Abstract
Aim To compare anterior fusion in standalone anterior lumbar interbody fusion (ALIF) using cage and screw constructs and anterior cage–alone constructs with posterior pedicle screw supplementation but without posterior fusion.
Methods Eighty-five patients underwent single- or two-level ALIF procedure for degenerative disk disease or lytic spondylolisthesis (SPL). Posterior instrumentation was performed without posterior fusion in all cases of lytic SPL and when the anterior cage used did not have anterior screw through cage fixation.
Results Seventy (82%) patients had adequate radiological follow-up at a mean of 19 months. Forty patients had anterior surgery alone (24 single level and 16 two levels) and 30 had front-back surgery (15 single level and 15 two levels). Anterior locked pseudarthrosis was only seen in the anterior surgery–alone group when using the STALIF cage (Surgicraft, Worcestershire, UK) (37 patients). This occurred in five of the single-level surgeries (5/22) and nine of the two-level surgeries (9/15). Fusion was achieved in 100% of the front-back group and only 65% (26/40) of the anterior surgery–alone group.
Conclusion Posterior pedicle screw supplementation without posterolateral fusion improves the fusion rate of ALIF when using anterior cage and screw constructs. We would recommend supplementary posterior fixation especially in cases where more than one level is being operated.
Keywords: anterior lumbar interbody fusion, ALIF, locked pseudarthrosis, spondylolisthesis
Many studies have shown that a solid fusion does not always produce clinical success, and others have shown that pseudarthrosis does correlate with poorer clinical outcomes.1 2 3 4 Recently in a study of 193 patients undergoing instrumented posterolateral fusions, Djurasovic et al5 have shown that solid arthrodesis, based on fine-cut computed tomography (CT) 2 years following surgery, is associated with clinically relevant improvement in low back–specific quality-of-life measures compared with those in whom fusion was not achieved. Therefore, a solid arthrodesis contributes to clinical outcome and is an important goal of fusion surgery.
Anterior lumbar interbody fusion (ALIF) surgery in patients with discogenic back pain and lytic spondylolisthesis (SPL) aims to remove the pain source, restore foraminal height, recreate and maintain a normal lumbar lordosis, and prevent movement of the painful motion segment. Indeed, discogenic pain has been shown to persist despite a solid posterolateral fusion.6 ALIF lends itself to fusion because the compressive environment encourages bone formation as opposed to the tensile environment seen in posterolateral fusion. Cages used in anterior lumbar fusions obviate the need for large corticocancellous grafts in which postoperative subsidence and donor site morbidity may be a problem. In 2004 the senior author published the results of 47 patients treated by ALIF with autogenous structural graft and posterior pedicle screw fixation without posterior fusion.7 A 97% fusion rate was achieved at 2-year follow-up based on plain radiographs with 72% satisfactory clinical outcome.
In 1998 Lund et al performed a biomechanical evaluation of three interference-fit anterior cages in human cadaveric spines. The results showed instability in extension and rotation.8 Standalone ALIF cages that utilize screws passing through the interbody cage and into the vertebral bodies were designed to obviate the need for a posterior procedure by increasing the anterior construct stability and fusion rate with a reduced operating time.3 In May 2006, Cappuccino and Cunningham presented the results of their cadaveric study in Montreal.9 This confirmed that the STALIF cage (Surgicraft, Worcestershire, UK) significantly reduced segmental motion in flexion extension and axial rotation compared with nonfixed cages.
The objective of this study was to compare anterior fusion in standalone ALIF, using cage and screw constructs, and anterior cage–alone constructs with posterior pedicle screw supplementation but without posterior fusion.
Methods
Between 2004 and 2009 the senior author performed 85 ALIF procedures at the Royal Devon and Exeter NHS Hospital, Exeter, United Kingdom. The procedures were performed for patients with low back pain secondary degenerative disk disease and low back pain with or without radicular pain secondary to grade 1 or 2 lytic SPL (with or without adjacent degenerative disk disease). Only single- or two-level fusions were included in this study. The senior author felt that provocative discography using a control level was potentially harmful and this has recently been authenticated in a study by Carragee et al.10 Operability was determined based on clinical history and examination and on magnetic resonance imaging (MRI) findings in the majority of cases and discography, when used, was very selective.
The surgery was performed by the senior author in all cases. The patient was initially placed supine and a left-sided anterior retroperitoneal approach was performed for the ALIF procedure. The iliac crests were made accessible during the draping procedure in all cases to permit harvesting if required. Diskectomy and end plate preparation was performed in a similar fashion in all cases and the cages were packed with morselized auto- or allograft prior to insertion. The patient was then turned prone in the same setting in cases where posterior instrumentation was performed. A minimal posterior exposure was performed allowing adequate exposure of pedicle screw entry points but protection of the facet joint capsules. The transverse processes and sacral alar were not exposed and posterolateral fusion was not performed. In selected cases, a posterior decompression was performed.
The study represents a sequential cohort with the evolution of different spinal implants. Initially the senior author performed autogenous structural graft ALIF and posterior pedicle screw fixation without posterior fusion.7 Based on his subjective experience with variable autogenous graft shape and strength, subsidence, donor site morbidity, and the evolution of implants and the early evidence to support them in the literature, he began to use anterior cage and screw constructs. Over time it was noted that several locked pseudarthroses developed, and the author changed to using anterior cages with posterior pedicle screw fixation without posterolateral fusion (around the end of 2006). In these cases, the cage was changed from the fixed STALIF cage (Surgicraft) to the nonfixed Antelys cage (Scient'x, Oxford, UK) because of the additional cost added by the posterior pedicle screw procedure and anterior cage fixation was deemed unnecessary. However, in all cases of lytic SPL, the senior surgeon performed the anterior procedure with a STALIF cage (Surgicraft) and also posterior pedicle screw fixation without posterolateral fusion because he felt that the condition harbored an unstable biomechanical environment and required the best possible fixation. Fig. 1 demonstrates the radiological appearances of the different implants used. Over the years, with the development of a bone bank in the Exeter Hip Unit, the use of femoral head allograft increased due to its availability and the reduction in operative time and additional morbidity from iliac crest graft harvesting.
Fig. 1.
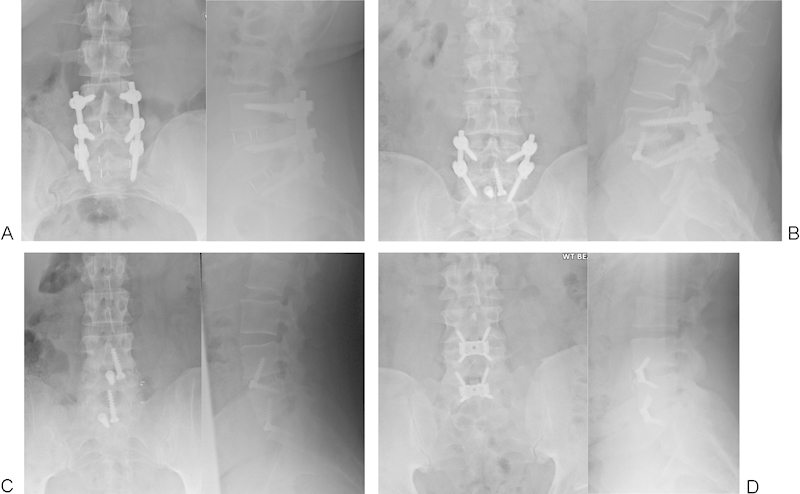
Examples of implants. (A) Antelys cage (Scient'x) with posterior instrumentation. (B) Lytic spondylolisthesis treated with STALIF (Surgicraft) and posterior instrumentation. (C) STALIF cage (Surgicraft). (D) Synfix cage (Synthes, Welwyn Garden City, UK).
The patients were routinely followed with radiographs at regular intervals, and a fine-cut CT scan to assess fusion across the cage was routinely intended at around the 1-year mark. However, this varied depending on the patients' clinical scenario, and MRI scanning was used in a small number of cases as the patients complained of neurological symptoms in the legs. All of the imaging was reviewed by M.J.H.M. and L.N., who had not been directly involved with the patients care. “Union” (yes or no) was defined as evidence of bridging bone across the cage on one or more images. Cross-reference was then made to the radiologists report and the senior authors' clinical interpretation of the scan as documented in the clinical notes. It was felt that subclassifying the stages of union would actually worsen reproducibility by virtue of random error through increasing the number of classification options (the error in selecting from four choices is greater than if given only two).
Results
Of the 85 operated patients, 70 (82%) had adequate radiological follow-up to be included in this study (Table 1). The remaining 15 patients had been lost to clinical and radiological follow-up before 1 year. The mean age was 43 years and there were 34 males and 36 females. Forty patients had anterior surgery alone (24 single level and 16 two levels) and 30 had front-back surgery (15 single level and 15 two levels). Of the 40 patients in the anterior surgery–alone group, 37 had STALIF cages (Surgicraft) and three had Synfix cages (Synthes, Welwyn Garden City, UK). Of the 30 patients in the front-back group, 15 had a lytic SPL and 15 operations were performed for degenerative disk disease (Fig. 2). Sixty-three patients had a CT and seven had an MRI at a mean of 19 months following surgery. Fourteen pseudarthroses of the locked type as described by Fagan et al11 were detected (bone growth through the adjacent end plates into the cage with no sign of lucency between the cage and the end plate; Figs. 3, 4). There were seven smokers in the cohort, and this did not appear to influence union (Table 1). The mean time to final follow-up imaging and confirmation of fusion was 17 months (n = 56, standard deviation 9) in the group that fused and 27 months (n = 14, standard deviation 12) in the locked pseudarthrosis group.
Table 1. Demographic data and fusion rates.
| Non-fixed Antelys cage and posterior instrumentation (no posterolateral fusion) | STALIF cage and posterior instrumentation (no posterolateral fusion) | STALIF cage alone | Synfix cage alone | |
|---|---|---|---|---|
| Number of patients | ||||
| Degenerative disk disease | 14 | 1 | 37 | 3 |
| Lytic spondylolisthesis | 0 | 15 | 0 | 0 |
| Previous posterior surgery | 3 | 0 | 8 | 3 |
| Number of levels | 23 | 22 | 52 | 4 |
| Age, y (standard deviation) | 43.6 (12.9) | 45 (11) | 42.3 (8.2) | 34 (2) |
| Sex (M:F) | 6:8 | 10:6 | 15:22 | 2:1 |
| Number of smokers | 2 | 0 | 4 | 1 |
| Graft material | ||||
| Autograft | 4 | 8 | 31 | 1 |
| Allograft | 9 | 8 | 5 | 2 |
| Both | 0 | 0 | 1 | 0 |
| Synthetic | 1 | 0 | 0 | 0 |
| Number of patients fused (%) | 14 (100) | 16 (100) | 23 (62) | 3 (100) |
| Number of levels fused (%) | 23 (100) | 22 (100) | 34 (65) | 4 (100) |
| Time to imaging, mo (standard deviation) | 16.1 (10.3) | 15 (6.9) | 21.3 (11.1) | 18.3 (14.5) |
Fig. 2.
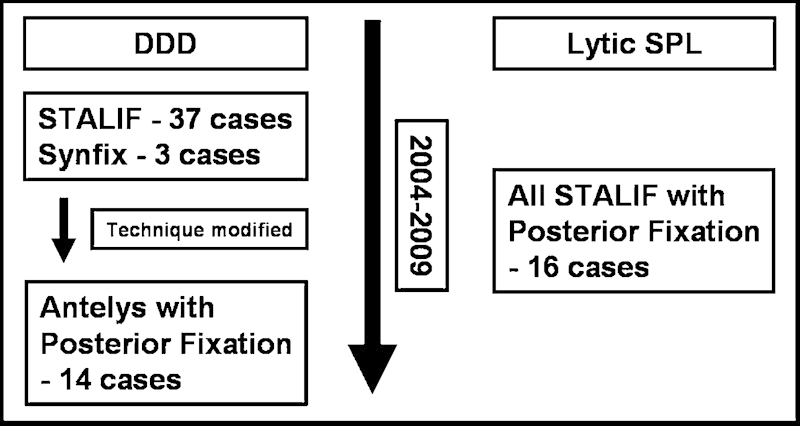
Summary of the cohorts. DDD, degenerative disk disease; SPL, spondylolisthesis.
Fig. 3.
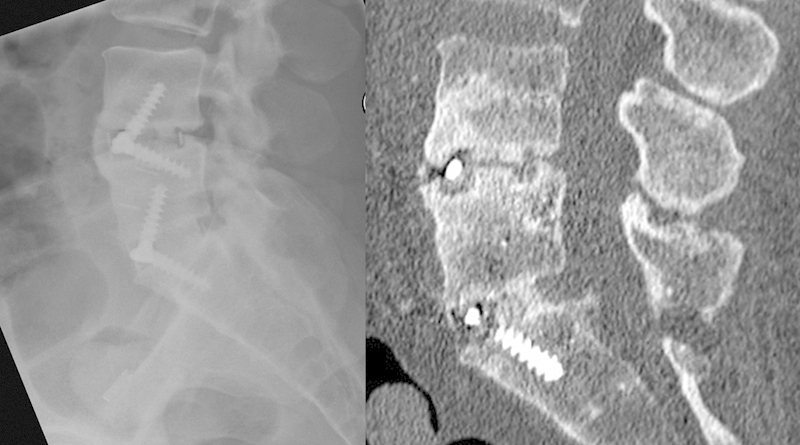
Plain radiographic evidence of locked pseudarthrosis at L4–5 and computed tomography confirming fusion at L5–S1.
Fig. 4.
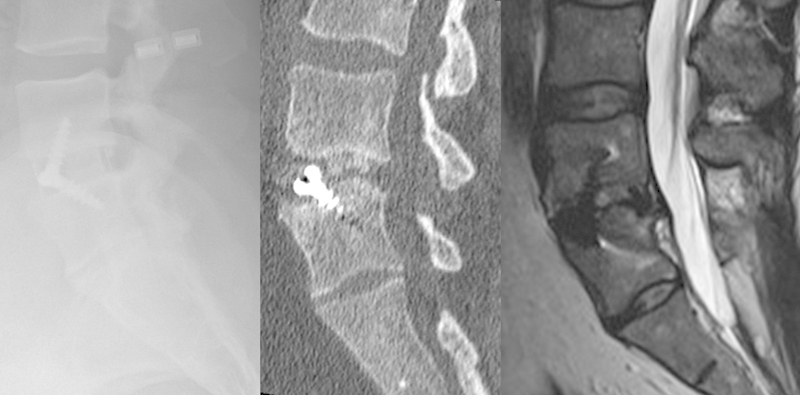
Satisfactory plain X-ray but computed tomography at 9 months reveals a locked pseudarthrosis and magnetic resonance imaging confirms persistence of this at 32 months.
Anterior locked pseudarthrosis was only seen in the 37 patients having anterior surgery–alone group with the STALIF cage (Surgicraft; Table 2). This occurred in five of the single-level surgeries (5/22) and nine of the two-level surgeries (9/15). In this last group, five patients had pseudarthroses at the L4–5 level and four had them at both the L4–5 and the L5–S1 levels. Twelve of these patients had iliac crest autograft, and two had ground femoral head allograft. The majority of STALIF cases utilized only two anterior screws. It was noted by the senior author that inserting three or more screws into the STALIF cage, as per the manufacturer's recommendation, was technically challenging in many cases. There were two cases of pseudarthrosis in which three or four anterior screws were used. Only 3 of the 14 patients underwent further surgery for ongoing back pain. One had posterior instrumentation without fusion and went on to unite anteriorly (Fig. 5), one had a posterior instrumented fusion and united posteriorly but not anteriorly (Fig. 6), and the third had posterior instrumentation without fusion and is still under follow-up.
Table 2. Locked pseudarthrosis rates in STALIF cage–alone group.
| Number of patients | Sex (M:F) | Graft | Smokers | Pseudarthrosis | Comments | |
|---|---|---|---|---|---|---|
| 1 level | 22 | 8:14 | 19 auto: 3 allo | 0/2 | 5 | 3 at L4–5 level |
| 2 levels | 15 | 7:8 | 12 auto: 2 allo: 1 both | 2/2 | 9 | 5 at L4–5 |
| Total | 37 | 15:22 | 31 auto: 5 allo: 1 both | 2/4 | 14 | 18 of 52 levels = 35% pseudarthrosis |
Fig. 5.
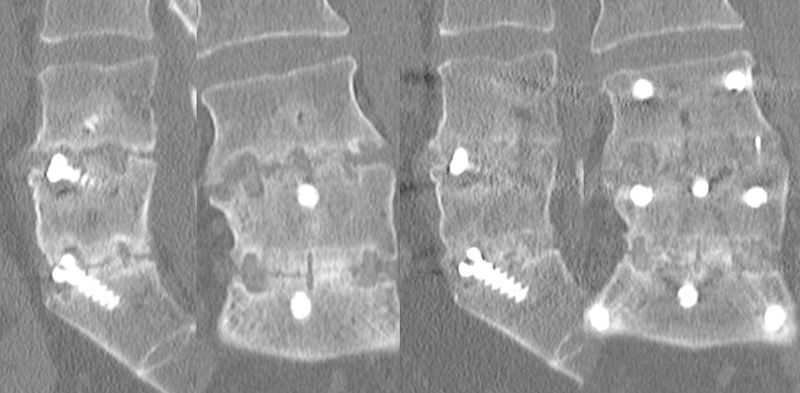
Union of a pseudarthrosis following posterior stabilization without posterolateral fusion (initial computed tomography at 36 months and subsequent computed tomography at 9 months post–revision surgery).
Fig. 6.
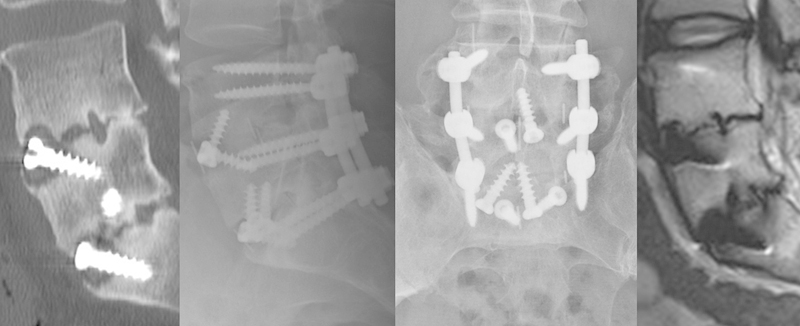
Persistent failure of anterior fusion and successful revision posterolateral fusion (initial computed tomography following STALIF alone at 24 months, X-ray and magnetic resonance imaging at 16 months post–revision surgery).
Union assessment showed no disagreement between the clinicians. It was subjectively noted by M.J.H.M. that the anterior fusion mass looked more substantial in cases when posterior instrumentation was used irrespective of the cage type. As demonstrated by the biomechanically unstable lytic SPL cohort using the fixed STALIF cage and the nonfixed Antelys group, both with additional posterior instrumentation, the addition of the posterior instrumentation without posterolateral fusion increased the anterior fusion rate compared with the STALIF-alone group (Tables 1 and 2). In summary, fusion was achieved in 100% of the front-back group and only 65% of the anterior surgery–alone group.
The complete data set can be seen in Table 3.
Table 3. Complete data set.
| Patient | Sex | Age (y) | Diagnosis | Operation | Levels | Type bone graft | No. of screws | FU (mo) | Fused | Imaging | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 45 | DDD | Antelys with PSF | L3–4 | Autograft | 0 | 24 | Yes | MRI | Previous left L3–4 microdecompression, facetectomy at time fusion |
| 2 | M | 36 | DDD | Antelys with PSF | L5–1 | Autograft | 0 | 46 | Yes | CT | Previous left L5–S1 microdiskectomy |
| 3 | M | 66 | DDD | Antelys with PSF | L4–5–1 | Allograft | 0 | 12 | Yes | CT | Right S1 screw removed 2 d later |
| 4 | F | 42 | DDD | Antelys with PSF | L4–5–1 | Allograft | 0 | 14 | Yes | CT | |
| 5 | M | 44 | DDD | Antelys with PSF | L5–1 | Autograft | 0 | 11 | Yes | CT | |
| 6 | F | 34 | DDD | Antelys with PSF | L4–5–1 | Allograft | 0 | 12 | Yes | CT | Previous left L5–S1 microdiskectomy |
| 7 | M | 35 | DDD | Antelys with PSF | L4–5–1 | Allograft | 0 | 14 | Yes | CT | |
| 8 | M | 61 | DDD | Antelys with PSF | L4–5–1 | Allograft | 0 | 4 | Yes | CT | |
| 9 | F | 51 | DDD | Antelys with PSF | L4–5 | Autograft | 0 | 14 | Yes | CT | |
| 10 | F | 36 | DDD | Antelys with PSF | L4–5–1 | Allograft | 0 | 12 | Yes | CT | |
| 11 | M | 32 | DDD | Antelys with PSF | L4–5–1 | Synthetic | 0 | 12 | Yes | MRI | CT at 3 and MRI at 12 mo |
| 12 | F | 33 | DDD | Antelys with PSF | L4–5–1 | Allograft | 0 | 14 | Yes | CT | |
| 13 | F | 29 | DDD | Antelys with PSF | L4–5–1 | Allograft | 0 | 27 | Yes | CT | |
| 14 | F | 67 | DDD | Antelys with PSF | L5–1 | Allograft | 2 buttress | 10 | Yes | CT | |
| 15 | M | 34 | DDD | Synfix | L4–5–1 | Autograft | 4, 4 | 35 | Yes | CT | Previous L5 laminectomy |
| 16 | M | 36 | DDD | Synfix | L5–1 | Autograft | 4 | 11 | Yes | CT | Previous left L5–1 microdiskectomy |
| 17 | M | 32 | DDD | Synfix | L5–1 | Allograft | 4 | 9 | Yes | MRI | Previous right L5–1 microdiskectomy |
| 18 | M | 42 | Lytic | STALIF with PSF | L4–5 | Autograft | 2 | 10 | Yes | CT | |
| 19 | F | 48 | Lytic | STALIF with PSF | L4–5 | Autograft | 2 | 28 | Yes | CT | |
| 20 | M | 61 | Lytic | STALIF with PSF | L4–5–1 | Autograft | 2 | 9 | Yes | CT | |
| 21 | M | 48 | Lytic | STALIF with PSF | L4–5–1 | Allograft | 2, 2 | 16 | Yes | CT | |
| 22 | M | 32 | DDD | STALIF with PSF | L5–1 | Autograft | 2 | 12 | Yes | CT | With posterior decompression |
| 23 | F | 45 | Lytic | STALIF with PSF | L4–5–1 | Allograft | 2, 2 | 14 | Yes | CT | |
| 24 | M | 62 | Lytic | STALIF with PSF | L3–4 | Autograft | 2 | 11 | Yes | CT | With left L3–4 and bilateral segmental L4–5 decompressions |
| 25 | F | 40 | Lytic | STALIF with PSF | l4–5–1 | Allograft | 2, 2 | 12 | Yes | CT | |
| 26 | M | 43 | Lytic | STALIF with PSF | L4–5 | Autograft | 2 | 14 | Yes | CT | |
| 27 | F | 48 | Lytic | STALIF with PSF | L4–5 | Autograft | 2 | 10 | Yes | CT | |
| 28 | F | 27 | Lytic | STALIF with PSF | L5–1 | Allograft | 2 | 22 | Yes | CT | |
| 29 | M | 41 | Lytic | STALIF with PSF | L4–5 | Allograft | 2 | 10 | Yes | CT | |
| 30 | M | 34 | Lytic | STALIF with PSF | L4–5 | Allograft | 2 | 11 | Yes | CT | |
| 31 | M | 46 | Lytic | STALIF with PSF | L5–1 | Allograft | 2 | 18 | Yes | CT | |
| 32 | F | 32 | Lytic | STALIF with PSF | L4–5–1 | Allograft | 2, 2 | 15 | Yes | MRI | MRI at 11 mo showed fusion only at L5–S1, MRI at 33 mo showed fusion at both levels |
| 33 | M | 66 | Lytic | STALIF with PSF | L4–5–1 | Autograft | 2, 2 | 33 | Yes | MRI | |
| 34 | F | 44 | DDD | STALIF | L4–5–1 | Autograft | 2, 2 | 46 | Yes | CT | |
| 35 | M | 47 | DDD | STALIF | L5–1 | Autograft | 4 | 23 | Yes | CT | |
| 36 | F | 60 | DDD | STALIF | L4–5 | Autograft | 2 | 21 | No | CT | Pseudarthrosis at L4–5 |
| 37 | F | 55 | DDD | STALIF | L4–5 | Autograft | 2 | 14 | Yes | CT | |
| 38 | M | 30 | DDD | STALIF | L5–1 | Autograft | 2 | 24 | No | CT | Pseudarthrosis at L5–S1 |
| 39 | M | 33 | DDD | STALIF | L4–5–1 | Autograft | 2, 2 | 23 | Yes | CT | |
| 40 | F | 32 | DDD | STALIF | L4–5–1 | Autograft | 3, 4 | 20 | No | CT | Pseudarthrosis at L4–5 |
| 41 | F | 35 | DDD | STALIF | L4–5 | Autograft | 2 | 10 | No | CT | Pseudarthrosis at L4–5 |
| 42 | M | 34 | DDD | STALIF | L4–5 | Autograft | 2 | 21 | Yes | CT | |
| 43 | F | 37 | DDD | STALIF | L4–5–1 | Autograft | 3, 3 | 12 | No | CT | Pseudarthrosis at L4–5 |
| 44 | F | 32 | DDD | STALIF | L5–1 | Autograft | 2 | 24 | No | CT | Pseudarthrosis at L5–S1 |
| 45 | F | 51 | DDD | STALIF | L5–1 | Autograft | 2 | 24 | Yes | CT | |
| 46 | M | 47 | DDD | STALIF | L4–5–1 | Autograft | 2, 3 | 43 | No | CT | CT 24 and 40 mo, flex/ext XRs stable, pseudarthrosis left alone at L4–5 |
| 47 | M | 46 | DDD | STALIF | L5–1 | Autograft | 2 | 12 | Yes | CT | |
| 48 | F | 51 | DDD | STALIF | L4–5–1 | Both | 2, 2 | 24 | Yes | CT | Previous L4 laminectomy |
| 49 | M | 43 | DDD | STALIF | L4–5–1 | Autograft | 2, 2 | 30 | No | CT | CT at 36 mo L4–5 and L5–S1 pseudarthrosis, had posterior instrumentation without posterior fusion, CT 9 mo later shows anterior fusion through cages |
| 50 | F | 48 | DDD | STALIF | L4–5–1 | Autograft | 2, 2 | 24 | Yes | CT | |
| 51 | F | 53 | DDD | STALIF | L5–1 | Autograft | 2 | 17 | Yes | CT | Previous right L5–1 microdecompression |
| 52 | M | 38 | DDD | STALIF | L4–5–1 | Autograft | 2, 4 | 24 | No | CT | Previous right L5–S1 microdiskectomy, Pseudarthrosis at both levels at 2 y, posterior decompression and instrumented fusion, persistent anterior pseudarthrosis at 16 mo, posterior has fused |
| 53 | M | 40 | DDD | STALIF | L4–5 | Allograft | 2 | 24 | Yes | CT | Previous left L4–5 diskectomy |
| 54 | M | 44 | DDD | STALIF | L5–1 | Autograft | 2 | 24 | Yes | CT | |
| 55 | F | 37 | DDD | STALIF | L4–5–1 | Autograft | 2, 2 | 11 | No | CT | Pseudarthrosis 4–5 |
| 56 | M | 62 | DDD | STALIF | L5–1 | Autograft | 2 | 10 | Yes | CT | Previous L5–1 microdecompression diskectomy |
| 57 | F | 40 | DDD | STALIF | L4–5–1 | Autograft | 2, 2 | 32 | No | CT | Pseudarthrosis at both levels |
| 58 | F | 42 | DDD | STALIF | L4–5 | Autograft | 2 | 32 | No | MRI | Pseudarthrosis L4–5, CT at 9 mo, MRI at 32 mo |
| 59 | M | 31 | DDD | STALIF | L5–1 | Autograft | 2 | 24 | Yes | CT | |
| 60 | F | 46 | DDD | STALIF | L5–1 | Autograft | 2 | 15 | Yes | CT | |
| 61 | M | 41 | DDD | STALIF | L4–5–1 | Autograft | 2, 2 | 8 | Yes | CT | Previous L5 laminectomy |
| 62 | F | 35 | DDD | STALIF | L4–5–1 | Allograft | 2, 2 | 48 | No | CT | Pseudarthrosis at L4–5 at 48 mo had posterior instrumentation with no posterior fusion |
| 63 | M | 40 | DDD | STALIF | L4–5–1 | Allograft | 2, 2 | 44 | No | CT | Pseudarthrosis at both levels |
| 64 | F | 34 | DDD | STALIF | L5–1 | Autograft | 2 | 9 | Yes | CT | |
| 65 | F | 42 | DDD | STALIF | L4–5 | Autograft | 2 | 9 | Yes | CT | |
| 66 | F | 44 | DDD | STALIF | L5–1 | Autograft | 2 | 10 | Yes | CT | |
| 67 | M | 40 | DDD | STALIF | L4–5–1 | Autograft | 2, 2 | 7 | Yes | CT | |
| 68 | F | 38 | DDD | STALIF | L5–1 | Autograft | 2 | 14 | Yes | MRI | Previous left L5–1 microdiskectomy |
| 69 | F | 37 | DDD | STALIF | L4–5 | Allograft | 2 | 26 | Yes | CT | Previous L4–5 microdiskectomy |
| 70 | F | 56 | DDD | STALIF | L5–1 | Allograft | 3 | 6 | Yes | MRI | CT at 5 mo, MRI at 26 mo |
Six patients had imaging at ≤ 9 mo follow-up. These all showed adequate union. Patient 8 had a CT scan at the 4-mo mark to check implant position and this showed clear union across the cages anteriorly. The ideal time to follow-up axial imaging for lumbar fusion is still unknown. Abbreviations: CT, computed tomography; DDD, degenerative disk disease; flex/ext, flexion/extension; FU, follow-up; MRI, magnetic resonance imaging; PSF, posterior screw fixation; XR, X-ray.
Discussion
Fine-cut CT scanning with sagittal and coronal reconstruction is becoming the imaging modality of choice in the postoperative assessment of fusion across cages in the lumbar spine.12 13 14 15 Despite this, there are still only a handful of studies using CT to assess fusion rate in anterior interbody cages, and the majority use plain and dynamic radiography. The reported fusion rates range from 32 to 95% and seem to be influenced by the presence of posterior pedicle screw fixation.12 14 15
Brantigan and Steffee16 published the first results of carbon fiber posterior lumbar interbody fusion (PLIF) cages and showed that they were successful in their design to prevent the problems of height loss, graft collapse, and pseudarthrosis based on plain radiography. Santos et al,14 however, showed that based on CT scanning ALIF standalone carbon fiber cages only achieve a 65% fusion rate based on CT at an average postoperative period of 64 months.
In 2003 the senior author concluded that transpedicular instrumentation without posterolateral fusion in combination with structural autograft ALIF acts to increase stability and can help to prevent subsidence.7 Indeed, Pradhan et al17 demonstrated graft collapse and pseudarthrosis in the absence of posterior pedicle stabilization when performing ALIF with femoral ring allograft and recombinant bone morphogenic protein type 2 (rhBMP-2). A subsequent study by Anjarwalla et al12 in 2006 showed that supplementary posterior fixation improves the anterior interbody fusion rate when using carbon fiber cages with iliac crest autograft. They studied four groups of patients with CT follow-up in 69% (81 of 117 patients). Group 1 consisted of ALIF cage alone and had an anterior fusion rate of 32% (8 of 25 patients). Group 2 consisted of ALIF plus bilateral translaminar screws and facet fusion and showed an anterior fusion rate of 47% (7 of 15 patients). Group 3 consisted of ALIF plus unilateral pedicular fixation including posterolateral autogenous iliac graft fusion. This group showed an anterior fusion rate through the cage of 82% (14 of 17 patients). Finally, group 4 consisted of ALIF plus bilateral pedicular fixation including posterolateral autogenous iliac graft fusion and the anterior fusion rate was 88% (21 of 24 patients). Li et al18 confirmed that a standalone carbon fiber cage, without screw fixation, for ALIF had poor clinical and radiographic results at 2 years and recommended adjunctive posterior stabilization. On plain radiographic follow-up, fusion was only achieved in 46 of 80 patients (57.5%).
Posterior fixation with interbody lumbar fusion surgery has been highlighted in earlier studies.19 20 Fusion rates based on plain radiography were quoted as greater than 90%. Faundez et al21 found an 82.4% fusion rate based on CT in 68 ALIF patients treated with anterior structural allograft and posterior instrumentation. However, as with Anjarwalla et al,12 these three studies performed simultaneous fusion in the posterolateral gutters.
Unlike Anjarwalla et al12 and Faundez et al,21 we did not perform a full exposure of the posterolateral gutters and a posterolateral fusion, and our data suggest that this is not required. The posterior screws act to improve the biomechanical environment in the cage and promote fusion across it. This is supported in the study by Shah et al,15 in which 53 patients were treated with titanium PLIF cages packed with autogenous graft and posterior pedicle screw fixation without posterolateral fusion. CT follow-up at 6 months showed a 95% fusion rate across cages. We feel that blood loss from epidural vessels and risk to the neural elements, as is often the case with PLIF and TLIF procedures, can be avoided if the spine can be approached anteriorly (although these procedures do have the advantage of a single approach without the need to turn the patient).
With this evidence and the development of minimally invasive techniques, it would seem reasonable to perform percutaneous posterior pedicle screw fixation in patients undergoing ALIF surgery. Anderson et al22 reported the results of 50 patients undergoing ALIF using femoral ring allograft and rhBMP-2 with percutaneous posterior pedicle screw fixation without posterior fusion for degenerative disk disease or SPL. The fusion rate based on plain radiography was reported as greater than 92%. Kim et al23 studied adult low-grade isthmic SPL treated with mini-ALIF and postpercutaneous fixation without posterolateral fusion versus mini-TLIF and postpercutaneous fixation without posterolateral fusion. There were 48 patients in the ALIF arm and 46 in the TLIF arm. The fusion rate based on plain and dynamic radiography and selective CT scanning was similar (95.8% and 91.3%, respectively). The ALIF group showed significant improvement in disk space height and segmental lordosis and a lower operative blood loss. All other clinical and radiological parameters showed similar results between the two techniques. Shim et al24 compared instrumented posterolateral fusion and ALIF (23 patients) with percutaneous pedicle screw fixation (no posterolateral fusion) and ALIF (26 patients) in elderly patients over the age of 65 years with symptomatic radicular pain from isthmic L5–S1 SPL and foraminal stenosis. They found that the clinical and radiological results were better at the 6-month mark in the posterolateral fusion group but at 2-year follow-up, there were no differences. However, the mean hospital stay, operative time, and blood loss were all significantly lower in the percutaneous fixation group. Again, they used a combination of pain and dynamic radiographs with selective CT scanning. The key difference between these studies and the present one is the follow-up imaging modality. In combination, they all support the concept of posterior instrumentation without posterolateral fusion when interbody fusion is performed.
Madan et al3 compared the clinical and plain radiographic outcomes of 27 noninstrumented single-level ALIF procedures using corticocancellous structural iliac crest autograft to 29 instrumented ALIF procedures using the fixed Hartshill Horseshoe cage instrumentation (the predecessor to the STALIF cage). Selective CT scanning was performed in patients with persistent symptoms and those with inconclusive radiographs. There were four obvious pseudarthroses in the uninstrumented group, and the authors felt it was reasonable to assume that there were no pseudarthroses in the Hartshill Horseshoe group. But we have found that the type of pseudarthrosis that occurs is “locked” and that plain radiographs not only fail to detect these but that they can be asymptomatic (Fig. 4).
Previous studies have shown that ALIF cages alone may not provide adequate stability for fusion.25 Combined anterior and posterior surgery can be time-consuming and an anterior surgery–alone approach offers several benefits. The locked pseudarthrosis rate in this study occurred in one-third of patients with STALIF cages and over 50% of those having two-level standalone surgery with the STALIF cage. There were no pseudarthroses in the lytic SPL group (STALIF plus posterior fixation but without posterolateral fusion) or the nonfixed Antelys cage group with posterior fixation but no posterolateral fusion. The time to final imaging confirming pseudarthrosis was clearly longer than in the fused group, which reflects the continued follow-up and also the confirmation of diagnosis. We must be absolutely clear that the senior author noted that inserting three or more screws into the STALIF cage, as per the manufacturer's guidelines, was technically challenging and hazardous in many cases. In the majority of cases, therefore, biomechanical stability may not have been adequately achieved, although one would anticipate that a cage fixed with two anterior screws would show a greater union rate than that seen in the study by Santos et al.14 Although this weakens our conclusions and the validity of the study, pseudarthrosis was still seen in two cases where three and four screws were used through the cage. We feel that it is important to report these results and highlight this fact to other surgeons so that they do not make similar errors of judgment when using the STALIF cage (i.e., only using two screws when using it as a standalone device). At the time of insertion, the senior author noted that the STALIF cages all had a good hold. We could not find any peer-reviewed publication of Cappuccino and Cunningham's biomechanical STALIF study presented in 2006.9 In this study, the senior surgeon performed STALIF-alone cases until the end of 2006 when he noted the high incidence of locked pseudarthroses and changed practice.
Several interesting points were noted with regards to fusion. The first was that not all patients with a pseudarthrosis were clinically symptomatic, and second, not all patients with a fusion showed a clinical improvement. Of note only 3 of the 14 patients with pseudarthrosis underwent further surgery for persistent symptoms. Although this study was mainly radiological, those patients achieving a fusion could be reassured and discharged as the surgical goal had been achieved (given the understanding that up to one-third of patients fails to improve despite adequate fusion). What remain unknown are the long-term effects of a locked pseudarthrosis. There was a trend that the pseudarthroses tended to occur more at the L4–5 level, which may reflect the excess intrinsic mobility at this level compared with L5–S1. Finally, one patient showing a locked pseudarthrosis at 48 months underwent posterior instrumentation without fusion and went on to unite anteriorly after 9 months (Fig. 5). A second patient has recently undergone this procedure and we are awaiting follow-up.
Biomechanical comparison of the STALIF (Surgicraft) and SYNFIX (Synthes) cages has shown that both implants have a significantly higher stiffness and hence stabilizing effect in all loading directions compared with the native disk. However, the Synfix cage had greater stiffness in lateral bending compared with the STALIF cage.26 The Synfix cage has divergent locking screws with a biconvex boxlike design, whereas the STALIF cage has non-angle-stable screws, which are larger and converge to the center of the vertebral body with a semicircular wedge-shaped cage design. Unfortunately, clinical comparison of these two cages as standalone devices in the current study is not possible.
A recent biomechanical study has shown that the use of an anterior plate in combination with femoral ring allograft ALIF significantly increases the stability but not as much as the addition of posterior pedicle instrumentation.25 Hence, presently there does not appear to be an anterior surgery–alone solution, although there are now several newer implants on the market including those utilizing blades and integrated plates.
This study has several strengths and weaknesses. It represents a large single-surgeon cohort with good radiological follow-up. Although the study was retrospective, the imaging was performed prospectively as part of routine follow-up. It is one of only a few studies that have used CT as a routine way of assessing ALIF. Unfortunately, as mentioned previously, the majority of cases using the STALIF cage utilized only two screws. The type of graft used in the cage varied but we do not believe this influenced the results. One would anticipate that autograft harvested at the time of surgery, as was the case in the majority of the STALIF-alone group, would perform better than allograft. This did not appear to be the case, and an explanation other than the addition of posterior instrumentation is not clearly apparent. We did not study the effects of recombinant bone morphogenic proteins as it is not routinely used in the UK National Health Service for cost reasons. Bone substitutes were not used or were deemed necessary as a bone bank was available.
The senior surgeon is experienced in microsurgical techniques. Table 3 shows that eight of the STALIF-alone group underwent previous posterior surgery. Only one patient from this group went on to develop a locked pseudarthrosis, and therefore we believe that prior microsurgery as performed by the lead author does not destabilize the spine and render it prone to locked pseudarthrosis.
Conclusions
Posterior pedicle screw supplementation without posterolateral fusion improves the fusion rate of ALIF when using anterior cage and screw constructs. We would recommend supplementary posterior fixation especially in cases where more than one level is being operated.
Footnotes
Disclosures M.J.H. McCarthy, None L. Ng, None G. Vermeersch, None D. Chan, None
References
- 1.Fritzell P Hägg O Wessberg P Nordwall A Swedish Lumbar Spine Study Group 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group Spine 2001262521–2532.; discussion 2532–2534 [DOI] [PubMed] [Google Scholar]
- 2.Fritzell P, Hägg O, Nordwall A. Swedish Lumbar Spine Study Group . Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish Lumbar Spine Study Group. Eur Spine J. 2003;12:178–189. doi: 10.1007/s00586-002-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madan S S, Harley J M, Boeree N R. Anterior lumbar interbody fusion: does stable anterior fixation matter? Eur Spine J. 2003;12:386–392. doi: 10.1007/s00586-003-0543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penta M, Fraser R D. Anterior lumbar interbody fusion. A minimum 10-year follow-up. Spine. 1997;22:2429–2434. doi: 10.1097/00007632-199710150-00021. [DOI] [PubMed] [Google Scholar]
- 5.Djurasovic M, Glassman S D, Dimar J R II, Howard J M, Bratcher K R, Carreon L Y. Does fusion status correlate with patient outcomes in lumbar spinal fusion? Spine. 2011;36:404–409. doi: 10.1097/BRS.0b013e3181fde2c4. [DOI] [PubMed] [Google Scholar]
- 6.Weatherley C R, Prickett C F, O'Brien J P. Discogenic pain persisting despite solid posterior fusion. J Bone Joint Surg Br. 1986;68:142–143. doi: 10.1302/0301-620X.68B1.2934399. [DOI] [PubMed] [Google Scholar]
- 7.El Masry M A, Badawy W S, Rajendran P, Chan D. Combined anterior interbody fusion and posterior pedicle screw fixation in patients with degenerative lumbar disc disease. Int Orthop. 2004;28:294–297. doi: 10.1007/s00264-004-0587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund T, Oxland T R, Jost B. et al. Interbody cage stabilisation in the lumbar spine: biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br. 1998;80:351–359. doi: 10.1302/0301-620x.80b2.7693. [DOI] [PubMed] [Google Scholar]
- 9.Cappuccino A Cunningham B W Multi-directional Flexibility Properties of the STALIF TTTM Device Versus Circumferential Spinal Arthrodesis: An In-Vitro Spine Model Paper presented at: SAS6; May 2006; Montreal, Canada
- 10.Carragee E J, Don A S, Hurwitz E L, Cuellar J M, Carrino J A, Herzog R. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine. 2009;34:2338–2345. doi: 10.1097/BRS.0b013e3181ab5432. [DOI] [PubMed] [Google Scholar]
- 11.Fagan A B, Fraser R D, Hall D J, Philadelphia, PA: Lippincott Williams and Wilkins; 1999. Carbon fiber cages for anterior lumbar interbody fusion: a prospective study. [Google Scholar]
- 12.Anjarwalla N K, Morcom R K, Fraser R D. Supplementary stabilization with anterior lumbar intervertebral fusion—a radiologic review. Spine. 2006;31:1281–1287. doi: 10.1097/01.brs.0000217692.90624.ab. [DOI] [PubMed] [Google Scholar]
- 13.Carreon L Y, Djurasovic M, Glassman S D, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32:892–895. doi: 10.1097/01.brs.0000259808.47104.dd. [DOI] [PubMed] [Google Scholar]
- 14.Santos E RG, Goss D G, Morcom R K, Fraser R D. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28:997–1001. doi: 10.1097/01.BRS.0000061988.93175.74. [DOI] [PubMed] [Google Scholar]
- 15.Shah R R, Mohammed S, Saifuddin A, Taylor B A. Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J. 2003;12:378–385. doi: 10.1007/s00586-002-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brantigan J W, Steffee A D. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18:2106–2107. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 17.Pradhan B B, Bae H W, Dawson E G, Patel V V, Delamarter R B. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine. 2006;31:E277–E284. doi: 10.1097/01.brs.0000216442.12092.01. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Dumonski M L, Liu Q. et al. A multicenter study to evaluate the safety and efficacy of a stand-alone anterior carbon I/F Cage for anterior lumbar interbody fusion: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine. 2010;35:E1564–E1570. doi: 10.1097/BRS.0b013e3181ef5c14. [DOI] [PubMed] [Google Scholar]
- 19.Christensen F B, Hansen E S, Eiskjaer S P. et al. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine. 2002;27:2674–2683. doi: 10.1097/00007632-200212010-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kozak J A, O'Brien J P. Simultaneous combined anterior and posterior fusion. An independent analysis of a treatment for the disabled low-back pain patient. Spine. 1990;15:322–328. doi: 10.1097/00007632-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Faundez A A, Schwender J D, Safriel Y. et al. Clinical and radiological outcome of anterior-posterior fusion versus transforaminal lumbar interbody fusion for symptomatic disc degeneration: a retrospective comparative study of 133 patients. Eur Spine J. 2009;18:203–211. doi: 10.1007/s00586-008-0845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson D G, Sayadipour A, Shelby K, Albert T J, Vaccaro A R, Weinstein M S. Anterior interbody arthrodesis with percutaneous posterior pedicle fixation for degenerative conditions of the lumbar spine. Eur Spine J. 2011;20:1323–1330. doi: 10.1007/s00586-011-1782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J S, Kang B U, Lee S H. et al. Mini-transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion augmented by percutaneous pedicle screw fixation: a comparison of surgical outcomes in adult low-grade isthmic spondylolisthesis. J Spinal Disord Tech. 2009;22:114–121. doi: 10.1097/BSD.0b013e318169bff5. [DOI] [PubMed] [Google Scholar]
- 24.Shim J H, Kim W S, Kim J H, Kim D H, Hwang J H, Park C K. Comparison of instrumented posterolateral fusion versus percutaneous pedicle screw fixation combined with anterior lumbar interbody fusion in elderly patients with L5–S1 isthmic spondylolisthesis and foraminal stenosis. J Neurosurg Spine. 2011;15:311–319. doi: 10.3171/2011.4.SPINE10653. [DOI] [PubMed] [Google Scholar]
- 25.Tzermiadianos M N, Mekhail A, Voronov L I. et al. Enhancing the stability of anterior lumbar interbody fusion: a biomechanical comparison of anterior plate versus posterior transpedicular instrumentation. Spine. 2008;33:E38–E43. doi: 10.1097/BRS.0b013e3181604644. [DOI] [PubMed] [Google Scholar]
- 26.Schleicher P, Gerlach R, Schär B. et al. Biomechanical comparison of two different concepts for stand alone anterior lumbar interbody fusion. Eur Spine J. 2008;17:1757–1765. doi: 10.1007/s00586-008-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


