Abstract
This article reviews cervical laminoplasty. The origin of cervical laminoplasty dates back to cervical laminectomy performed in Japan ~50 years ago. To overcome poor surgical outcomes of cervical laminectomy, many Japanese orthopedic spine surgeons devoted their lives to developing better posterior decompression procedures for the cervical spine. Thanks to the development of a high-speed surgical burr, posterior decompression procedures for the cervical spine showed vast improvement from the 1970s to the 1980s, and the original form of cervical laminoplasty was determined. Since around 2000, surgeons performing cervical laminoplasty have been adopting less invasive procedures for the posterior cervical muscle structures so as to minimize postoperative axial neck pain and obtain better functional outcomes of the cervical spine. This article covers the history of cervical laminoplasty, surgical procedures, the benefits and limitation of this procedure, and surgery-related complications.
Keywords: cervical spine, surgical treatment, laminoplasty, spinal canal stenosis, ossification of posterior longitudinal ligament, axial pain, cervical kyphosis
History of Laminoplasty
Before the development of laminoplasty, laminectomy had been extensively used for treatment of cervical myelopathy caused by multisegmental spondylosis, ossification of the posterior longitudinal ligament (OPLL), and developmental spinal canal stenosis. The clinical results of laminectomy for the cervical spine were not satisfactory because of intraoperative spinal cord injury, postoperative progression of cervical kyphosis, and worsening of neurological functions in relation to the formation of scar tissue, so-called “laminectomy membrane,” over the dural sac. Intraoperative neurological complications could be attributed to the insertion of Kerrison rongeurs or curettes in the severely narrowed spinal canal without special attention. Progression of local kyphosis was a major complication following cervical laminectomy, especially in patients younger than 50 years of age. To improve surgical outcomes of cervical laminectomy, Japanese orthopedic spine surgeons had been trying to refine or improve surgical procedures of laminectomy.
One of the improvements was brought about in 1968 by Miyazaki and Kirita, who developed extensive multisegmental laminectomy for cervical OPLL by resecting the edges of remaining laminae that hindered the posterior shift of the spinal cord after decompression procedures. Miyazaki and Kirita reported the technique in 1986,1 almost 20 years after the first clinical application of this technique.
Another attempt was made by Oyama and Hattori,2 who developed the first expansive laminoplasty called an “expansive Z-shaped laminoplasty.” Their surgical technique consisted of preserving the posterior spinal canal with Z-shaped plasty of the thinned laminae. The purpose of restoring the spinal canal with the thinned laminae was to prevent the scar formation over the dural sac and to minimize the destruction of spinal stability. Their technique was published in 1973. The advent of a high-speed automated surgical burr played a major role in establishing this procedure.
In 1981, Hirabayashi et al3 introduced a unilateral open-door laminoplasty in the journal Spine. The benefits of this procedure were twofold: (1) to allow simultaneous decompression for multiple segments, and (2) to preserve the posterior muscle structures that would prevent postoperative progression of cervical kyphosis and segmental instability.4 Building on this procedure, many modified procedures of cervical laminoplasty have been developed and reported in Japan due to urgent clinical needs to treat cervical myelopathy caused by spondylosis and OPLL.
Aims of Laminoplasty
Cervical laminoplasty had been developed in Japan for many years to overcome the drawbacks of laminectomy. There were several hopes for cervical laminoplasty: (1) to achieve adequate multilevel spinal cord decompression with expansion of the spinal canal, (2) to prevent formation of postoperative severe scar formation over the dural sac, (3) to avoid destabilization of the posterior structures of the spine, and (4) to preserve physiological mobility of the cervical spine.
Other requirements for cervical laminoplasty are: (1) there is less need of structural bone graft after decompression procedures, (2) it is easy to add decompression procedures for nerve roots, and (3) simultaneous posterior instrumentation can be added immediately after multilevel decompression.
Indications and Contraindications for Laminoplasty
The best indication of cervical laminoplasty is cervical myelopathy due to developmental canal stenosis, spondylotic changes at more than three segments, and continuous or mixed type of OPLL compressing the spinal cord at multiple levels. The definite spinal canal stenosis at the cervical spine is defined as the anteroposterior spinal canal diameter of 12 mm or less and that of relative spinal canal stenosis, 12 to 14 mm. There are reports showing that the thickness of OPLL will increase after posterior decompression procedures especially in younger patients.5 6 If patients have radiculopathy in combination with myelopathy, posterior foraminotomy can be performed at the same time with laminoplasty to decompress both the dural sac and the nerve roots. To maintain the stability of the cervical spine, the amount of resection of the facet joint should be limited to less than 25%.7 8
Laminoplasty is not indicated for patients who have cervical kyphotic deformity, because the spinal cord is unable to expand posteriorly without the cervical lordosis.9 Suda et al10 reported that if the patients have local kyphosis in the cervical spine more than 13 degrees, the recovery of neural functions is limited after laminoplasty. A beak-shaped OPLL with a huge bony prominence also should not be treated by laminoplasty because neural decompression would not be enough even after posterior shift of the spinal cord could be achieved to some extent.
The age of the patient does not affect the indication of laminoplasty. Any type of laminoplasty for four to five spinal levels can be performed by experienced spine surgeons within 2 hours with blood loss less than 200 mL. However, patients treated with anticoagulants before surgery are at high risk of postoperative hematoma, which significantly deteriorates neural functions if it occurs. Special attention to postoperative hematoma should be paid after laminoplasty with patients who have been receiving anticoagulants for their comorbid medical problems.
Techniques of Laminoplasty
Unilateral Open-Door Laminoplasty
Hirabayashi's Method
The patient is placed in the prone position with the head slightly raised (Fig. 1A, B). The spinous processes are exposed with special care not to cause any damage to the supraspinous and interspinous ligaments. A gutter is created at the junction between the articular process and the laminae with an automated steel burr followed by a diamond burr. The gutter should be made just medial to the pedicles without any harm to the facet joints. The inner cortex of the laminae should be thinned by automated high-speed burrs. The range of laminoplasty is one above and one below the stenotic segments, considering the posterior shift of the dural sac after posterior decompression. After making deep gutters of ~2-mm width down to thin the inner cortex of the laminae, the gutter on the dominant side of the symptoms should be cut completely with a thin Kerrison rongeur. After cutting one side of the laminae completely, the spinous processes and the laminae can be pushed laterally with a hinge of the gutter on the opposite side so that the spinal canal will be enlarged by opening the posterior bony elements of the spine. If there is any fibrous adhesion between the dural sac and the ventral surface of the laminae, the fibrous tissues should be released with small dissectors. The pulsation of the dural sac can then be observed. The laminae should be kept open with three to four suture threads on the facet capsules on the hinge side.3 11 12
Figure 1.
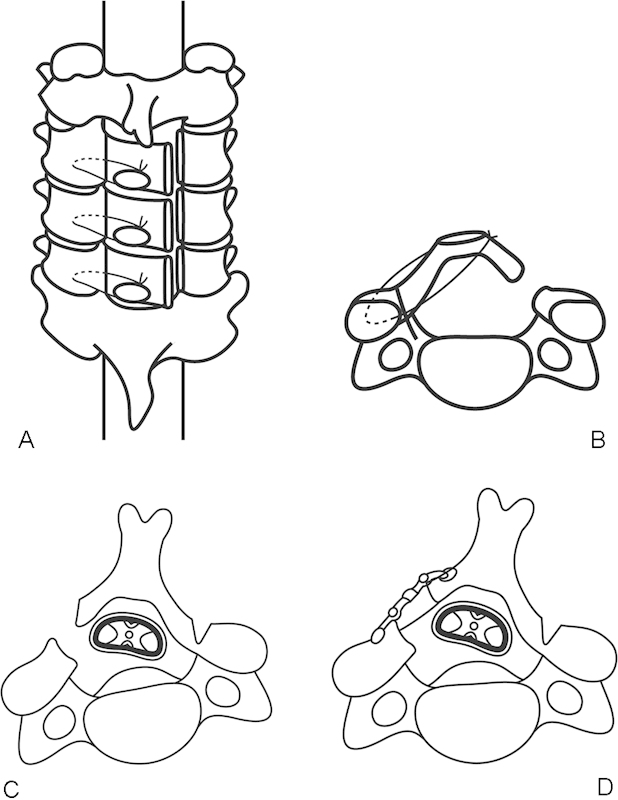
(A) The top view of unilateral open-door laminoplasty (Hirabayashi's method). Three laminae are lifted bilaterally. (B) The axial view of unilateral open-door laminoplasty. The lamina is kept open with a wire. (C) The axial view of en -bloc laminoplasty (Ito and Tsuji's method). (D) A graft bone and a miniplate are placed at the gap to maintain the canal patency.
En Bloc Laminoplasty (Itoh and Tsuji's Method)
After the posterior elements are exposed, each spinous process is removed in en bloc from the junction between the lamina and the spinous process (Fig. 1C, D). These resected spinous processes can be used for bone grafts at the gaps between the opened laminae and the opposite facet joint to maintain the enlarged spinal canal. Tunnels through the laminae and at the facet joints are made either with an awl or a bone perforator for wires, which will maintain the positions of the lifted laminae. A braided stainless steel wire (diameter 0.32 mm) is commonly used for this purpose. The wire should be placed within the opened laminae first and then through the grafted bone and around the facet joints. The graft bone is thereby firmly placed within the gap between the opened laminae and facet joint. If foraminotomy is required on one side, the laminae on the side of foraminotomy should be opened and additional decompression for the foramen can be added after lifting the laminae.13 There have been several materials used for spacers between the opened laminae, such as ceramic blocks, hydroxyapatite blocks, and allografts.14 15 16
Middle Line or French-Door Laminoplasty
Spinous Process Splitting (Kurokawa's Method)
After exposure from the spinous process to the medial border of the facet joints, the midline of the spinous process is drilled down with a high-speed automated burr after resecting the interspinous ligament (Fig. 2A, B). For this purpose, a small tip of burr 1.5 to 2.0 mm in diameter is used. When getting close to the junction between the spinous process and the lamina, the lamina should be thinned around the midline and then the inner cortex cut with a diamond burr. Two gutters on both sides of the laminae are made with a steel cutting burr just medial to the facet joint. At both lateral gutters, the inner cortex of the laminae should be thinned but not cut, thereby allowing both sides of the laminae to open at the same time with hinges at both lateral gutters. After completing the enlargement of the spinal canal, the pulsation of the dural sac can be observed.
Figure 2.
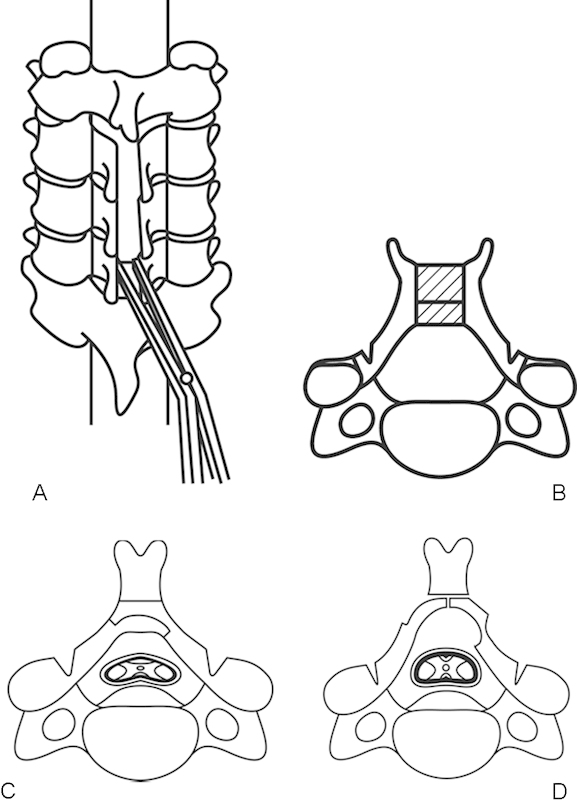
(A) Bilateral open-door laminoplasty. The top view of Kurokawa's method. The spinous processes and laminae are split at the midline and opened. (B) A block of bone graft is placed between the split spinous process. (C) The axial view of Tomita's method. The lamina can be cut as desired by a T-saw. (D) After cutting the lamina, the spinal canal is enlarged by opening the lamina bilaterally.
The tip of the spinous process can be used for a spacer between the opened lamina to keep the laminae open. The spinous process should be trimmed to a suitable shape at the gap between the spinous processes. Enlargement of the spinal canal depends on the size of the spacer between the opened spinous processes. Iliac bone grafts or hydroxyapatite spacers with appropriate sizes and shapes can be placed between the spinous processes to maintain the patency of the spinal canal. Bone grafts or synthetic spacers are firmly placed by wires. Another technique to keep the laminae open is using small metal plates between the gaps.17
Opening Arch Laminoplasty (Tomita's Method)
Tomita and his coworkers developed an opening arch laminoplasty by cutting the laminae using a threaded wire saw (T-saw, or Tomita saw; Fig. 2C, D). This wire saw can be used just like a Gigli saw to cut the bone without any difficulty. After cutting the spinous processes at their base, the posterior outer surface of the laminae is exposed. The T-saw is placed under each lamina and the lamina is sawed at the center over the dural sac by the wire. The lamina can be cut wherever the surgeon desires so that the final shape of the enlarged lamina can be more anatomic than those created by other techniques. Two or three laminae can be cut at the same time if the T-saw can be placed under multiple laminae. Special care must be taken when the wire is passed through the spinal canal with severe stenosis. Especially in patients with large cervical OPLL, when passing the T-saw under the laminae into the spinal canal, there are potential risks to the spinal cord, which has been already damaged by the presence of OPLL.18 19
Comparisons between Posterior Decompression Procedures for Multilevel Cervical Myelopathy
There were several reports comparing the clinical results of different poster decompression procedures for multilevel cervical myelopathy. Tsuzuki et al20 compared two laminoplasty procedures, such as en bloc laminoplasty and spinous process-splitting laminoplasty, in patients with cervical spondylotic myelopathy and cervical OPLL and concluded that there was no statistical difference between the two procedures in the degree of improvement of long tract signs. Recovery rate of the en bloc laminoplasty was 51.2% and that of the spinous process-splitting laminoplasty was 49.0%. The reported recovery rates of various types of cervical laminoplasty ranged from 20 to 80% with an average of 55%.21 There was no difference in neurological improvement based on different laminoplasty techniques or when laminoplasty was compared with laminectomy.21 Approximately 35% of patients with laminoplasty showed postoperative worsening of cervical alignment, and 10% of them developed cervical kyphosis in long-term follow-up studies.21 22 Cervical range of motion showed substantial decrease after laminoplasty ranging from 17 to 80%. One of the reasons for the decrease in cervical range of motion is spontaneous facet joint fusion, which occurred at least 10 years after Kurokawa-type laminoplasty.23 Another study compared laminoplasty with laminectomy and fusion in patients with multiple cervical myelopathy. The results indicated that laminoplasty provided better functional outcomes and fewer complications than laminectomy with fusion did.24
Minimally Invasive Approach for Laminoplasty
Cervical laminoplasty is effective in multilevel spinal decompression for patients with spinal canal stenosis causing myelopathy in the cervical spine (Fig. 3). Most of the previous procedures detached muscles from the posterior elements of the spine, which is considered to have negative effects on neck pain, loss of cervical motion, and progression of cervical kyphosis after surgery.1 25 26 There had been considerable need for preservation of posterior muscle structures, which would be able to lessen postsurgical persisting axial neck pain and maintain physiological cervical lordosis.27 To overcome these problems of expansive laminoplasty, Shiraishi and coworkers28 29 30 31 introduced a new surgical technique called “skip laminectomy” or a technique for muscle-preserving double-door laminoplasty (TEMPL).
Figure 3.
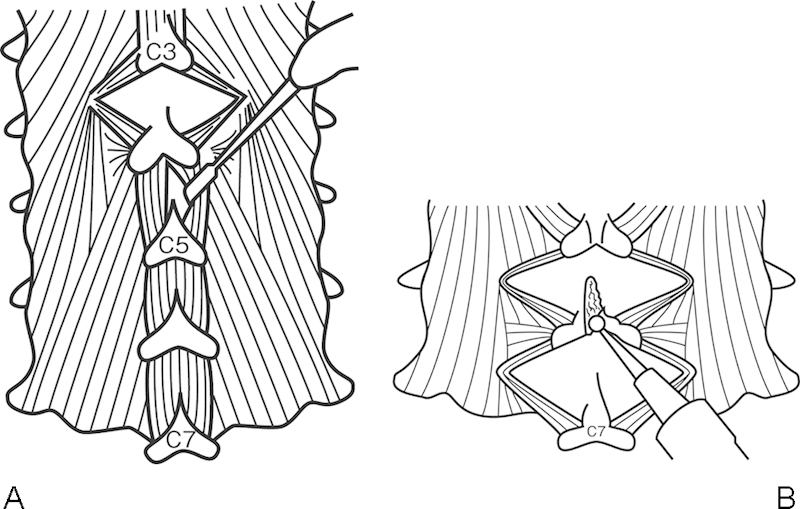
(A) Muscle-preservation approach for cervical laminoplasty (Shiraishi's method). Divide the interspinalis muscles by a pair of nerve retractors. (B) Split the spinous processes with a high-speed burr with a small tip.
This surgical technique requires minimum resection of the muscle attachments to the cervical spine. During posterior exposure of the muscles, the interspinalis muscles should be divided bluntly at the middle line with two nerve root retractors. Then minimal dissection of the multifidus muscle is done by an electric cautery. Because the insertion of the multifidus muscle is not so wide and tight, it is easy to expose entire posterior aspects of laminae with only gentle retraction of these muscles. After completion of gentle retraction of the interspinalis and multifidus muscles, the spinous processes should be cut at the midline with a small cutting burr to the base of the spinous processes and then divided to the sides.29 After complete exposure of the posterior surface of the laminae, bilateral open-door laminoplasty should be performed. After opening the laminae on the both sides, divided spinous processes are sutured. Among the decompression levels, some of the spinous processes need not be opened, especially for patients with mild spinal canal stenosis. Shiraishi and colleagues named this procedure “skip laminoplasty.”28 29 30 The decision how to select the skip levels calls for clinical experience. In patients with severe canal compromise such as OPLL, developmental canal stenosis, and hemodialysis-related canal stenosis, it is safer not to perform skip laminoplasty because the skipped lamina may hinder the posterior shift of the dural sac after surgery. However, a muscle-preserving technique for posterior exposure is applicable and beneficial for any patient with cervical myelopathy requiring posterior multilevel decompression procedures (Fig. 4).
Figure 4.
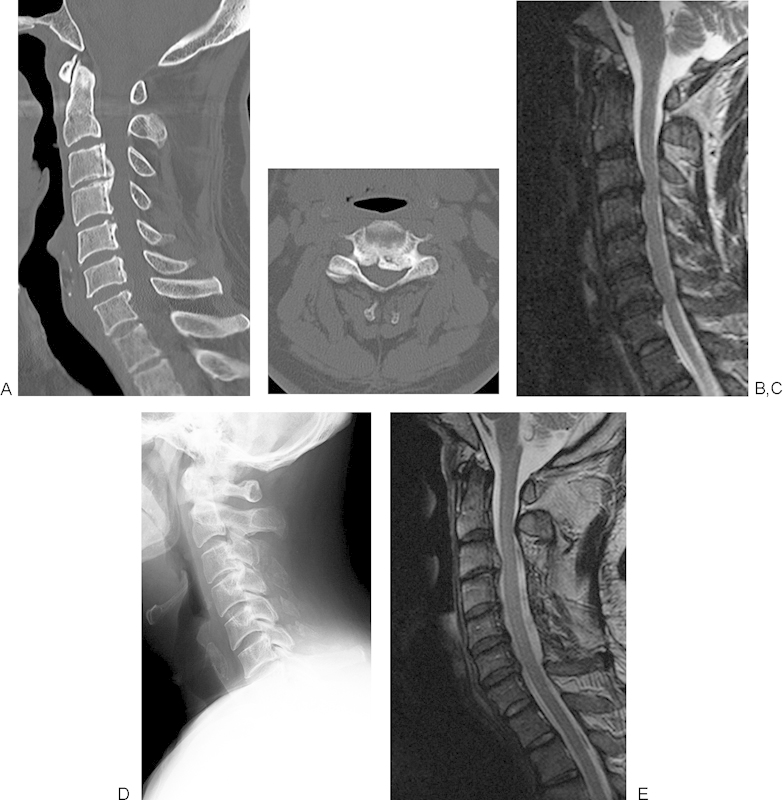
(A) A preoperative sagittal computed tomography (CT) image of the cervical spine of a 62-year-old man shows cervical ossification of the posterior longitudinal ligament (OPLL) from C3 to C6 and spinal cord compression at multiple levels. (B) A preoperative axial CT image at C3–4 level shows a marked spinal canal stenosis due to OPLL. (C) A preoperative magnetic resonance (MR) sagittal image shows spinal cord compression from C3–4 to C6–7 level. (D) A postoperative lateral radiograph of the cervical spine. Muscle preserving double-door laminoplasty from C3 to C6 was performed. Split spinous processes from C3 to C6 were seen. (E) A postoperative sagittal MR image showed the expansion of the dural sac at the site of laminoplasty from C3 to C6.
In our series of TEMPL procedures, compared with conventional bilateral open-door laminoplasty, the TEMPL group showed less axial neck pain, better range of motion of the cervical spine, and better quality of life with a statistically significant difference. Regarding deep extensor muscle volume at postoperative 2 years, the TEMPL group showed 88% of preoperative muscle volume and the conventional group showed 56%. There was a statistically significant difference between the two groups.32
Reduction of Postoperative Persistent Axial Neck Pain
There have been several attempts to reduce axial neck pain after laminoplasty. One of these is to start isometric muscle exercise as soon as possible, just several days after surgery. No type of cervical orthosis is necessary after cervical laminoplasty. Because prolonged rest for cervical muscles may cause muscle atrophy, early accelerated rehabilitation program is recommended to obtain better functional outcomes after cervical laminoplasty.
Minimization of muscle damage and focal decompression for radicular symptoms with minimized destabilization to the spine are becoming more common in Japan. This type of motion-preservation technology avoids using artificial intervertebral discs to maintain flexibility of the spine.
Preservation of muscle structures of the posterior cervical spine is very important to reduce postsurgical persistent axial neck pain and maintain cervical alignment.33 The C2 spinous process especially is one of the important structures to influence postoperative axial neck pain.30 The reasons for this maybe due to many muscle attachments from the occiput. Another important structure is the C7 spinous process and its attached muscles. Hosono et al27 reported that axial neck pain after laminoplasty from C3 to C7 is worse than that from C3 to C6. Sakaura et al34 reported that preservation of the nuchal ligament at C6 and C7 prevents postoperative cervical kyphosis. Ono et al35 reported the surgical anatomy of the nuchal muscles in the posterior cervicothoracic junction. If C7 spinous process is not resected for laminoplasty, the rhomboideus minor, the serratus posterior superior, and the splenius capitis can be left intact. All of the muscles play an important role in cervical motion and maintenance of sagittal alignment and axial neck pain.
Need for Instrumentation Surgery
Cervical laminoplasty is indicated for patients with multilevel spinal cord compression under the presence of cervical lordosis. Preoperative cervical kyphosis is a poor prognostic factor of cervical laminoplasty. Chiba et al36 reported a 14-year follow-up study of expansive open-door laminoplasty for cervical myelopathy. Their results showed that OPLL patients with cervical kyphosis had lower recovery rates. Suda et al10 reported that laminoplasty was effective for patients with cervical local kyphosis less than 13 degrees. For those with cervical kyphosis exceeding 13 degrees, Suda et al recommended posterior instrumentation surgery to correct the kyphotic deformity in combination with cervical laminoplasty for neural decompression (Fig. 5).10
Figure 5.
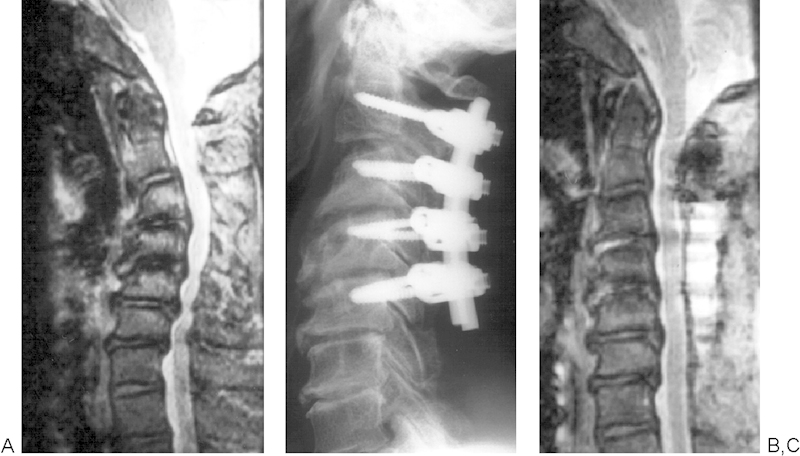
(A) Kyphosis correction for patients with cervical myelopathy and kyphotic deformity. A preoperative sagittal image of the cervical spine shows 35-degree kyphosis between C2 and C5. (B) A postoperative radiograph shows that the kyphotic deformity from C2 to C5 decreased by posterior instrumentation surgery using pedicle screws. (C) A postoperative magnetic resonance image shows the expansion of the dural sac.
There are conflicting reports regarding the postoperative progression of kyphosis and neurological recovery with patients who had undergone cervical laminoplasty. Though there were several reports showing that there was no relation between postoperative progression of cervical kyphosis and neurological decline,23 26 37 Sakaura et al34 reported that progression of kyphosis had a negative impact on the functional outcomes of patients with cervical myelopathy who had undergone laminoplasty.
In our institute, for patients needing cervical posterior decompression and having cervical kyphosis exceeding 13 degrees, we usually perform both laminoplasty and correction of kyphosis simultaneously by using posterior instrumentation such as pedicle screws and rods or lateral mass screws and rods.
Complications Related to Laminoplasty
One of the common complications arising from cervical laminoplasty is postoperative hematoma. Reported incidence of postoperative hematoma related to cervical laminoplasty is 0.44%.38 Postoperative hematoma commonly occurs within postoperative 48 hours. Risk factors of this complication are preoperative administration of anticoagulants, excessive epidural bleeding during surgery, and high blood pressure.39 40 For patients with anticoagulants, postoperative hematoma may occur even at 1 week after surgery.41 Surgeons should keep the patients informed about the possibility of postsurgical hematoma, which will deteriorate neurological status and frequently require emergency surgery consisting of decompression and coagulation.
Another postsurgical complication related to cervical laminoplasty is C5 nerve root palsy. Reported incidence of postoperative C5 palsy is 4.6%: 5.3% in unilateral open-door laminoplasty and 4.3% in bilateral French-door open laminoplasty.42 The occurrence of C5 nerve palsy is unrelated to surgical procedures and to disease etiologies. Clinical presentation of C5 nerve root palsy is weakness of deltoid muscle and sensory disturbance at C5 area. The prognosis of C5 nerve palsy is generally good, and most patients with this complication will recover completely several months later. However, patients with severe motor deficits with deltoid muscle will take a longer period for full recovery. The causes of this complication are still unknown. Many reasons can be presumed, including nerve root traction due to posterior shift of the spinal cord, spinal cord ischemia, reperfusion injury to the spinal cord or segmental spinal cord disorders.
Footnotes
Disclosures Manabu Ito, None Ken Nagahama, None
References
- 1.Miyazaki K, Kirita Y. Extensive simultaneous multisegment laminectomy for myelopathy due to the ossification of the posterior longitudinal ligament in the cervical region. Spine. 1986;11:531–542. doi: 10.1097/00007632-198607000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Oyama M, Hattori S. A new method of posterior decompression [in Japanese] Central Japan Journal of Orthopedic and Traumatic Surgery (Chubuseisai) 1973;16:792. [Google Scholar]
- 3.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6:354–364. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Satomi K, Nishu Y, Kohno T, Hirabayashi K. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine. 1994;19:507–510. doi: 10.1097/00007632-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi Y, Kanamori M, Ishihara H. et al. Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am. 2001;83-A:1798–1802. doi: 10.2106/00004623-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki M, Kawaguchi Y, Kimura T, Yonenobu K. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg. 2002;96(2, Suppl):180–189. [PubMed] [Google Scholar]
- 7.Zdeblick T A, Zou D, Warden K E, McCabe R, Kunz D, Vanderby R. Cervical stability after foraminotomy. A biomechanical in vitro analysis. J Bone Joint Surg Am. 1992;74:22–27. [PubMed] [Google Scholar]
- 8.Raynor R B, Pugh J, Shapiro I. Cervical facetectomy and its effect on spine strength. J Neurosurg. 1985;63:278–282. doi: 10.3171/jns.1985.63.2.0278. [DOI] [PubMed] [Google Scholar]
- 9.Aita I, Hayashi K, Wadano Y, Yabuki T. Posterior movement and enlargement of the spinal cord after cervical laminoplasty. J Bone Joint Surg Br. 1998;80:33–37. doi: 10.1302/0301-620x.80b1.7919. [DOI] [PubMed] [Google Scholar]
- 10.Suda K, Abumi K, Ito M, Shono Y, Kaneda K, Fujiya M. Local kyphosis reduces surgical outcomes of expansive open-door laminoplasty for cervical spondylotic myelopathy. Spine. 2003;28:1258–1262. doi: 10.1097/01.BRS.0000065487.82469.D9. [DOI] [PubMed] [Google Scholar]
- 11.Hirabayashi K, Satomi K. Operative procedure and results of expansive open-door laminoplasty. Spine. 1988;13:870–876. doi: 10.1097/00007632-198807000-00032. [DOI] [PubMed] [Google Scholar]
- 12.Hirabayashi K, Watanabe K, Wakano K, Suzuki N, Satomi K, Ishii Y. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine. 1983;8:693–699. doi: 10.1097/00007632-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Itoh T, Tsuji H. Technical improvements and results of laminoplasty for compressive myelopathy in the cervical spine. Spine. 1985;10:729–736. doi: 10.1097/00007632-198510000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Shaffrey C I, Wiggins G C, Piccirilli C B, Young J N, Lovell L R. Modified open-door laminoplasty for treatment of neurological deficits in younger patients with congenital spinal stenosis: analysis of clinical and radiographic data. J Neurosurg. 1999;90(2, Suppl):170–177. doi: 10.3171/spi.1999.90.2.0170. [DOI] [PubMed] [Google Scholar]
- 15.Nakano K, Harata S, Suetsuna F, Araki T, Itoh J. Spinous process-splitting laminoplasty using hydroxyapatite spinous process spacer. Spine. 1992;17(3, Suppl):S41–S43. doi: 10.1097/00007632-199203001-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kamo Y, Takemitsu Y, Hamada O. Cervical laminoplasty by splitting the spinous process using a AW glass-ceramic lamina spacer [in Japanese] Rinsho Seikeigeka. 1992;10:1115–1122. [Google Scholar]
- 17.Kurokawa T, Tsuyama N, Tanaka H. Enlargement of spinal canal by sagittal splitting of the spinal processes [in Japanese] Bessatsu Seikeigeka. 1984;2:234–240. [Google Scholar]
- 18.Tomita K, Nomura S, Umeda S, Baba H. Cervical laminoplasty to enlarge the spinal canal in multilevel ossification of the posterior longitudinal ligament with myelopathy. Arch Orthop Trauma Surg. 1988;107:148–153. doi: 10.1007/BF00451594. [DOI] [PubMed] [Google Scholar]
- 19.Tomita K, Kawahara N, Toribatake Y, Heller J G. Expansive midline T-saw laminoplasty (modified spinous process-splitting) for the management of cervical myelopathy. Spine. 1998;23:32–37. doi: 10.1097/00007632-199801010-00007. [DOI] [PubMed] [Google Scholar]
- 20.Tsuzuki N, Abe R, Saiki K, Okai K. Paralysis of the arm after posterior decompression of the cervical spinal cord. II. Analyses of clinical findings. Eur Spine J. 1993;2:197–202. doi: 10.1007/BF00299446. [DOI] [PubMed] [Google Scholar]
- 21.Ratliff J K, Cooper P R. Cervical laminoplasty: a critical review. J Neurosurg. 2003;98(3, Suppl):230–238. doi: 10.3171/spi.2003.98.3.0230. [DOI] [PubMed] [Google Scholar]
- 22.Koshu K Tominaga T Yoshimoto T Spinous process-splitting laminoplasty with an extended foraminotomy for cervical myelopathy Neurosurgery 199537430–434.; discussion 434–435 [DOI] [PubMed] [Google Scholar]
- 23.Seichi A, Takeshita K, Ohishi I. et al. Long-term results of double-door laminoplasty for cervical stenotic myelopathy. Spine. 2001;26:479–487. doi: 10.1097/00007632-200103010-00010. [DOI] [PubMed] [Google Scholar]
- 24.Heller J G, Edwards C C II, Murakami H, Rodts G E. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine. 2001;26:1330–1336. doi: 10.1097/00007632-200106150-00013. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi Y, Kanamori M, Ishihara H, Ohmori K, Nakamura H, Kimura T. Minimum 10-year followup after en bloc cervical laminoplasty. Clin Orthop Relat Res. 2003;(411):129–139. doi: 10.1097/01.blo.0000069889.31220.62. [DOI] [PubMed] [Google Scholar]
- 26.Wada E Suzuki S Kanazawa A Matsuoka T Miyamoto S Yonenobu K Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years Spine 2001261443–1447.; discussion 1448 [DOI] [PubMed] [Google Scholar]
- 27.Hosono N, Sakaura H, Mukai Y, Yoshikawa H. The source of axial pain after cervical laminoplasty-C7 is more crucial than deep extensor muscles. Spine. 2007;32:2985–2988. doi: 10.1097/BRS.0b013e31815cda83. [DOI] [PubMed] [Google Scholar]
- 28.Shiraishi T. Skip laminectomy—a new treatment for cervical spondylotic myelopathy, preserving bilateral muscular attachments to the spinous processes: a preliminary report. Spine J. 2002;2:108–115. doi: 10.1016/s1529-9430(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 29.Shiraishi T. A new technique for exposure of the cervical spine laminae. Technical note. J Neurosurg. 2002;96(1, Suppl):122–126. doi: 10.3171/spi.2002.96.1.0122. [DOI] [PubMed] [Google Scholar]
- 30.Shiraishi T, Yato Y. New double-door laminoplasty procedure for the axis to preserve all muscular attachments to the spinous process: technical note. Neurosurg Focus. 2002;12:E9. doi: 10.3171/foc.2002.12.1.10. [DOI] [PubMed] [Google Scholar]
- 31.Shiraishi T, Fukuda K, Yato Y, Nakamura M, Ikegami T. Results of skip laminectomy-minimum 2-year follow-up study compared with open-door laminoplasty. Spine. 2003;28:2667–2672. doi: 10.1097/01.BRS.0000103340.78418.B2. [DOI] [PubMed] [Google Scholar]
- 32.Kotani Y, Abumi K, Ito M. et al. Minimum 2-year outcome of cervical laminoplasty with deep extensor muscle-preserving approach: impact on cervical spine function and quality of life. Eur Spine J. 2009;18:663–671. doi: 10.1007/s00586-009-0892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato M, Nakamura H, Konishi S. et al. Effect of preserving paraspinal muscles on postoperative axial pain in the selective cervical laminoplasty. Spine. 2008;33:E455–E459. doi: 10.1097/BRS.0b013e318178e607. [DOI] [PubMed] [Google Scholar]
- 34.Sakaura H, Hosono N, Mukai Y, Oshima K, Iwasaki M, Yoshikawa H. Preservation of the nuchal ligament plays an important role in preventing unfavorable radiologic changes after laminoplasty. J Spinal Disord Tech. 2008;21:338–343. doi: 10.1097/BSD.0b013e3181453de4. [DOI] [PubMed] [Google Scholar]
- 35.Ono A, Tonosaki Y, Yokoyama T. et al. Surgical anatomy of the nuchal muscles in the posterior cervicothoracic junction: significance of the preservation of the C7 spinous process in cervical laminoplasty. Spine. 2008;33:E349–E354. doi: 10.1097/BRS.0b013e31817152cc. [DOI] [PubMed] [Google Scholar]
- 36.Chiba K, Ogawa Y, Ishii K. et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy—average 14-year follow-up study. Spine. 2006;31:2998–3005. doi: 10.1097/01.brs.0000250307.78987.6b. [DOI] [PubMed] [Google Scholar]
- 37.Morio Y, Yamamoto K, Teshima R, Nagashima H, Hagino H. Clinicoradiologic study of cervical laminoplasty with posterolateral fusion or bone graft. Spine. 2000;25:190–196. doi: 10.1097/00007632-200001150-00008. [DOI] [PubMed] [Google Scholar]
- 38.Aono H, Ohwada T, Hosono N. et al. Incidence of postoperative symptomatic epidural hematoma in spinal decompression surgery. J Neurosurg Spine. 2011;15:202–205. doi: 10.3171/2011.3.SPINE10716. [DOI] [PubMed] [Google Scholar]
- 39.Sokolowski M J, Garvey T A, Perl J II. et al. Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine. 2008;33:108–113. doi: 10.1097/BRS.0b013e31815e39af. [DOI] [PubMed] [Google Scholar]
- 40.Kebaish K M, Awad J N. Spinal epidural hematoma causing acute cauda equina syndrome. Neurosurg Focus. 2004;16:e1. [PubMed] [Google Scholar]
- 41.Uribe J, Moza K, Jimenez O, Green B, Levi A D. Delayed postoperative spinal epidural hematomas. Spine J. 2003;3:125–129. doi: 10.1016/s1529-9430(02)00535-1. [DOI] [PubMed] [Google Scholar]
- 42.Sakaura H, Hosono N, Mukai Y, Ishii T, Yoshikawa H. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine. 2003;28:2447–2451. doi: 10.1097/01.BRS.0000090833.96168.3F. [DOI] [PubMed] [Google Scholar]


