Abstract
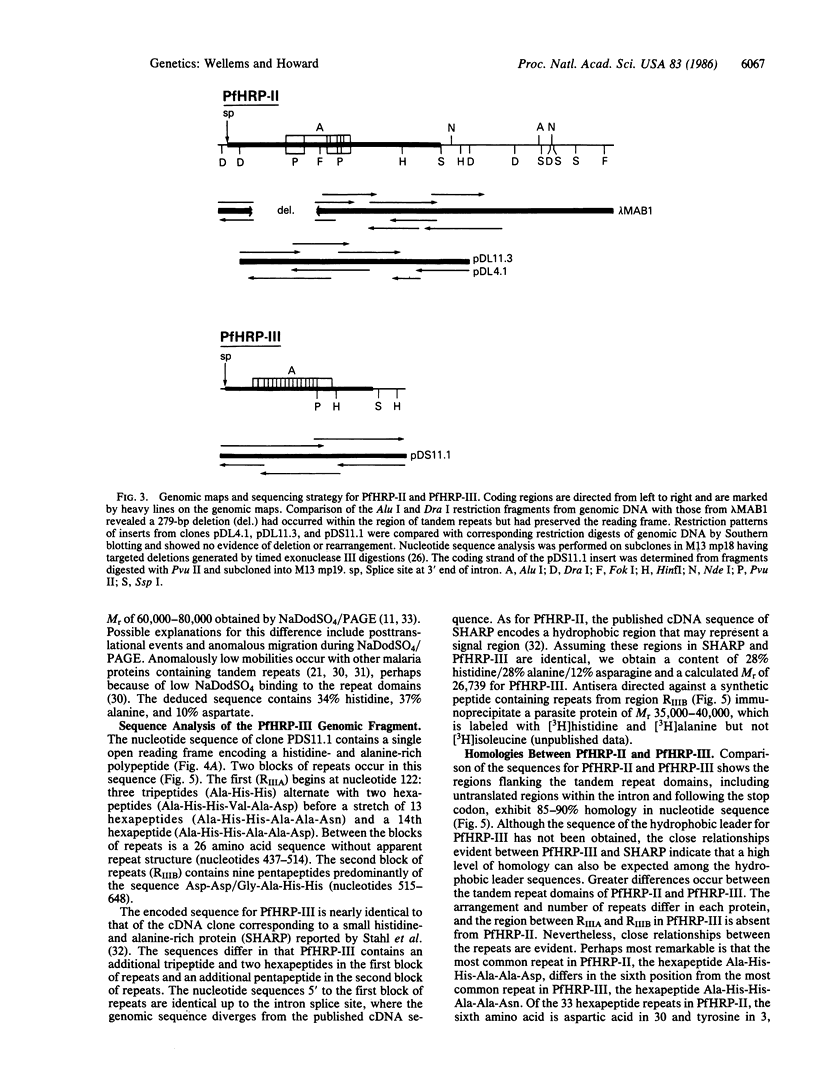
Two genes encoding distinct histidine-rich proteins in a Plasmodium falciparum clone exhibit high levels of homology, suggesting they have originated by duplication and divergence from a common ancestral sequence. Both genes have a similar interrupted structure and an exon that encodes closely related tandem repeats of very high histidine and alanine content. The most common repeat encoded by one gene, the hexapeptide Ala-His-His-Ala-Ala-Asp, differs in the sixth position from the most common repeat encoded by the other gene, the hexapeptide Ala-His-His-Ala-Ala-Asn. The divergence of the repeat domains is greater than that of the flanking regions, which exhibit 85-90% homology, including untranslated sequences. This suggests the tandem repeats are relatively labile elements within the genome that may provide the parasite with a means of rapid evolutionary change.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfort M., Pedersen-Lane J., West D., Ehrenman K., Maley G., Chu F., Maley F. Processing of the intron-containing thymidylate synthase (td) gene of phage T4 is at the RNA level. Cell. 1985 Jun;41(2):375–382. doi: 10.1016/s0092-8674(85)80010-6. [DOI] [PubMed] [Google Scholar]
- Burkot T. R., Williams J. L., Schneider I. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the same isolate. Trans R Soc Trop Med Hyg. 1984;78(3):339–341. doi: 10.1016/0035-9203(84)90114-7. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Feder R., Blobel G. In vitro biosynthesis and core glycosylation of the histidine-rich protein of Plasmodium lophurae. Mol Biochem Parasitol. 1983 Dec;9(4):351–362. doi: 10.1016/0166-6851(83)90091-9. [DOI] [PubMed] [Google Scholar]
- Hadley T. J., Leech J. H., Green T. J., Daniel W. A., Wahlgren M., Miller L. H., Howard R. J. A comparison of knobby (K+) and knobless (K-) parasites from two strains of Plasmodium falciparum. Mol Biochem Parasitol. 1983 Nov;9(3):271–278. doi: 10.1016/0166-6851(83)90102-0. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Zolg J. W., Scaife J. G. Isolation and characterisation of ribosomal RNA from the human malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 1981 Dec 31;4(5-6):283–290. doi: 10.1016/0166-6851(81)90061-x. [DOI] [PubMed] [Google Scholar]
- Irving D. O., Cross G. A., Feder R., Wallach M. Structure and organization of the histidine-rich protein gene of Plasmodium lophurae. Mol Biochem Parasitol. 1986 Feb;18(2):223–234. doi: 10.1016/0166-6851(86)90040-x. [DOI] [PubMed] [Google Scholar]
- Kilejian A. A unique histidine-rich polypeptide from the malaria parasite, Plasmodium lophurae. J Biol Chem. 1974 Jul 25;249(14):4650–4655. [PubMed] [Google Scholar]
- Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A. Immunological cross-reactivity of the histidine-rich protein of Plasmodium lophurae and the knob protein of Plasmodium falciparum. J Parasitol. 1983 Apr;69(2):257–261. [PubMed] [Google Scholar]
- Kilejian A. The biosynthesis of the knob protein and a 65 000 dalton histidine-rich polypeptide of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Jun;12(2):185–194. doi: 10.1016/0166-6851(84)90134-8. [DOI] [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Aikawa M., Miller L. H., Howard R. J. Plasmodium falciparum malaria: association of knobs on the surface of infected erythrocytes with a histidine-rich protein and the erythrocyte skeleton. J Cell Biol. 1984 Apr;98(4):1256–1264. doi: 10.1083/jcb.98.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., Hansen J. L., Dame J. B., Mullins J. A. Mung bean nuclease cleaves Plasmodium genomic DNA at sites before and after genes. Science. 1984 Aug 10;225(4662):625–628. doi: 10.1126/science.6330899. [DOI] [PubMed] [Google Scholar]
- Mehdy M. C., Ratner D., Firtel R. A. Induction and modulation of cell-type-specific gene expression in Dictyostelium. Cell. 1983 Mar;32(3):763–771. doi: 10.1016/0092-8674(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki L. S., Svec P., Nussenzweig R. S., Nussenzweig V., Godson G. N. Structure of the plasmodium knowlesi gene coding for the circumsporozoite protein. Cell. 1983 Oct;34(3):815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Feder R., Pavlovec A., Blobel G. Primary structure and genomic organization of the histidine-rich protein of the malaria parasite Plasmodium lophurae. Nature. 1984 Dec 13;312(5995):616–620. doi: 10.1038/312616a0. [DOI] [PubMed] [Google Scholar]
- Sanderson A., Walliker D., Molez J. F. Enzyme typing of Plasmodium falciparum from African and some other Old World countries. Trans R Soc Trop Med Hyg. 1981;75(2):263–267. doi: 10.1016/0035-9203(81)90331-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. Automated preparation of DNA sequences for publication. Nucleic Acids Res. 1986 Jan 10;14(1):65–73. doi: 10.1093/nar/14.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H. D., Kemp D. J., Crewther P. E., Scanlon D. B., Woodrow G., Brown G. V., Bianco A. E., Anders R. F., Coppel R. L. Sequence of a cDNA encoding a small polymorphic histidine- and alanine-rich protein from Plasmodium falciparum. Nucleic Acids Res. 1985 Nov 11;13(21):7837–7846. doi: 10.1093/nar/13.21.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]