Abstract
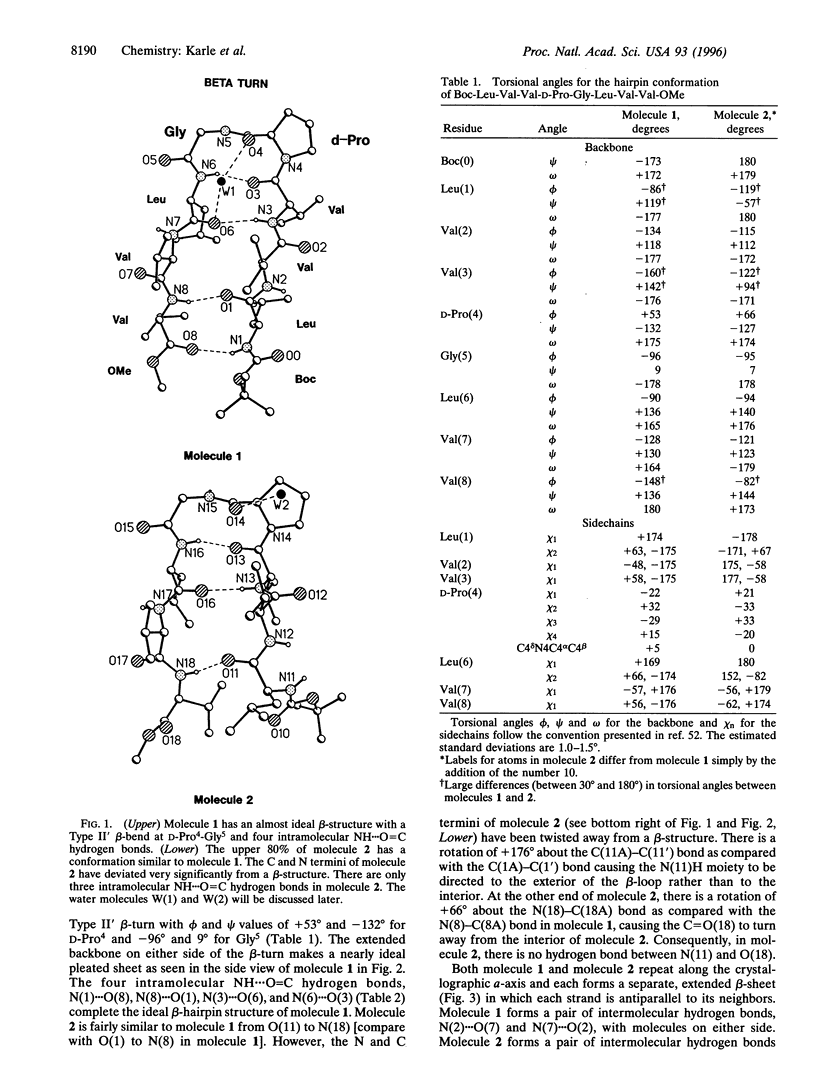
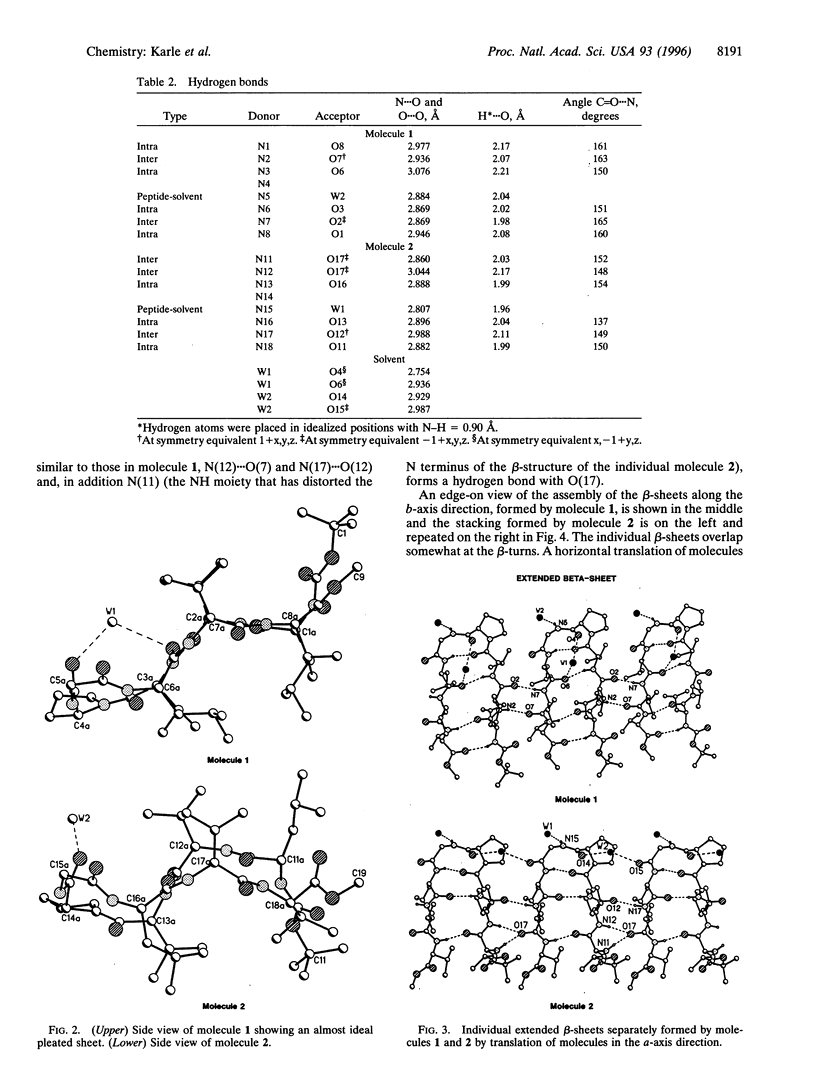
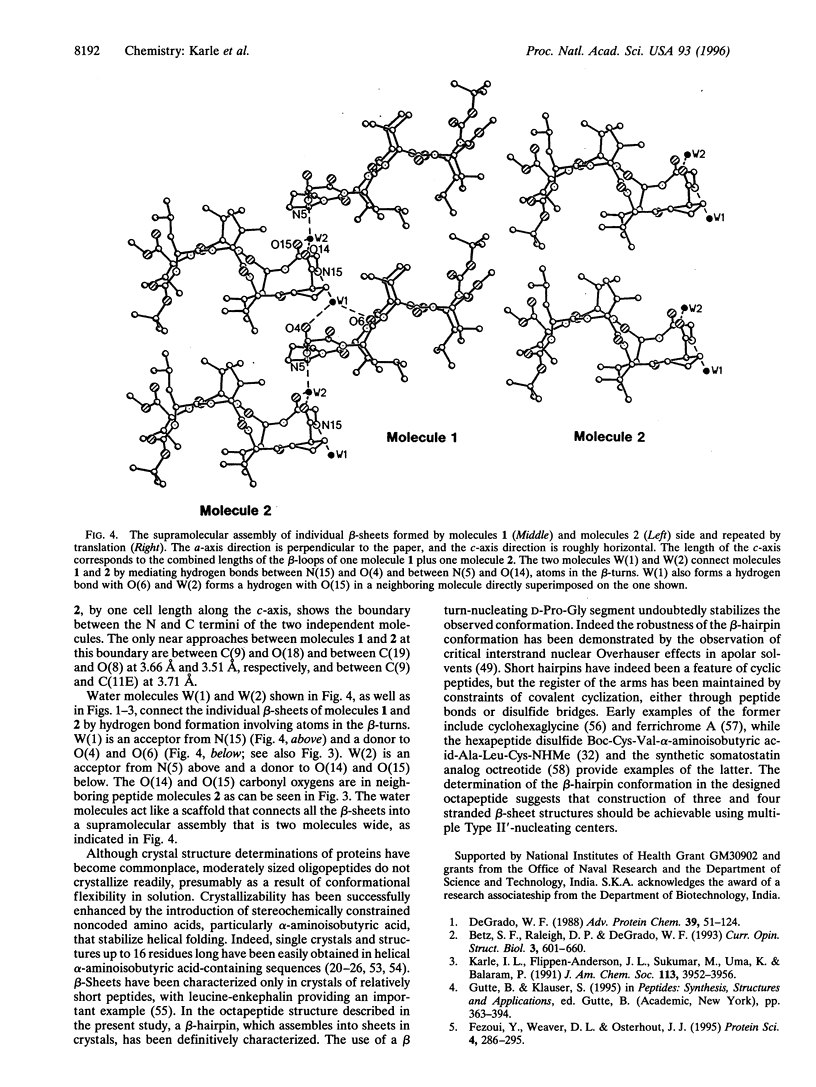
Beta-hairpin structures have been crystallographically characterized only in very short acyclic peptides, in contrast to helices. The structure of the designed beta-hairpin, t-butoxycarbonyl-Leu-Val-Val-D-Pro-Gly-Leu-Val-Val-OMe in crystals is described. The two independent molecules of the octapeptide fold into almost ideal beta-hairpin conformations with the central D-Pro-Gly segment adopting a Type II' beta-turn conformation. The definitive characterization of a beta-hairpin has implications for de novo peptide and protein design, particularly for the development of three- and four-stranded beta-sheets.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awasthi S. K., Raghothama S., Balaram P. A designed beta-hairpin peptide. Biochem Biophys Res Commun. 1995 Nov 2;216(1):375–381. doi: 10.1006/bbrc.1995.2634. [DOI] [PubMed] [Google Scholar]
- Blanco F. J., Rivas G., Serrano L. A short linear peptide that folds into a native stable beta-hairpin in aqueous solution. Nat Struct Biol. 1994 Sep;1(9):584–590. doi: 10.1038/nsb0994-584. [DOI] [PubMed] [Google Scholar]
- Bodkin M. J., Goodfellow J. M. Competing interactions contributing to alpha-helical stability in aqueous solution. Protein Sci. 1995 Apr;4(4):603–612. doi: 10.1002/pro.5560040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Degrado W. F. Design of peptides and proteins. Adv Protein Chem. 1988;39:51–124. doi: 10.1016/s0065-3233(08)60375-7. [DOI] [PubMed] [Google Scholar]
- Doig A. J., Baldwin R. L. N- and C-capping preferences for all 20 amino acids in alpha-helical peptides. Protein Sci. 1995 Jul;4(7):1325–1336. doi: 10.1002/pro.5560040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fezoui Y., Weaver D. L., Osterhout J. J. Strategies and rationales for the de novo design of a helical hairpin peptide. Protein Sci. 1995 Feb;4(2):286–295. doi: 10.1002/pro.5560040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M. H. De novo design of beta-sheet proteins. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8729–8730. doi: 10.1073/pnas.91.19.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M. H., Richardson J. S., Richardson D. C., Ogden R. C. De novo design, expression, and characterization of Felix: a four-helix bundle protein of native-like sequence. Science. 1990 Aug 24;249(4971):884–891. doi: 10.1126/science.2392678. [DOI] [PubMed] [Google Scholar]
- Ishida T., Kenmotsu M., Mino Y., Inoue M., Fujiwara T., Tomita K., Kimura T., Sakakibara S. X-ray diffraction studies of enkephalins. Crystal structure of [(4'-bromo) Phe4,Leu5]enkephalin. Biochem J. 1984 Mar 15;218(3):677–689. doi: 10.1042/bj2180677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamtekar S., Hecht M. H. Protein Motifs. 7. The four-helix bundle: what determines a fold? FASEB J. 1995 Aug;9(11):1013–1022. doi: 10.1096/fasebj.9.11.7649401. [DOI] [PubMed] [Google Scholar]
- Kamtekar S., Schiffer J. M., Xiong H., Babik J. M., Hecht M. H. Protein design by binary patterning of polar and nonpolar amino acids. Science. 1993 Dec 10;262(5140):1680–1685. doi: 10.1126/science.8259512. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Balaram P. Structural characteristics of alpha-helical peptide molecules containing Aib residues. Biochemistry. 1990 Jul 24;29(29):6747–6756. doi: 10.1021/bi00481a001. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J. L., Agarwalla S., Balaram P. Crystal structure of [Leu1]zervamicin, a membrane ion-channel peptide: implications for gating mechanisms. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5307–5311. doi: 10.1073/pnas.88.12.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J. L., Gurunath R., Balaram P. Facile transition between 3(10)- and alpha-helix: structures of 8-, 9-, and 10-residue peptides containing the -(Leu-Aib-Ala)2-Phe-Aib- fragment. Protein Sci. 1994 Sep;3(9):1547–1555. doi: 10.1002/pro.5560030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J., Sukumar M., Balaram P. Conformation of a 16-residue zervamicin IIA analog peptide containing three different structural features: 3(10)-helix, alpha-helix, and beta-bend ribbon. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5087–5091. doi: 10.1073/pnas.84.15.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy B., Choe S., Cascio D., McRorie D. K., DeGrado W. F., Eisenberg D. Crystal structure of a synthetic triple-stranded alpha-helical bundle. Science. 1993 Feb 26;259(5099):1288–1293. doi: 10.1126/science.8446897. [DOI] [PubMed] [Google Scholar]
- Marraud M., Aubry A. Crystal structures of peptides and modified peptides. Biopolymers. 1996;40(1):45–83. doi: 10.1002/(sici)1097-0282(1996)40:1<45::aid-bip3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Milner-White E. J., Poet R. Four classes of beta-hairpins in proteins. Biochem J. 1986 Nov 15;240(1):289–292. doi: 10.1042/bj2400289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor D. L., Jr, Kim P. S. Context is a major determinant of beta-sheet propensity. Nature. 1994 Sep 15;371(6494):264–267. doi: 10.1038/371264a0. [DOI] [PubMed] [Google Scholar]
- Pohl E., Heine A., Sheldrick G. M., Dauter Z., Wilson K. S., Kallen J., Huber W., Pfäffli P. J. Structure of octreotide, a somatostatin analogue. Acta Crystallogr D Biol Crystallogr. 1995 Jan 1;51(Pt 1):48–59. doi: 10.1107/S0907444994006104. [DOI] [PubMed] [Google Scholar]
- Quinn T. P., Tweedy N. B., Williams R. W., Richardson J. S., Richardson D. C. Betadoublet: de novo design, synthesis, and characterization of a beta-sandwich protein. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8747–8751. doi: 10.1073/pnas.91.19.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L. L., Johnson P. L. Solid state conformation of the C-terminal tripeptide of oxytocin, L-Pro-L-Leu-Gly-NH2 0.5H2O. J Am Chem Soc. 1973 Oct 31;95(22):7523–7524. doi: 10.1021/ja00803a062. [DOI] [PubMed] [Google Scholar]
- Regan L., DeGrado W. F. Characterization of a helical protein designed from first principles. Science. 1988 Aug 19;241(4868):976–978. doi: 10.1126/science.3043666. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C., Tweedy N. B., Gernert K. M., Quinn T. P., Hecht M. H., Erickson B. W., Yan Y., McClain R. D., Donlan M. E. Looking at proteins: representations, folding, packing, and design. Biophysical Society National Lecture, 1992. Biophys J. 1992 Nov;63(5):1185–1209. [PMC free article] [PubMed] [Google Scholar]
- Rose G. D., Gierasch L. M., Smith J. A. Turns in peptides and proteins. Adv Protein Chem. 1985;37:1–109. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]
- Scholtz J. M., Baldwin R. L. The mechanism of alpha-helix formation by peptides. Annu Rev Biophys Biomol Struct. 1992;21:95–118. doi: 10.1146/annurev.bb.21.060192.000523. [DOI] [PubMed] [Google Scholar]
- Searle M. S., Williams D. H., Packman L. C. A short linear peptide derived from the N-terminal sequence of ubiquitin folds into a water-stable non-native beta-hairpin. Nat Struct Biol. 1995 Nov;2(11):999–1006. doi: 10.1038/nsb1195-999. [DOI] [PubMed] [Google Scholar]
- Sibanda B. L., Blundell T. L., Thornton J. M. Conformation of beta-hairpins in protein structures. A systematic classification with applications to modelling by homology, electron density fitting and protein engineering. J Mol Biol. 1989 Apr 20;206(4):759–777. doi: 10.1016/0022-2836(89)90583-4. [DOI] [PubMed] [Google Scholar]
- Sibanda B. L., Thornton J. M. Beta-hairpin families in globular proteins. Nature. 1985 Jul 11;316(6024):170–174. doi: 10.1038/316170a0. [DOI] [PubMed] [Google Scholar]
- Smith C. K., Withka J. M., Regan L. A thermodynamic scale for the beta-sheet forming tendencies of the amino acids. Biochemistry. 1994 May 10;33(18):5510–5517. doi: 10.1021/bi00184a020. [DOI] [PubMed] [Google Scholar]
- Struthers M. D., Cheng R. P., Imperiali B. Design of a monomeric 23-residue polypeptide with defined tertiary structure. Science. 1996 Jan 19;271(5247):342–345. doi: 10.1126/science.271.5247.342. [DOI] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Analysis and prediction of the different types of beta-turn in proteins. J Mol Biol. 1988 Sep 5;203(1):221–232. doi: 10.1016/0022-2836(88)90103-9. [DOI] [PubMed] [Google Scholar]
- Yan Y., Erickson B. W. Engineering of betabellin 14D: disulfide-induced folding of a beta-sheet protein. Protein Sci. 1994 Jul;3(7):1069–1073. doi: 10.1002/pro.5560030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin A., Forrester J. D., Templeton D. H. Ferrichrome-A tetrahydrate. Determination of crystal and molecular structure. J Am Chem Soc. 1966 Apr 20;88(8):1810–1814. doi: 10.1021/ja00960a040. [DOI] [PubMed] [Google Scholar]
- de Alba E., Blanco F. J., Jiménez M. A., Rico M., Nieto J. L. Interactions responsible for the pH dependence of the beta-hairpin conformational population formed by a designed linear peptide. Eur J Biochem. 1995 Oct 1;233(1):283–292. doi: 10.1111/j.1432-1033.1995.283_1.x. [DOI] [PubMed] [Google Scholar]



