Abstract
Purpose
We conducted a phase III trial in patients with previously untreated metastatic prostate cancer to test the hypothesis that three 8-week cycles of ketoconazole and doxorubicin alternating with vinblastine and estramustine, given in addition to standard androgen deprivation, would delay the appearance of castrate-resistant disease.
Patients and Methods
Eligible patients had metastatic prostate cancer threatening enough to justify sustained androgen ablation and were fit enough for chemotherapy. The primary end point was time to castrate-resistant progression as shown by increasing prostate-specific antigen, new radiographic lesions, worsening cancer-related symptoms, or receipt of any other systemic therapy.
Results
Three hundred six patients were registered; 286 are reported. Median time to progression was 24 months (95% CI, 18 to 39 months) in the standard therapy arm, and 35 months (95% CI, 26 to 44 months) in the chemohormonal group (P = .39). At median follow-up of 6.4 years, overall survival was 5.4 years (95% CI, 4.7 to 7.8 years) in the standard therapy arm versus 6.1 years (95% CI, 5.1 to 10.1 years; P = .41). Prostate-specific antigen kinetics at the time of androgen ablation and the nadir after hormone treatment were strongly correlated with survival. Chemotherapy significantly increased the burden of therapy, with 51% of patients experiencing an adverse event of grade 3 or worse, especially thromboembolic events.
Conclusion
There is no role for ketoconazole and doxorubicin alternating with vinblastine and estramustine before emergence of a castrate-resistant phenotype.
INTRODUCTION
The front-line paradigm for the treatment of metastatic prostate cancer remains, as it has been for more than half a century, to disrupt androgen receptor signaling. Despite reliable initial response, all patients eventually exhibit castrate-resistant progression, a disease state responsible for slightly more than 27,000 deaths per year in the United States.1
Since the early 1970s, scores of phase II studies found a monotonous median survival of approximately 11 months for patients with castrate-resistant prostate cancer.2,3 The University of Texas M.D. Anderson Cancer Center experience with weekly treatment consisting of ketoconazole and doxorubicin alternating with vinblastine and estramustine (KA/VE), given 6 of 8 weeks, suggested an incremental advance. This regimen produced obvious palliation in the majority of treated patients, and a median survival of 18 months, with 10% alive at 3 years.4 Similar reports for taxane-based therapy soon appeared5,6 and were confirmed in a community-based randomized phase II trial.7 More recently, docetaxel has emerged as a useful single agent, and two large phase III trials have now rigorously demonstrated a modest survival advantage for docetaxel-based therapy.8,9
Many investigators have considered early application of cytotoxic therapy in an attempt to forestall progression to overt castrate resistance. In fact, addition of cytotoxics to hormone therapy was first tested more than 30 years ago, and there are at least 10 published randomized trials of this design, involving more than 1,900 patients (Table 1). None of these trials included cytotoxic therapy that prolongs survival in the setting of metastatic castrate-resistant disease, so the availability of regimens in the mid 1990s that seemed to be more active suggested to us that it was prudent to revisit the issue of early intervention with what seemed to be better chemotherapy. In particular, we designed a trial to test the hypothesis that the addition of KA/VE to standard, sustained, androgen ablation would delay the emergence of castrate-resistant disease, and ultimately, prolong survival. Here we report results of this phase III trial in a patient population with nonlocalized prostate cancer felt to be sufficiently threatening to justify sustained androgen ablation. The primary end point was time to castrate-resistant progression; secondary end points were overall and cause-specific survival.
Table 1.
Summary of 10 Published Randomized Trials of Sustained Androgen Ablation With or Without Immediate Chemotherapy
| First Author and Year | Accrual | No. of Patients |
Therapy | Median TTP | Median OS | ||
|---|---|---|---|---|---|---|---|
| Reg | Rep | ||||||
| Murphy,10 1983 | July 1976 to September 1980 | 301 | 246 | A: DES, orch; B: DES, CTX; C: Estramustine, CTX | Not reported | 21 months in all arms | |
| Murphy,11 1986 | July 1980 to June 1983 | 319 | 296 | A: DES or orch; B: CTX/FU/DES;C: Estramustine | 15 months in all arms | 33 months in all arms | |
| Osborne,12 1990 | September 1982 to October 1986 | 143 | 137 | A: DES, orch; B: DES or orch + CTX/dox | A: 15 months; B: 18 months (P = .8) | A: 26 months; B: 22 months (P = .55) | |
| Pummer,13 1997 | April 1988 to January 1991 | 145 | 114 | A: orch/FLT; B: orch/FLT + epirubicin | A: 12 months; B: 22 months (P = .02) | A: 18 months; B: 30 months (P = .12) | |
| Janknegt,14 1997 | January 1989 to July 1990 | 419 | 385 | A: orch; B: orch + estramustine | A: 17 months; B: 24 months (P = .3) | A: 24 months; B: 27 months (NS) | |
| Fontana,15 1998 | June 1990 to May 1993 | 63 | 55 | A: LHRH superagonist; B: LHRH + mitomycin | A: 19 months; B: 25 months (NS) | A: 32 months; B: 29 months (NS) | |
| Boel,16 1999 | June 1988 to December 1991 | 178 | 148 | A: orch; B: orch + mitomycin | A: 29 months; B: 26 months (NS) | A: 31 months; B: 31 months (NS) | |
| de Reijke,17 1999 | February 1990 to May 1995 | 189 | 184 | A: orch; B: orch + mitomycin | A: 12 months; B: 12 months (NS) | A: 26 months; B: 22 months (P = .04) | |
| Kuriyama,18 2001 | April 1990 to December 1992 | 142 | 136 | A: DES or orch; B: DES or orch + UFT | A: 30 months; B: 72 months (P = .06) | A: 5.7 years; B: > 8.2 years (P = .13) | |
| Noguchi,19 2004 | June 1995 to March 1998 | 57 | 51 | A: LHRH superagonist + FLT; B: LHRH + estramustine | A: 14.6 months; B: 25.4 months (P = .03) | A: 30 months; B: 30 months (NS) | |
Abbreviations: Reg, No. of patients registered; Rep, No. of patients reported; orch, bilateral orchiectomy; DES, diethyl stilbestrol; dox, doxorubicin; NS, not significant; LHRH, super agonist of luteinizing hormone–releasing hormone; CTX, cyclophosphamide; FU, fluorouracil; UFT, uracil plus tegafur (an oral fluoropyrimidine); FLT, flutamide.
PATIENTS AND METHODS
Patients
Eligible patients had nonlocalized prostate cancer conventionally treated with sustained androgen ablation and were also fit enough for chemotherapy. If increasing serum prostate-specific antigen (PSA) after definitive local therapy was the only clinical evidence of metastatic disease, then the PSA doubling time (PSADT) had to be less than 9 months. Specific eligibility criteria included histologic proof of acinar adenocarcinoma; PSA more than 1.0 ng/mL; life expectancy from comorbid illness in excess of 3 years; Eastern Cooperative Oncology Group performance status of 2 or better, an absolute neutrophil count of at least 1,500/μL; platelet count of at least 150,000/μL; transaminases less than 4× the upper limit of normal; an estimated creatinine clearance of at least 40 mL/min; and no evidence of active ischemia or bifascicular block by ECG. Patients with a history of vagotomy or who required continuous therapy with antacids, histamine receptor blockers, or proton pump inhibitors were excluded, as were patients taking terfenadine, astemizole, omeprazole, or cisapride. Patients were excluded for any history of transient ischemic attack within the previous 6 months, a requirement for regular antianginal therapy, or any history of deep venous thrombosis or pulmonary embolism. Prior hormone therapy was allowed if it was an adjunct to definitive local therapy, was given for 6 months or less, and was completed at least 12 months before initiating therapy for metastatic disease.
All patients provided written informed consent to participate in this trial, which was approved by the M.D. Anderson Cancer Center institutional review board, and overseen by the institutional Data Monitoring Committee.
Patients with metastatic prostate cancer are typically started on hormonal therapy before being seen at M.D. Anderson. Accordingly, eligible patients could be registered up to 3 months from the date hormonal therapy was initiated, and time-to-event data were calculated from the start of hormonal therapy, not the date of protocol registration.
Treatment
All patients received androgen ablation therapy, either by means of luteinizing hormone–releasing hormone super-agonist (of any formulation) or surgical castration. Use of androgen receptor antagonists was suggested in the protocol but was left to the discretion of the treating physicians. Patients assigned to the experimental arm also received three cycles of KA/VE, repeated every 8 weeks: ketoconazole 400 mg orally (PO) three times a day times 7 days/wk in weeks 1, 3, and 5; doxorubicin 20 mg/m2 administered intravenously on days 1, 15, and 29; vinblastine 4 mg/m2 administered intravenously on days 8, 22, and 35; estramustine 140 mg PO three times a day times 7 days/wk in weeks 2, 4, and 6; and hydrocortisone 20 mg PO every morning and 10 mg PO every evening.
Concurrent use of antiandrogens and ketoconazole was not allowed secondary to concerns about cumulative hepatic toxicity; therefore, patients on the experimental arm did not start antiandrogens until after three cycles of KA/VE.
Clinical End Points
The primary end point was time to castrate-resistant progression, defined by any of the following: symptomatic or radiographic progression, increasing PSA greater than 25% above the nadir on hormonal therapy (or > 1 ng/mL for those with a nadir < 0.5 ng/mL), or receipt of any additional therapy. As usual, these progression-defining events required a castrate testosterone level (< 50 ng/dL) and, if applicable, withdrawal of androgen-receptor blockers.
Descriptive end points included documentation of adverse events (according to the criteria of the National Cancer Institute Common Toxicity Criteria version 2.0) and documentation of evolution to an anaplastic phenotype, which was declared when any of the following clinical features were present: lytic bone metastases, hypercalcemia, biopsy-proven neuroendocrine cytologic features, or symptomatic or radiographic progression occurring without an increase in PSA concentration.
Statistical Considerations
Patients were randomly assigned (1:1) to two arms, with enforced balance with respect to four prospectively defined strata: high-volume bone or visceral disease, low-volume bone (ie, one or two areas of presumably pathologic uptake on bone scan), local/nodal with prior definitive local therapy, and local/nodal without prior definitive therapy. The trial was designed to test the hypothesis that addition of KA/VE to standard androgen ablation would result in an improvement in median time to castrate-resistant phenotype from 24 months (the anticipated result from hormone therapy only) to 36 months. The initial target accrual was 368 patients, providing 80% power to detect this difference with type I error of 5%. Although arbitrary, a 12-month difference was considered to have a reasonable likelihood of being associated with a difference in overall survival and to represent a clinically relevant difference to most clinicians.
Because the trial was designed in the midst of PSA-driven stage migration, we planned (and performed) a single interim analysis for the purpose of reassessing the accrual target in view of the actual performance of the control group. This analysis was performed in 2001, after total accrual of 229 patients. Given the observed median time to progression (at that time) of 21 months in the control arm, the accrual goal was revised downward to 300 patients, which preserved the original target power and type I error.
Fisher's exact test20 was used to assess the independence between two categoric variables. The Wilcoxon rank sum test21 was used to assess whether there is a difference between two groups for continuous variables. For all time-to-event data, survival curves were estimated using the Kaplan-Meier method, and log-rank test was used to assess the difference between groups. Multivariate analysis was performed using the Cox proportional hazards regression model 22 to estimate adjusted hazard ratios on overall survival and progression to castrate-resistant disease. The statistical analyses were carried out using S-Plus 2000 (Insightful Corporation, Seattle, WA) and StatXact 4.0.1 (Cytel Software Corporation, Cambridge, MA). All statistical tests were two-sided.
The analysis was based on intention to treat but does exclude patients immediately withdrawing consent, because there are no data for such patients. Patients who died without evidence of recurrent prostate cancer or were lost to follow-up before evidence of castrate-resistant progression were censored for the analysis of time to progression and cause-specific survival. Patients lost to follow-up after they were confirmed to have progressive, castrate-resistant disease were treated in the most conservative way (ie, they were counted as dead as of the day after their last follow-up).
RESULTS
Between September 1996 and April 2003, 306 patients were registered (Fig 1). Of these, 17 patients immediately withdrew consent (related to dissatisfaction with the arm to which they were randomly assigned). Two patients were discovered to be ineligible before starting therapy and were excluded, and one international patient was immediately lost to follow-up. This leaves 286 patients (93% of those registered) in the analysis. Baseline characteristics are listed in Table 2. There were no statistically significant imbalances for any of the baseline covariates considered.
Fig 1.
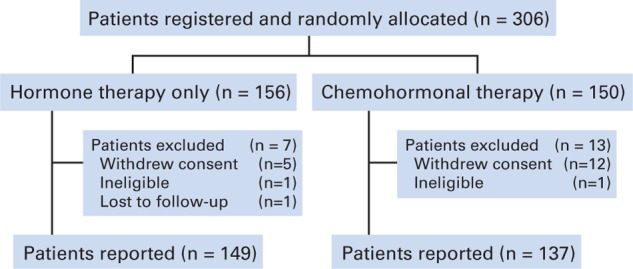
Enrollment and reporting.
Table 2.
Baseline Characteristics of Patients Included in Analysis
| Feature | Hormone Rx Group | Chemohormonal Rx Group | Total | % |
|---|---|---|---|---|
| No. randomly assigned | 156 | 150 | 306 | |
| No. in analysis | 149 | 137 | 286 | |
| Age at diagnosis, years | ||||
| Median | 59 | 58 | ||
| Range | 42-80 | 37-78 | ||
| Time from diagnosis to hormonal therapy, months | ||||
| Median | 20 | 28 | ||
| Range | 0.3-104 | 0.1-109 | ||
| Ethnicity | ||||
| Hispanic | 15 | 8 | 23 | 8 |
| Non-Hispanic | 134 | 129 | 263 | 92 |
| Race | ||||
| White | 139 | 131 | 270 | 94 |
| African American | 8 | 6 | 14 | 5 |
| Asian | 2 | 0 | 2 | 1 |
| Prior local therapy | ||||
| Radical prostatectomy | 45 | 35 | 80 | 28 |
| Aborted prostatectomy | 6 | 5 | 11 | 4 |
| External-beam radiotherapy | 10 | 6 | 16 | 6 |
| Brachytherapy | 1 | 4 | 5 | 2 |
| Cryotherapy | 1 | 0 | 1 | 0 |
| None | 86 | 87 | 173 | 60 |
| PSA at diagnosis, ng/mL | ||||
| < 10 | 36 | 34 | 70 | 24 |
| 10-20 | 27 | 26 | 53 | 19 |
| > 20-100 | 48 | 42 | 90 | 31 |
| > 100 | 37 | 33 | 70 | 24 |
| Unknown | 1 | 2 | 3 | 1 |
| PSADT at hormone therapy, months | ||||
| Undefined | 41 | 45 | 86 | 30 |
| < 3 | 40 | 44 | 84 | 31 |
| 3 to 9 | 44 | 32 | 76 | 26 |
| > 9 | 24 | 16 | 40 | 14 |
| Stratification | ||||
| Local, with prior definitive therapy | 29 | 22 | 51 | 18 |
| Local, without definitive therapy | 25 | 28 | 53 | 19 |
| Low-volume bone | 30 | 26 | 56 | 20 |
| High-volume bone/visceral | 65 | 61 | 126 | 44 |
Abbreviations: Rx, therapy; PSA, prostate-specific antigen; PSADT, PSA doubling time.
Among 137 patients assigned to the experimental arm, 110 patients (80%) completed three cycles of KA/VE as planned, 20 patients (15%) completed two cycles, and seven patients (5%) received one cycle or fewer of treatment. Fatigue and thromboembolic events were the most common reasons for discontinuation of chemotherapy. Despite our intent for sustained androgen deprivation, 28 patients (19%) on the standard therapy arm and 67 patients (49%) on the experimental arm are known to have used intermittent androgen ablation, and it is quite likely that some patients have taken this course of action unknown to us.
Adverse events observed more than once, and of grade 3 or 4, are summarized in Table 3. There were no treatment-related deaths. Overall, 148 events of grade 3 or worse were recorded in 83 patients (29%). The toxicity observed with KA/VE was substantial: 70 patients (51%) exposed to KA/VE had at least one grade 3 event, and most were attributed to treatment. By contrast, only 13 patients (9%) in the control arm had an adverse event of grade 3 or worse, and most were considered unrelated to treatment. As anticipated, the most frequently observed adverse events were thromboembolic, with a total of 23 such events of grade 3 or worse in the KA/VE arm versus only one event in the control arm.
Table 3.
Summary of Observed Adverse Events
| Event | Androgen Ablation
Only |
Chemohormonal
Rx |
||||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |||
| Abdominal pain | 1 | 0 | 3 | 2 | ||
| Bone pain | 0 | 1 | 8 | 1 | ||
| Cardiac ischemia/infarction | 0 | 2 | 0 | 1 | ||
| Catheter-related infection | 0 | 0 | 3 | 0 | ||
| Catheter-related thrombosis | 0 | 0 | 8 | 1 | ||
| Chest pain | 0 | 0 | 1 | 1 | ||
| CNS ischemia | 0 | 1 | 1 | 0 | ||
| Depression | 0 | 0 | 2 | 1 | ||
| Fatigue | 0 | 0 | 6 | 0 | ||
| Infection | 0 | 0 | 15 | 0 | ||
| Left ventricular function | 0 | 0 | 2 | 0 | ||
| Neuropathy | 1 | 0 | 5 | 0 | ||
| Neutropenia | 0 | 0 | 6 | 7 | ||
| Neutropenic fever | 0 | 0 | 2 | 0 | ||
| Odynophagia | 0 | 0 | 2 | 0 | ||
| Pneumonitis | 1 | 0 | 1 | 0 | ||
| Sinus bradycardia | 0 | 0 | 2 | 0 | ||
| Stomatitis | 0 | 0 | 4 | 0 | ||
| Supraventricular tachycardia | 0 | 0 | 1 | 2 | ||
| Syncope | 1 | 0 | 1 | 0 | ||
| Thrombosis/embolism | 1 | 0 | 13 | 1 | ||
| Ureteral obstruction | 0 | 1 | 0 | 1 | ||
| Totals | 5 | 5 | 86 | 18 | ||
NOTE. Only events observed in more than one patient with maximum grade of 3 or 4 are shown. There were no toxic deaths.
Abbreviation: Rx, therapy.
The primary end point of the trial was time to castrate-resistant progression. As of December 2006, 194 patients (68%) had reached this end point, and another 12 patients (4%) had died without evidence of active prostate cancer. Eighty patients (28% of the total) remain at risk for progression. Progression was declared by PSA criteria in 122 patients (63%), radiographic criteria in 56 patients (29%), initiation of therapy in 10 patients (5%), and progressive symptoms in six patients (3%). Median time to progression was 24 months (95% CI, 18 to 39 months) for the androgen ablation arm, and 35 months (95% CI, 26 to 44 months) for those receiving chemohormonal therapy (P = .39; Fig 2A). Note that the curves happen to artifactually diverge at the median; the proportional hazards assumption is not valid for the observed curves.
Fig 2.
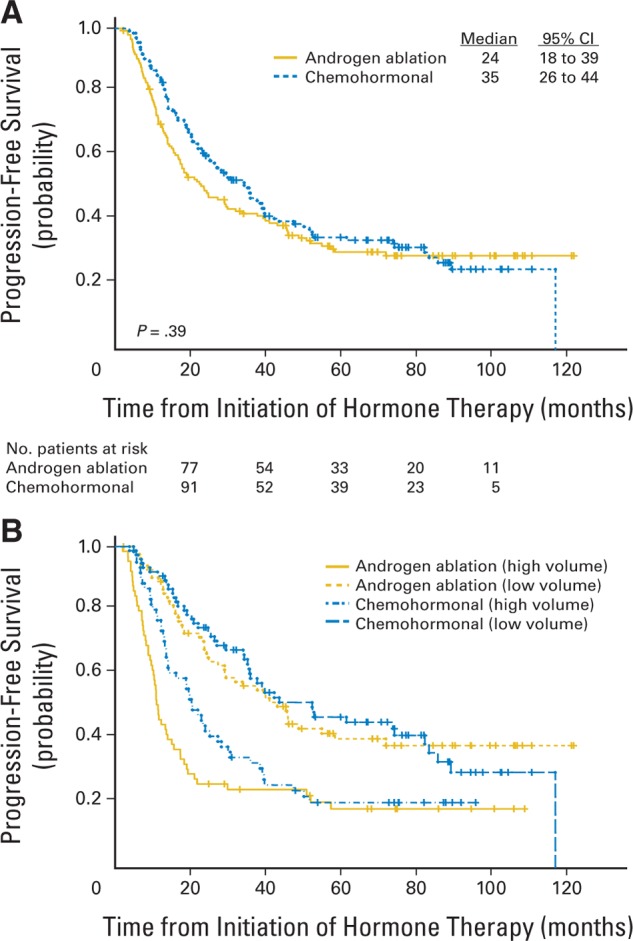
Time to progression (TTP) as defined by appearance of castrate-resistant phenotype. See text for definition of progression and details of stratification. (A) TTP by assigned treatment. (B) TTP by treatment, stratified by disease volume at entry.
As expected, extent of disease at the time hormone therapy was initiated was strongly related to outcome. Among all patients, the median time to progression was 58.3 months (95% CI, 38 to 120 months) for those with local/regional disease and prior definitive local therapy, 42.9 months (95% CI, 29 to 89 months) for those with local/regional disease without prior definitive local therapy, 39.4 months (95% CI, 26 to 120 months) for patients with one or two lesions by bone scan, and 14.2 months (95% CI, 12 to 20 months) for patients with three or more bone lesions or involvement of viscera. It is convenient to combine the first three groups as the low-volume subset (n = 160) and consider the last group (n = 126) as the high-volume subset. In the low-volume subset, the median time to castrate-resistant progression was 45.4 months (95% CI, 29 to 120 months) for the control arm and 52.4 months (95% CI, 36 to 86 months) for patients assigned to the chemohormonal arm (P = .94; Fig 2B). In the high-volume subset, the median time to progression was 11.2 months (95% CI, 10 to 17 months) in the control group and 20.5 months (95% CI, 14 to 30 months) in the experimental arm (P = .08; Fig 2B).
We found no evidence that early exposure to chemotherapy contributes to the evolution of more aggressive cancers, as an anaplastic phenotype developed in five patients in each arm.
Overall, 156 patients (55%) have died, including 142 deaths resulting from prostate cancer and 14 deaths from unrelated causes. Median follow-up for survival was 6.4 years. Median overall survival for patients in the control arm was 5.4 years (95% CI, 4.7 to 7.8 years), versus 6.1 years (95% CI, 5.1 to 10.1 years) for patients receiving KA/VE (P = .41; Fig 3A). In the low-volume subset, the median survival in the hormone therapy arm was 7.8 years (95% CI, 5.5 to 10.1 years) versus 7.8 years (95% CI, 6.9 to 10.1 years) among patients treated initially with KA/VE (P = .68; Fig 3B). In the high-volume subset, the median survival in the hormone therapy arm was 3.1 years (95% CI, 2.6 to 5.5 years), versus 4.4 years (95% CI, 3.2 to 6.1 years) among patients treated initially with KA/VE (P = .29; Fig 3B).
Fig 3.
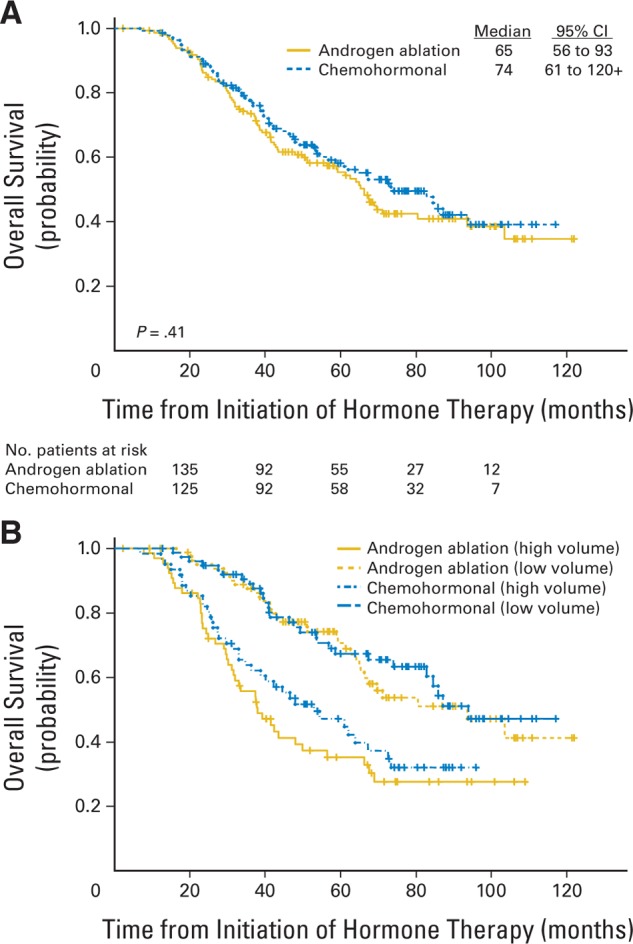
Overall survival. See text for details of stratification. (A) Survival by assigned treatment. (B) Survival by treatment, stratified by disease volume at entry.
Survival from the date of progression was virtually identical in the two arms, with a median value of 26 months. Nearly all patients (in either arm) did receive cytotoxic therapy for castrate-resistant disease, usually including a taxane.
A standard Cox model including indicators for treatment arm, prior definitive local therapy, age, disease volume stratum, nadir PSA,23,24 PSA at entry, and PSADT24 before initiation of hormone therapy was fitted to both the time-to-progression and survival data. All of these covariates remained significant in the multivariate model, except age and treatment arm. The most powerful predictor for both outcomes was achievement of PSA less than 0.2 ng/mL at 8 months from initiation of hormone therapy (Fig 4).
Fig 4.
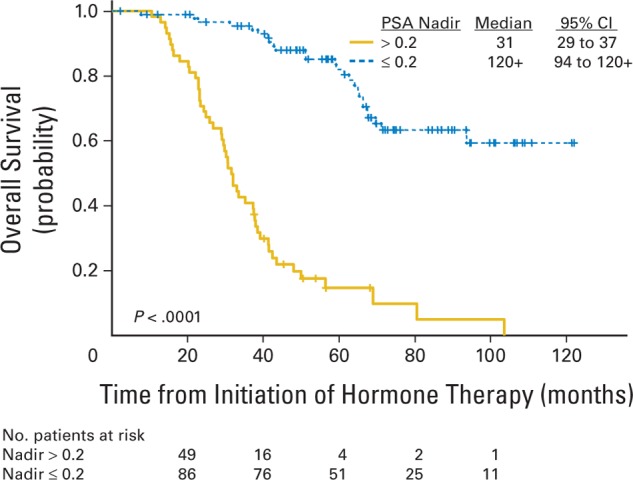
Overall survival among all patients by prostate-specific antigen (PSA) nadir after androgen ablation.
We had sufficient PSA data before the initiation of androgen ablation to calculate PSADT for 200 patients (70%). Patients with a PSADT less than 3 months (n = 84) had median time to progression of 16 months (95% CI, 14 to 25 months), compared with 46 months (95% CI, 36 to 74 months) for 116 patients with PSADT of 3 months or longer (P < .0001). The corresponding values for cause-specific survival were 3.5 years (95% CI, 3.2 to 5.5 years) in the short PSADT group versus 7.8 years (95% CI, 6.2 to 10.1 years) for those with a long PSADT (P < .0001). Among these 200 patients, there was no hint of any difference in outcome according to assigned treatment after accounting for PSADT at baseline.
DISCUSSION
At first blush, our primary result of a difference in median time to progression of 11 months may seem provocative, despite the fact that the P value for this result is far from significance. There are three concerns: the trial could simply be underpowered for a difference of this magnitude; baseline covariates could be nonrandomly distributed; or the median values could be poor surrogates for the information in the survival curve. Taking the last point first, we know that the proportional hazards assumption is not met, and it is clear from inspection that the separation at the median is not representative of the overall difference in the curves. Furthermore, any hint of difference disappears with covariate adjustment in the Cox model. Finally, even though this was a small trial, we did have reasonable power to find a difference of this magnitude. Under the usual assumptions, the power to detect the difference we observed was approximately 78%, and, in the high-volume subset (n = 126), we have 82% power to detect the observed difference of median time to progression (11.2 v 20.5 months) at a significance level of .05. Thus it is clear that there is no hint of improved outcome with KA/VE. In fact, in retrospective, exploratory analyses, we examined subsets with short PSADT, young age, extensive disease, and a short interval from diagnosis to hormonal therapy and found no subset where early application of KA/VE improves outcome.
Importantly, our results provide documentation of outcome in the modern era. For example, patients with only regional disease after prior local therapy had an estimated cause-specific survival of 70% at 6 years. Even among patients with small-volume bone involvement (ie, one or two lesions on bone scan), only 33 (59%) of 56 patients have experienced disease progression, and just 25 patients (45%) have died of prostate cancer. The estimated median cause-specific survival of this cohort exceeds 7 years.
Our results also confirm the prognostic implications of PSADT when hormonal therapy is initiated24 and the prognostic importance of a clinical response to androgen ablation.23,24 Not only did PSA nadir after hormone therapy correlate with subsequent time to progression, but the duration of hormonal response was the most powerful predictor of survival after progression to a castrate-resistant phenotype, as we have previously reported.7
Our results were mildly surprising, given the fact that the response rate and modest survival prolongation seen with KA/VE applied to patients with far advanced disease are similar to treatment effects seen in patients with metastatic colon or breast cancer. Importantly, however, in those diseases, the benefit of early (ie, adjuvant) therapy is well established, but a corresponding effect in prostate cancer has not been observed. This suggests to us that sensitivity to chemotherapy is a function of how far the cancer has evolved toward castrate resistance. In keeping with this notion, the time to progression observed in this trial was significantly delayed in the high-volume group but not in those with less advanced disease. The hypothesis that hormone-dependent prostate cancers are resistant to chemotherapy also arises from recent results with preoperative chemotherapy, in which no pathologic complete responses were seen, and indeed, little impact on the primary tumor was appreciated with either KA/VE25 or docetaxel.26
In summary, these data are consistent with at least 10 previous randomized trials of chemohormonal therapy in providing no support for adding cytotoxics to androgen ablation for androgen-driven prostate cancer. For the present, it is remarkable that more than half a century after its introduction, androgen suppression remains the preferred front-line approach to the treatment of metastatic prostate cancer, and that so far, there is no cytotoxic regimen with clinically relevant activity against hormone-sensitive prostate cancer.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Randall E. Millikan, Lance C. Pagliaro, Christopher J. Logothetis
Financial support: Christopher J. Logothetis
Administrative support: Randall E. Millikan, Christopher J. Logothetis
Provision of study materials or patients: Randall E. Millikan, Lance C. Pagliaro, Christopher J. Logothetis
Collection and assembly of data: Randall E. Millikan, Melissa A. Brown, Brenda Moomey
Data analysis and interpretation: Randall E. Millikan, Sijin Wen, Kim-Anh Do
Manuscript writing: Randall E. Millikan, Christopher J. Logothetis
Final approval of manuscript: Randall E. Millikan, Sijin Wen, Lance C. Pagliaro, Melissa A. Brown, Brenda Moomey, Kim-Anh Do, Christopher J. Logothetis
Footnotes
published online ahead of print at www.jco.org on November 24, 2008.
Presented in part at the Annual Meeting of the American Urologic Association May 8-13, 2004, San Francisco, CA.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002855.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al: Cancer Statistics, 2007. CA Cancer J Clin 57:43-66, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Yagoda A, Petrylak D: Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer 71:1098-1109, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Beer T, Raghavan D: Chemotherapy for hormone-refractory prostate cancer: Beauty is in the eye of the beholder. Prostate 45:184-193, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Ellerhorst JA, Tu SM, Amato RJ, et al: Phase II trial of alternating weekly chemohormonal therapy for patients with androgen-independent prostate cancer. Clin Cancer Res 3:2371-2376, 1997 [PubMed] [Google Scholar]
- 5.Smith DC, Esper P, Strawderman M, et al: Phase II trial of oral estramustine, oral etoposide, and intravenous paclitaxel in hormone-refractory prostate cancer. J Clin Oncol 17:1664-1671, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Kelly WK, Curley T, Slovin S, et al: Paclitaxel, estramustine phosphate, and carboplatin in patients with advanced prostate cancer. J Clin Oncol 19:44-53, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Millikan RE, Thall PF, Lee SJ, et al: Randomized multicenter phase II trial of two multicomponent regimens in androgen independent prostate cancer. J Clin Oncol 21:878-883, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Petrylak DP, Tangen CM, Hussain MH, et al: Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513-1520, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Tannock IF, de Wit R, Berry WR, et al: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502-1512, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Murphy GP, Beckley S, Brady MF, et al: Treatment of newly diagnosed metastatic prostate cancer patients with chemotherapy agents in combination with hormones versus hormones alone. Cancer 51:1264-1272, 1983 [DOI] [PubMed] [Google Scholar]
- 11.Murphy GP, Huben RP, Priore R: Results of another trial of chemotherapy with and without hormones in patients with newly diagnosed metastatic prostate cancer. Urology 28:36-40, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Osborne CK, Blumenstein B, Crawford ED, et al: Combined versus sequential chemo-endocrine therapy in advanced prostate cancer: Final results of a randomized Southwest Oncology Group study. J Clin Oncol 8:1675-1682, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Pummer K, Lehnert M, Stettner H, et al: Randomized comparison of total androgen blockade alone versus combined with weekly epirubicin in advanced prostate cancer. Eur Urol 32:81-85, 1997. (suppl) [PubMed] [Google Scholar]
- 14.Janknegt RA, Boon TA, van de Beek C, et al: Combined hormono/chemotherapy as primary treatment for metastatic prostate cancer: A randomized, multicenter study of orchiectomy alone versus orchiectomy plus estramustine phosphate. Urology 49:411-420, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Fontana D, Bertetto O, Fasolis G, et al: Randomized comparison of goserelin acetate versus mitomycin C plus goserelin acetate in previously untreated prostate cancer patients with bone metastases. Tumori 84:39-44, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Boel K, Van Poppel H, Goethuys H, et al: Mitomycin C for metastatic prostate cancer: Final analysis of a randomized trial. Anticancer Res 19:2157-2161, 1999 [PubMed] [Google Scholar]
- 17.de Reijke TM, Keuppens FI, Whelan P, et al: Orchiectomy and orchiectomy plus mitomycin C for metastatic prostate cancer in patients with poor prognosis: The final results of a European organization for research in cancer therapy genitourinary group trial. J Urol 162:1658-1664, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kuriyama M, Takanhashi Y, Sahashi M, et al: Prospective and randomized comparison of combined androgen blockade versus combination with oral UFT as an initial treatment for prostate cancer. Jpn J Clin Oncol 31:18-24, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Noguchi M, Noda Shinshi, Yoshida M, et al: Chemohormonal therapy as primary treatment for metastatic prostate cancer: A randomized study of estramustine phosphate plus luteinizing hormone-releasing hormone agonist versus flutamide plus luteinizing hormone-releasing hormone agonist. Int J Urol 11:103-109, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Fisher LD, Belle GV: Biostatistics: A Methodology For the Health Sciences. New York, NY, John Wiley, 1993
- 21.Randles RH, Wolfe DA: Introduction to the Theory of Nonparametric Statistics. New York, NY, John Wiley, 1979
- 22.Lawless JF: Statistical Models and Methods for Lifetime Data. New York, NY, John Wiley, 1982
- 23.Hussain M, Tangen CM, Higano C, et al: Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from the Southwest Oncology Group trial 9346 (INT-0162). J Clin Oncol 24:3984-3990, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Stewart AJ, Scher HI, Chen M-H, et al: Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J Clin Oncol 23:6556-6560, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Pettaway CA, Pisters LL, Troncoso P, et al: Neoadjuvant chemotherapy and hormonal therapy followed by radical prostatectomy: Feasibility and preliminary results. J Clin Oncol 18:1050-1057, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Friedman J, Dunn RL, Wood D, et al: Neoadjuvant docetaxel and capecitabine in patients with high risk prostate cancer. J Urol 179:911-916, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]


