol. 2010;84:12691–702. doi: 10.1128/JVI.00769-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lay M, Callejo B, Chang S, et al. Cationic lipid/DNA complexes (JVRS-100) combined with influenza vaccine (Fluzone (R)) increases antibody response, cellular immunity, and antigenically drifted protection. Vaccine. 2009;27:3811–20. doi: 10.1016/j.vaccine.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaks K, Jordan M, Guth A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–45. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 11.Carroll TD, Matzinger SR, Barro M, et al. Alphavirus replicon-based adjuvants enhance the immunogenicity and effectiveness of Fluzone (R) in rhesus macaques. Vaccine. 2011;29:931–40. doi: 10.1016/j.vaccine.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll TD, Matzinger SR, Geneseca M, et al. Interferon-induced expression of MxA in the respiratory tract of rhesus macaques is suppressed by influenza virus replication. J Immunol. 2008;180:2385–95. doi: 10.4049/jimmunol.180.4.2385. [DOI] [PubMed] [Google Scholar]
- 13.Webster RG, Cox N, Stöhr K. 1st ed. World Health Organization; 2002. WHO manual on animal influenza diagnosis and surveillance; pp. 28–36. [Google Scholar]
- 14.Brignolo L, Spinner A, Yee JL, Lerche NW. Subsets of T cells in healthy rhesus macaques (Macaca mulatta) infected with simian T-lymphotropic virus type 1. Comp Med. 2004;54:271–4. [PubMed] [Google Scholar]
- 15.Genesca M, Rourke T, Li J, et al. Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8 plus; T cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J Immunol. 2007;179:4732–40. doi: 10.4049/jimmunol.179.7.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- 17.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–61. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 18.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–43. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol. 2011;12:467–71. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansari AA, Reimann KA, Mayne AE, et al. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011;186:1044–59. doi: 10.4049/jimmunol.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keitel WA, Couch RB, Cate TR, et al. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J Clin Microbiol. 1994;32:2468–73. doi: 10.1128/jcm.32.10.2468-2473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–74. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 24.Wood JM, Newman RW, Ploss K. The use of correlates of immunity in European Union licensing of influenza vaccines. Dev Biol. 2003;115:9–16. [PubMed] [Google Scholar]
- 25.Gardner EM, Bernstein ED, Dran S, et al. Characterization of antibody responses to annual influenza vaccination over four years in a healthy elderly population. Vaccine. 2001;19:4610–7. doi: 10.1016/s0264-410x(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 26.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–7. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007;25:7656–63. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asquith M, Haberthur K, Brown M, et al. Age-dependent changes in innate immune phenotype and function in rhesus macaques (Macaca mulatta) Pathobiol Aging Age Related Dis. 2012;2:18052–66. doi: 10.3402/pba.v2i0.18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panda A, Qian F, Mohanty S, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–27. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saurwein-Teissl M, Lung TL, Marx F, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–9. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28- T cells. Int Immunol. 1998;10:775–83. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 32.Pallikkuth S, Parmigiani A, Silva SY, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120:985–93. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu M, Li G, Lee WW, et al. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc Natl Acad Sci U S A. 2012;109:E879–88. doi: 10.1073/pnas.1109797109. [DOI] [PMC free article] [PubMed] [Google Scholar]