Abstract
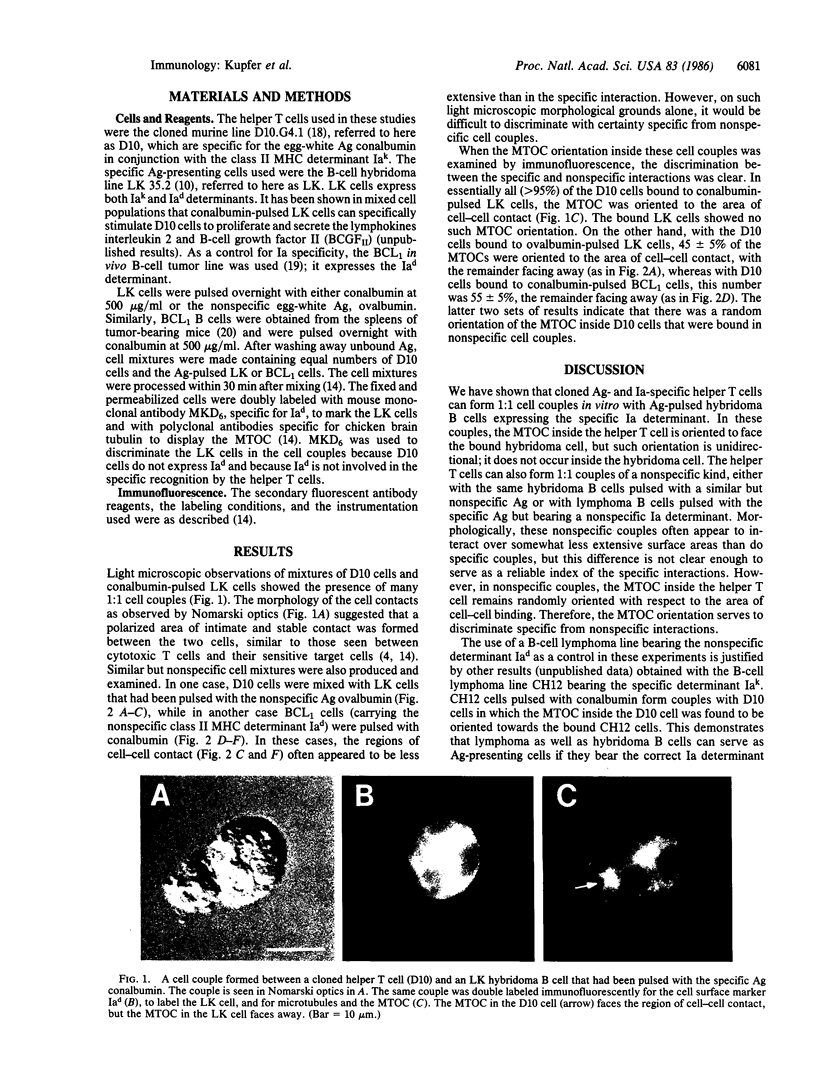
Cell couples have been formed by mixing an antigen- and Ia-specific cloned helper T-cell line with a B-cell hybridoma presenting the antigen. By immunofluorescence observations, we have shown that the microtubule-organizing center (MTOC) inside the helper T cell, but not in the bound antigen-presenting cell, becomes oriented to face the area of specific cell-cell contact. This MTOC orientation is antigen- and Ia-specific, and thus provides direct evidence for the specific interaction of a helper T cell with a B cell. It is presumed that the function served by this MTOC orientation, which is accompanied by the coordinate reorientation of the Golgi apparatus, is to target Golgi apparatus-derived secretory vesicles, containing putative lymphokines and/or growth factors, from the helper T cell directly to the antigen-presenting cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berke G. Cytotoxic T-lymphocytes. How do they function? Immunol Rev. 1983;72:5–42. doi: 10.1111/j.1600-065x.1983.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Bottomly K., Jones B., Kaye J., Jones F., 3rd Subpopulations of B cells distinguished by cell surface expression of Ia antigens. Correlation of Ia and idiotype during activation by cloned Ia-restricted T cells. J Exp Med. 1983 Aug 1;158(2):265–279. doi: 10.1084/jem.158.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayberger C., DeKruyff R. H., Fay R., Cantor H. Identification of a unique B-cell-stimulating factor produced by a cloned dendritic cell. Proc Natl Acad Sci U S A. 1985 Jan;82(1):183–187. doi: 10.1073/pnas.82.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Matis L. A., Hecht T. T., Samelson L. E., Longo D. L., Heber-Katz E., Schwartz R. H. The fine specificity of antigen and Ia determinant recognition by T cell hybridoma clones specific for pigeon cytochrome c. Cell. 1982 Aug;30(1):141–152. doi: 10.1016/0092-8674(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Henkart P. A., Millard P. J., Reynolds C. W., Henkart M. P. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984 Jul 1;160(1):75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Witmer M. D., Steinman R. M. Clustering of dendritic cells, helper T lymphocytes, and histocompatible B cells during primary antibody responses in vitro. J Exp Med. 1984 Sep 1;160(3):858–876. doi: 10.1084/jem.160.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Janeway C. A., Jr Cooperative interaction of B lymphocytes with antigen-specific helper T lymphocytes is MHC restricted. Nature. 1981 Aug 6;292(5823):547–549. doi: 10.1038/292547a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., White J., Wegmann D., Mustain E., Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3604–3607. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Hamaoka T., Dorf M. E., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. The H-2 gene complex determines successful physiologic lymphocyte interactions. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2624–2628. doi: 10.1073/pnas.70.9.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M. R., Severinson-Gronowicz E., Schröder J., Strober S. Characterization of a spontaneous murine B cell leukemia (BCL1). II. Tumor cell proliferation and IgM secretion after stimulation by LPS. J Immunol. 1979 Sep;123(3):1000–1006. [PubMed] [Google Scholar]
- Kupfer A., Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984 Nov;133(5):2762–2766. [PubMed] [Google Scholar]
- Kupfer A., Dennert G., Singer S. J. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Dennert G., Singer S. J. The reorientation of the Golgi apparatus and the microtubule-organizing center in the cytotoxic effector cell is a prerequisite in the lysis of bound target cells. J Mol Cell Immunol. 1985;2(1):37–49. [PubMed] [Google Scholar]
- Kupfer A., Louvard D., Singer S. J. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J., Dennert G. On the mechanism of unidirectional killing in mixtures of two cytotoxic T lymphocytes. Unidirectional polarization of cytoplasmic organelles and the membrane-associated cytoskeleton in the effector cell. J Exp Med. 1986 Mar 1;163(3):489–498. doi: 10.1084/jem.163.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. II. Antigen-mediated physical interactions between immune guinea pig lymph node lymphocytes and syngeneic macrophages. J Exp Med. 1975 Jan 1;141(1):138–154. doi: 10.1084/jem.141.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Skidmore B., Kappler J. W. Binding of antigen-specific, H-2-restricted T cell hybridomas to antigen-pulsed adherent cell monolayers. J Immunol. 1983 May;130(5):2088–2092. [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C., Uhr J. W., Vitetta E. S. Activation of antigen-specific B cells: role of T cells, cytokines, and antigen in induction of growth and differentiation. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6628–6631. doi: 10.1073/pnas.80.21.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. Interleukin-induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984 Sep 1;160(3):679–694. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Marrack P., Kappler J. W. Helper signals in the plaque-forming cell response to protein-bound haptens. J Exp Med. 1983 Aug 1;158(2):317–333. doi: 10.1084/jem.158.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski A. A., Singer S. J. Associations of elements of the Golgi apparatus with microtubules. J Cell Biol. 1984 Sep;99(3):1092–1100. doi: 10.1083/jcb.99.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Shultz L. D., Gray P. W., Johnson H. M. Gamma-interferon is one of several direct B cell-maturing lymphokines. 1984 Jun 28-Jul 4Nature. 309(5971):801–804. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]
- Sredni B., Schwartz R. H. Antigen-specific, proliferating T lymphocyte clones. Methodology, specificity, MHC restriction and alloreactivity. Immunol Rev. 1981;54:187–223. doi: 10.1111/j.1600-065x.1981.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Dennert G., Warner J. F., Dutton R. W. Culture supernatants of a stimulated T-cell line have helper activity that acts synergistically with interleukin 2 in the response of B cells to antigen. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2517–2521. doi: 10.1073/pnas.78.4.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Dutton R. W. Production of a B cell growth-promoting activity, (DL)BCGF, from a cloned T cell line and its assay on the BCL1 B cell tumor. J Exp Med. 1982 Dec 1;156(6):1821–1834. doi: 10.1084/jem.156.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Howard M., Kappler J., Marrack P., Watson J., Booth R., Wetzel G. D., Dutton R. W. Evidence for two distinct classes of murine B cell growth factors with activities in different functional assays. J Exp Med. 1983 Sep 1;158(3):822–835. doi: 10.1084/jem.158.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L. Role of BCGFII in the differentiation to antibody secretion normal and tumor B cells. J Immunol. 1985 Jun;134(6):3934–3943. [PubMed] [Google Scholar]
- Swierkosz J. E., Rock K., Marrack P., Kappler J. W. The role of H-2 linked genes in helper T-cell function. II. Isolation on antigen-pulsed macrophages of two separate populations of F1 helper T cells each specific for antigen and one set of parental H-2 products. J Exp Med. 1978 Feb 1;147(2):554–570. doi: 10.1084/jem.147.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Kaye J., Jones B. The role of B cell surface Ia antigen recognition by T cells in B cell triggering. Analysis of the interaction of cloned helper T cells with normal B cells in differing states of activation and with B cells expressing the xid defect. Eur J Immunol. 1984 Jun;14(6):553–561. doi: 10.1002/eji.1830140613. [DOI] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. The specific binding of Listeria monocytogenes-immune T lymphocytes to macrophages. I. Quantitation and role of H-2 gene products. J Exp Med. 1979 Nov 1;150(5):1143–1160. doi: 10.1084/jem.150.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. H-2 compatability requirement for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. Different cytotoxic T-cell specificities are associated with structures coded for in H-2K or H-2D;. J Exp Med. 1975 Jun 1;141(6):1427–1436. doi: 10.1084/jem.141.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Lowenthal J. W., Erard F., Hashimoto N., Devos R., MacDonald H. R. Activated B cells express receptors for, and proliferate in response to, pure interleukin 2. J Exp Med. 1984 Oct 1;160(4):1170–1183. doi: 10.1084/jem.160.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]