Abstract
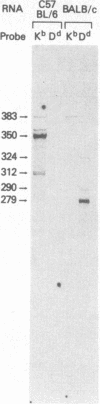
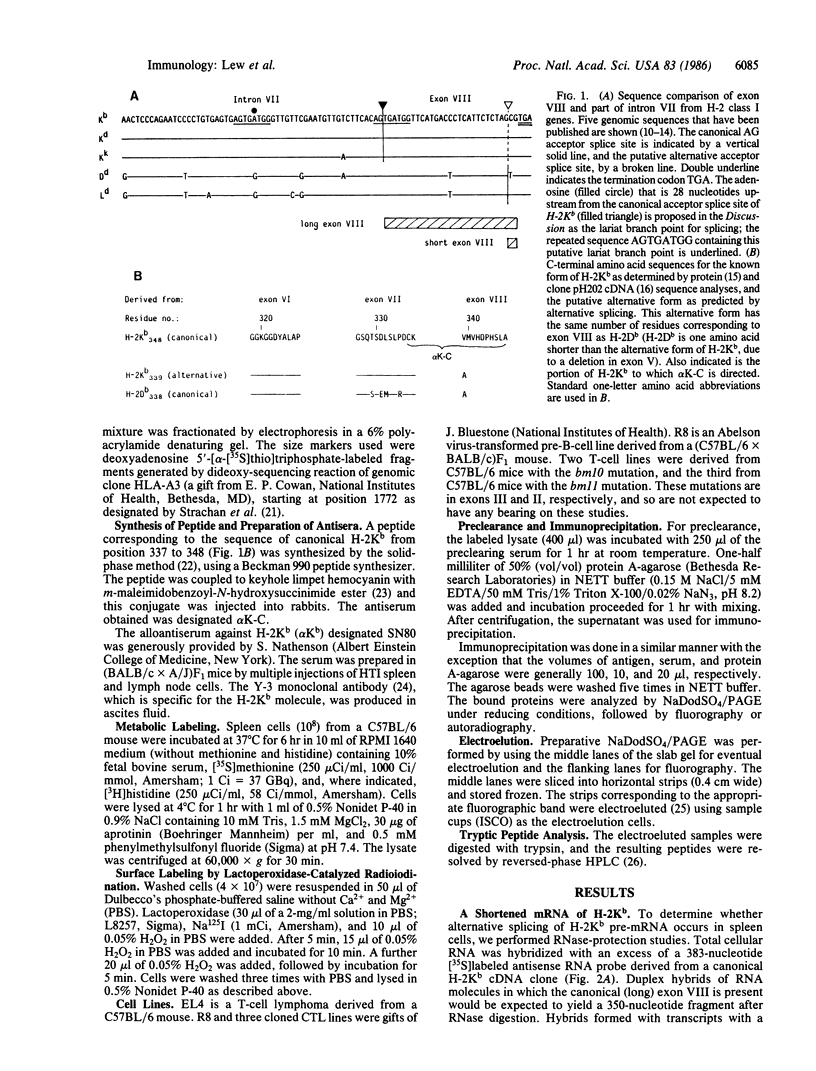
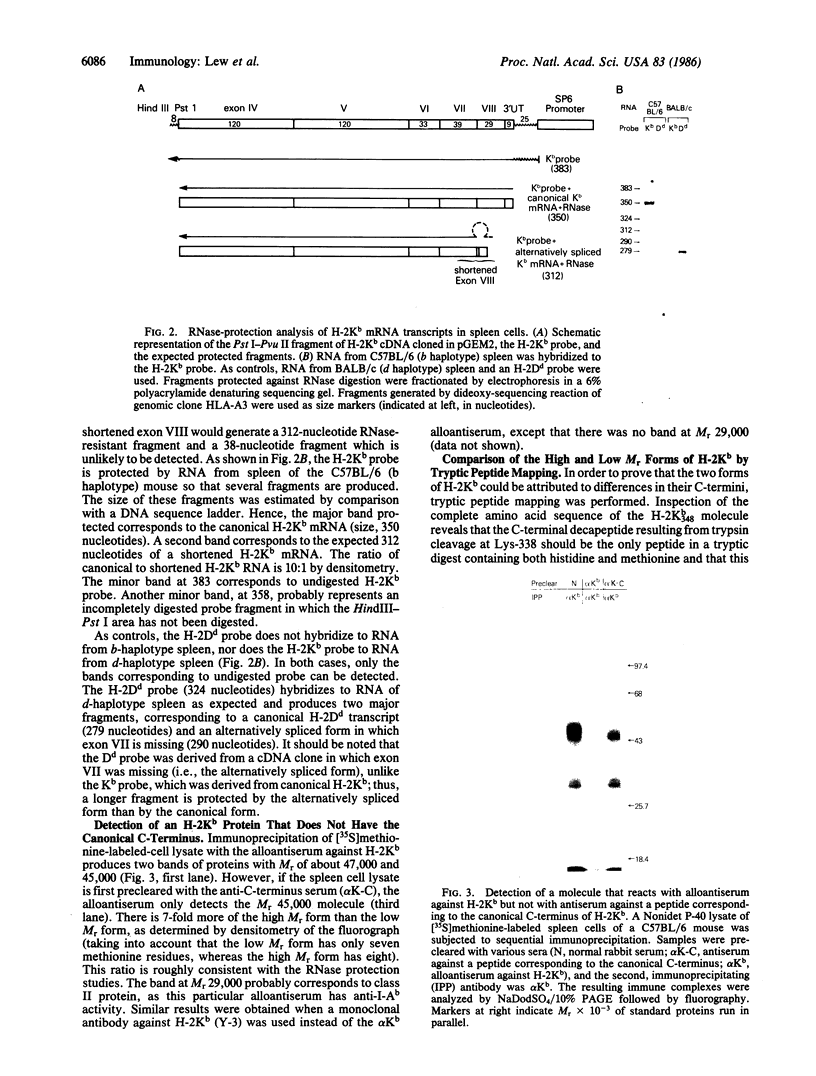
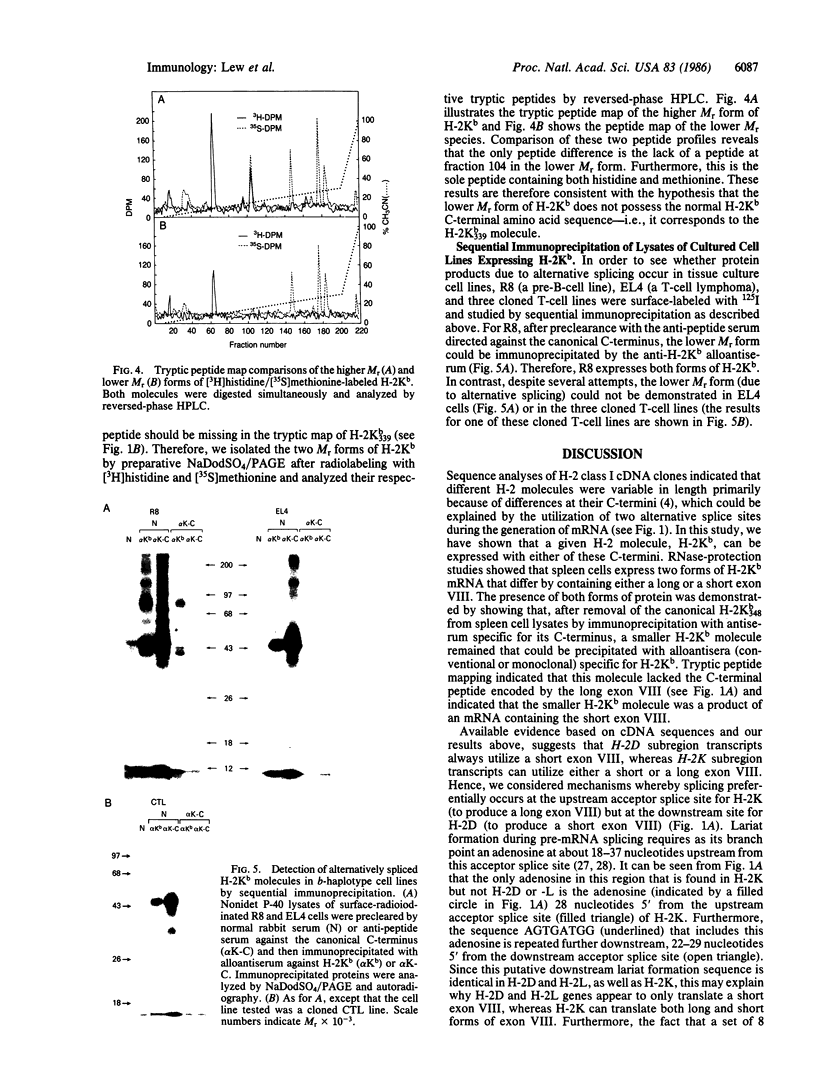
In addition to mRNA encoding the canonical form of the murine class I antigen H-2Kb (348 amino acid residues), mRNA that would encode a shortened form of H-2Kb (missing 9 amino acids from the C-terminus) has been identified in C57BL/6 spleen cells by RNase-protection studies. The alternative transcripts of H-2Kb arise through the use of different AG acceptor splice sites for exon VIII. The existence of a shortened H-2Kb protein was demonstrated by sequential immunoprecipitation. Lysates of spleen cells that had been labeled with [35S]methionine and [3H]histidine were precleared with rabbit anti-peptide serum reactive with the C-terminus of the canonical H-2Kb. The shortened form of H-2Kb was immunoprecipitated from this lysate with H-2Kb alloantiserum. Both forms of H-2Kb were isolated by NaDodSO4/PAGE. Tryptic peptide mapping confirmed that these molecules differed only at their C-terminus. The shortened form of H-2Kb is also found in a B-cell line (R8) but not in three cloned T-cell lines or in a T-cell lymphoma (EL4), suggesting that regulatory events are involved in producing the two forms of H-2Kb. Putative lariat branch points involved in these alternative splicing events for the 3' coding region of H-2 class I pre-mRNAs are proposed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Burgert H. G., Archibald A. L., Kvist S. Complete nucleotide sequence of the murine H-2Kk gene. Comparison of three H-2K locus alleles. Nucleic Acids Res. 1984 Dec 21;12(24):9473–9487. doi: 10.1093/nar/12.24.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. The class I major histocompatibility antigen gene activated in a line of SV40-transformed mouse cells is H-2Dd, not Qa/Tla. Nature. 1985 Jul 11;316(6024):162–163. doi: 10.1038/316162a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Peyron J. F., Samson M., Van Obberghen E., Brandenburg D., Brossette N. Molecular association between major histocompatibility complex class I antigens and insulin receptors in mouse liver membranes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8634–8637. doi: 10.1073/pnas.82.24.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Jones B., Janeway C. A., Jr Cooperative interaction of B lymphocytes with antigen-specific helper T lymphocytes is MHC restricted. Nature. 1981 Aug 6;292(5823):547–549. doi: 10.1038/292547a0. [DOI] [PubMed] [Google Scholar]
- Kimball E. S., Coligan J. E. Structure of class I major histocompatibility antigens. Contemp Top Mol Immunol. 1983;9:1–63. doi: 10.1007/978-1-4684-4517-6_1. [DOI] [PubMed] [Google Scholar]
- Kress M., Glaros D., Khoury G., Jay G. Alternative RNA splicing in expression of the H-2K gene. Nature. 1983 Dec 8;306(5943):602–604. doi: 10.1038/306602a0. [DOI] [PubMed] [Google Scholar]
- Kvist S., Roberts L., Dobberstein B. Mouse histocompatibility genes: structure and organisation of a Kd gene. EMBO J. 1983;2(2):245–254. doi: 10.1002/j.1460-2075.1983.tb01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Brown M. S., Russell D. W., Schneider W. J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985 Jul;41(3):735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- Lew A. M., Lillehoj E. P., Cowan E. P., Maloy W. L., van Schravendijk M. R., Coligan J. E. Class I genes and molecules: an update. Immunology. 1986 Jan;57(1):3–18. [PMC free article] [PubMed] [Google Scholar]
- Lillehoj E. P., Myers N. B., Lee D. R., Hansen T. H., Coligan J. E. Structural definition of a family of Ld-like molecules distributed among four of seven haplotypes compared. J Immunol. 1985 Aug;135(2):1271–1275. [PubMed] [Google Scholar]
- Marglin A., Merrifield R. B. Chemical synthesis of peptides and proteins. Annu Rev Biochem. 1970;39:841–866. doi: 10.1146/annurev.bi.39.070170.004205. [DOI] [PubMed] [Google Scholar]
- McCluskey J., Bluestone J. A., Coligan J. E., Maloy W. L., Margulies D. H. Serologic and T cell recognition of truncated transplantation antigens encoded by in vitro deleted class I major histocompatibility genes. J Immunol. 1986 Feb 15;136(4):1472–1481. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., Sher B. T., Sun Y. H., Eakle K. A., Hood L. DNA sequence of a gene encoding a BALB/c mouse Ld transplantation antigen. Science. 1982 Feb 5;215(4533):679–682. doi: 10.1126/science.7058332. [DOI] [PubMed] [Google Scholar]
- Murre C., Reiss C. S., Bernabeu C., Chen L. B., Burakoff S. J., Seidman J. G. Construction, expression and recognition of an H-2 molecule lacking its carboxyl terminus. Nature. 1984 Feb 2;307(5950):432–436. doi: 10.1038/307432a0. [DOI] [PubMed] [Google Scholar]
- Reddy E. S., Pan J. Y. Molecular cloning and sequencing of H-2Kk cDNA: comparison with other H-2 genes and evidence for alternative splicing. Gene. 1985;38(1-3):239–244. doi: 10.1016/0378-1119(85)90223-9. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Itakura K., Wallace R. B. Isolation of a cDNA clone for the murine transplantation antigen H-2Kb. Proc Natl Acad Sci U S A. 1982 May;79(10):3270–3274. doi: 10.1073/pnas.79.10.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. On the origin of RNA splicing and introns. Cell. 1985 Sep;42(2):397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]
- Sher B. T., Nairn R., Coligan J. E., Hood L. E. DNA sequence of the mouse H-2Dd transplantation antigen gene. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1175–1179. doi: 10.1073/pnas.82.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan T., Sodoyer R., Damotte M., Jordan B. R. Complete nucleotide sequence of a functional class I HLA gene, HLA-A3: implications for the evolution of HLA genes. EMBO J. 1984 Apr;3(4):887–894. doi: 10.1002/j.1460-2075.1984.tb01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transy C., Lalanne J. L., Kourilsky P. Alternative splicing in the 5' moiety of the H-2Kd gene transcript. EMBO J. 1984 Oct;3(10):2383–2386. doi: 10.1002/j.1460-2075.1984.tb02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zuniga M. C., Malissen B., McMillan M., Brayton P. R., Clark S. S., Forman J., Hood L. Expression and function of transplantation antigens with altered or deleted cytoplasmic domains. Cell. 1983 Sep;34(2):535–544. doi: 10.1016/0092-8674(83)90386-0. [DOI] [PubMed] [Google Scholar]