Abstract
Despite promising advances in basic spinal cord repair research, no effective therapy resulting in major neurological or functional recovery after traumatic spinal cord injury (tSCI) is available to date. The neurological examination according to the International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients (International Standards) has become the cornerstone in the assessment of the severity and level of the injury. Based on parameters from the International Standards, physicians are able to inform patients about the predicted long-term outcomes, including the ability to walk, with high accuracy. In those patients who cannot participate in a reliable physical neurological examination, magnetic resonance imaging and electrophysiological examinations may provide useful diagnostic and prognostic information. As clinical research on this topic continues, the prognostic value of the reviewed diagnostic assessments will become more accurate in the near future. These advances will provide useful information for physicians to counsel tSCI patients and their families during the catastrophic initial phase after the injury.
Keywords: spinal cord injury, diagnosis, prognosis, review
Traumatic spinal cord injury (SCI) is a serious disorder that has a profound impact on a patient’s physical and psychosocial well-being. The incidence of tSCI is estimated to be 11 to 53 new cases per million population.1 2 Epidemiological data from the 1980s show that spinal cord injury (SCI) primarily affects young adults (mean age: 29 years). During the last three decades, however, the proportion of elderly SCI subjects increased considerably. Currently, the average age at injury is estimated to be 45 years.3–5 For all age groups, people with incomplete tetraplegia made up the highest number (30.1%), followed by complete paraplegia (25.6%), complete tetraplegia (20.4%), and incomplete paraplegia (18.5%).1
Although promising advances in basic spinal cord repair research have been made, no effective therapy resulting in major neurological or functional recovery after tSCI is available to date.6 Despite the absence of a cure, significant progress has been made with regard to the care of SCI patients during the 21st century. Since the discovery and use of antibiotics, the prevention of complications, and the introduction of specialized care by the founding fathers of SCI rehabilitation, Dr. Donald Munro and Sir Ludwig Guttmann, survival rates in the SCI population increased dramatically.7
After the initial medical stabilization of a patient with tSCI, the following aspects are of importance: (1) invasive monitoring and hemodynamic support to maintain mean blood pressure above 90 mm Hg,8 (2) preventing occurrence of complications, and (3) determining long-term outcomes as accurately as possible. In the early days after the injury, patients and their families want to know whether they will be able to walk again and whether they will be able to perform self-care activities such as feeding, bathing, and clothing.9
An accurate assessment of the level and severity of the tSCI is the key for predicting functional outcomes. This review will present the prognostic value and clinical utility of contemporary diagnostic instruments for tSCI.
Diagnosis
The Neurological Examination
The initial neurological examination is the most important instrument for the assessment of the severity and level of the injury. For optimal reliability of the initial examination, the patient must be able to cooperate and follow the instructions of the examiner and should not have major distracting injuries such as a complicated tibia midshaft fracture.
Since its introduction in 1969, the Frankel scale, a 5-point severity scale, has commonly been used to determine the severity of the SCI ( Table 1 ).10 Patients are classified as complete (grade A), sensory only (grade B), motor useless (grade C), motor useful (grade D), or no neurological deficit/complete recovery (grade E). This scale provided a simple, though nonspecific, scheme for the categorization of SCI. Two major limitations of this scale have been identified: (1) the level of the injury is not incorporated into the classification and (2) the scale’s inherent subjectivity in judging what constitutes “useful” motor strength. Moreover, the Frankel scale has limited responsiveness to subtle neurological improvements during recovery.11
Table 1. The Frankel Scale for Spinal Cord Injury That Classifies the Extent of the Neurological/Functional Deficit into Five Grades10 .
| Frankel Scale | ||
|---|---|---|
| A | Complete | No motor or sensory function below level of lesion |
| B | Sensory only | No motor function, but some sensation preserved below level of lesion |
| C | Motor useless | Some motor function without practical application |
| D | Motor useful | Useful motor function below level of lesion |
| E | Recovery | Normal motor and sensory function, may have reflex abnormalities |
These methodological shortcomings of the Frankel scale were recognized by the classification committee of the American Spinal Injury Association and in 1992 a major revision of the International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients (International Standards) was published.12 Today, the most recent 2002 revision of the International Standards are used worldwide for the assessment of the severity and level of the injury.13 The testing of myotomes and dermatomes are the key components of this classification ( Fig. 1 ).
Figure 1.
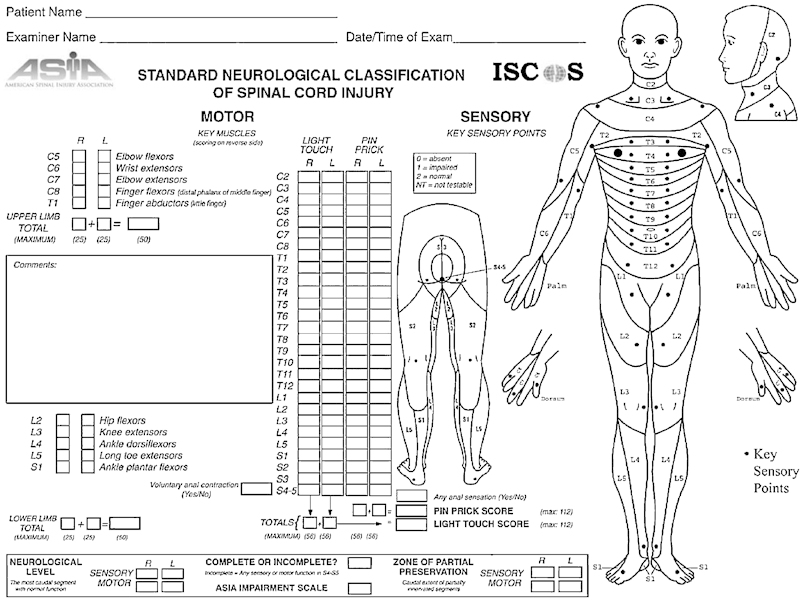
The scoring form of the International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients, available on the following Web site: http://www.asia-spinalinjury.org/publications/59544_Sc_Exam_Sheet_r4.pdf
Motor function testing according to the International Standards encompasses 10 myotomes, specifically C5 to T1 and L2 to S1, corresponding to the five key muscles each in the left and right arms and legs. Motor score testing of the key muscles is graded on a 5-point scale adapted from the Medical Research Council scale.13
Sensory examination comprises testing of what are known as key points in each of the 28 dermatomes on both the left and right sides of the body ( Fig. 1 ). The key points correspond with a defined area of skin in each dermatome where overlapping innervation to adjacent dermatomes is at a minimum, thereby making these areas most suitable for testing the function of each specific dermatome. The dermatomes extend from level C2 to S5, where S4 and S5 are considered as one dermatome. Each key point, including the anal and perianal region, is tested for light touch (with a cotton tip applicator or similar object) and pain (using a pin or similar object). Sensory function is graded as follows: normal = 2; impaired/ distorted = 1; absent = 0; not testable = NT. The latter may be due to a local injury, amputation, or a cast covering the area.14
Based on the sensorimotor scores, the level and the severity of the SCI can be determined. The scale most commonly used to classify the severity of the injury is the American Spinal Injury Association (ASIA)/International Spinal Cord Society (ISCoS) neurological standard scale (AIS), better known as the ASIA Impairment Scale. The AIS is a modification of the previously used Frankel scale, and the infralesional function is graded on a 5-point scale from A to E ( Table 2 ).
Table 2. The American Spinal Injury Association/International Spinal Cord Society Neurological Standard Scale (Better known as the “ASIA Impairment Scale”)13 .
| ASIA Impairment Scale | Lesion | |
|---|---|---|
| A | No motor or sensory function is preserved in the sacral segments S4–S5 | Complete |
| B | Sensory but not motor function is preserved below the neurological level and includes the sacral segments S4–S5 | Incomplete |
| C | Motor function is preserved below the neurological level, and more than half of key muscles below the neurological level have a muscle grade less than 3 | Incomplete |
| D | Motor function is preserved below the neurological level, and at least half of key muscles below the neurological level have a muscle grade of 3 or more | Incomplete |
| E | Motor and sensory functions are normal | Normal |
Among adult patients with SCI, the intrarater and interrater correlation coefficients for the ASIA motor score assessment have been reported as high as 0.98 and 0.97, respectively.15 The intrarater and interrater correlation coefficients for the ASIA sensory scores varied from 0.76 to 0.98 and 0.88 to 0.96, respectively. Furlan et al15 demonstrated that the neurological classification on the whole has a good responsiveness to change.
Diagnostic testing of reflex arcs in acute tSCI is only of limited value. Immediately after the injury, “spinal shock” develops below the level of injury. This may result in reflexes being diminished or even absent within the first 24 to 72 hours after the injury.16
Diagnostic Imaging
Magnetic resonance imaging (MRI) is the technique of choice for the imaging of the spinal cord ( Fig. 2 ). The typical SCI lesion on MRI is spindle shaped, containing an epicenter of hemorrhage surrounded by a halo of edema; the latter has a greater rostral-caudal extent than the central hemorrhage.17 Although clearly specified indications have not been postulated yet, several authors advise that patients with a suspected spinal cord injury should undergo an MRI examination as soon as possible.18–20 Given currently available evidence, however, MRI does not provide additional prognostic information on neurological outcomes in a fully cooperative patient with tSCI with a stable neurological condition and an uncomplicated injury of the spinal column.21–23
Figure 2.
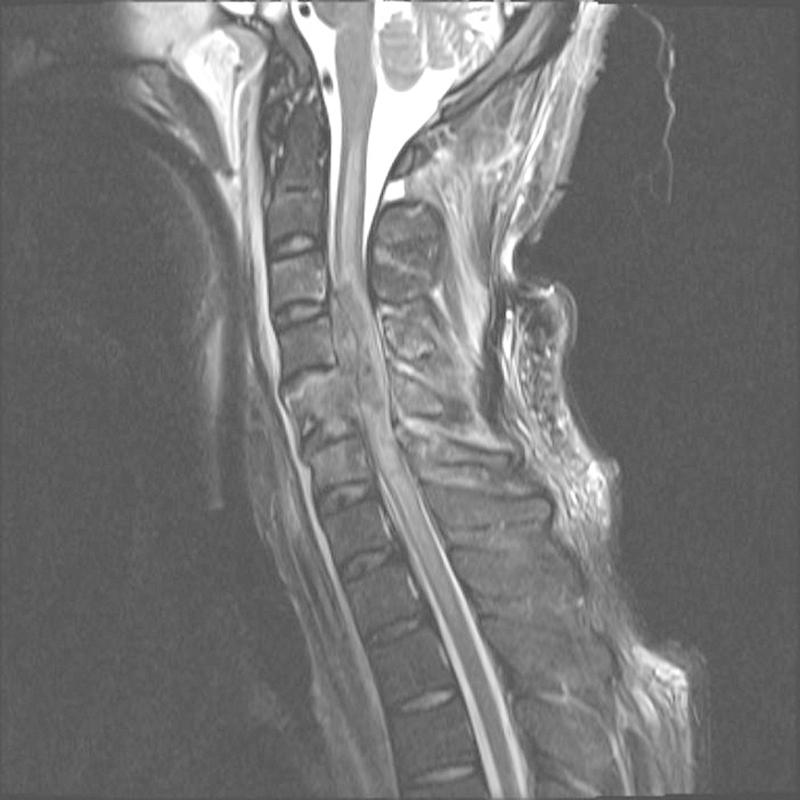
Sagittal T2-weighted magnetic resonance image of the cervical spinal cord in a patient with a traumatic spinal cord injury. The three classical features of a severe spinal cord injury, including spinal cord hemorrhage (C4–C6), spinal cord edema (C1–T3, very distinct), and spinal cord swelling (C1–T3, not very distinct) are present.
If, however, a spinal column injury has been detected on computed tomography and an accurate examination of the neurological status is not possible, MRI may provide some prognostic information. In 2007, Miyanji et al demonstrated that the extent of (1) maximal spinal cord compression, (2) spinal cord hemorrhage, and (3) cord swelling are associated with a poor prognosis for neurological recovery.24 However, clinically utilizable predictive values of MRI have not been published yet ( Table 3 ).
Table 3. Predictive Value of Various Prognostic Approaches for Independent Ambulation Outcomes 6 Months or 1 Year Postinjury.
| Predictor | Distance (Timing) | N (total) | Subgroups | n (%) | NPV (%) | 95% CI | PPV (%) | 95% CI | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Complete versus incomplete SCI | 10 m (1 y, 6 mo) | 492 | 32 | ||||||
| Complete | 240 (49) | 91.7 | 87.4–94.8 | 8.3 | 5.2–12.6 | ||||
| Incomplete | 252 (51) | 28.6 | 23.0–34.2 | 71.4 | 65.9–77.0 | ||||
| AIS grades | 10 m (1 y, 6 mo) | 492 | 32 | ||||||
| A | 240 (49) | 91.7 | 87.4–94.8 | 8.3 | 5.2–12.6 | ||||
| B | 66 (13) | 60.6 | 47.8–72.4 | 39.4 | 27.6–52.2 | ||||
| C | 76 (16) | 38.2 | 27.3–50.0 | 61.8 | 50.0–72.8 | ||||
| D | 110 (22) | 2.7 | 0.6–7.8 | 97.3 | 92.2–99.4 | ||||
| SSEP (tibial nerve) | 500 m (6 mo) | 31 | 29 | ||||||
| Absent | ? | 93 | — | 7 | — | ||||
| Present, altered | ? | 30 | — | 70 | — | ||||
| Normal | ? | 0 | — | 100 | — | ||||
| ≥ Household distances (1 y) | 22 | 24 | |||||||
| Absent | 9 (41) | 78 | 40.0–97.2 | 22 | 2.8–60.0 | ||||
| Present | 13 (59) | 8 | 0.0–36.0 | 92 | 64.0–99.8 | ||||
| MEP (anterior tibial muscle) | 500 m (6 mo) | 36 | 25 | ||||||
| Absent | ? | 78 | — | 22 | — | ||||
| Normal | ? | 0 | — | 100 | — | ||||
| MRI (no data available) | — | — | — |
SCI, spinal cord injury; AIS, American Spinal Injury Association/International Spinal Cord Society neurological standard scale; MRI, magnetic resonance imaging; NPV, negative predictive value; PPV, positive predictive value; CI, confidence interval; MEP, motor evoked potential; SSEP, somatosensory evoked potential.
Electrophysiological Examination
The integrity and function of axons in the spinal cord can also be measured with us electrophysiological recordings such as somatosensory evoked potentials and motor evoked potentials. These instruments are particularly valuable in patients who cannot participate in a reliable physical examination. Based on the latency and amplitude of the evoked response, an estimation can be made on the severity and prognosis of the injury ( Table 3 ).25–27 Although it has been demonstrated that somatosensory evoked potentials are strongly related to ambulation outcomes, this technique does not offer additional prognostic accuracy over that provided by the clinical neurological examination.25 It is for this reason that electrophysiological examinations of the limbs are currently not indicated in the evaluation of cooperative patients with tSCI.
Prognosis
In 2008, Ditunno et al published the results of a panel study in which the priorities for recovery of independent functional activities after tSCI were questioned.28 Recovery preferences for bladder and bowel function were the highest, closely followed by recovery of walking. In clinical practice, one of the most prominent questions patients and their families ask during the early days after the injury is: “Will I (he/she) ever be able to walk again?” Until recently, physicians experienced the greatest difficulties in answering this question accurately. Recent advances in clinical SCI research have led to the introduction of valuable tools for the prediction of functional outcomes after tSCI.
Recently, Goodwin-Wilson et al introduced the use of “evidence-based process maps” for SCI rehabilitation.29 In these process maps, the range of daily activities of patients with a specified severity (AIS) and level of injury are presented for each week postinjury. Using this method, physicians are able to provide patients with a framework for expected short-, intermediate- and long-term outcomes. This benchmarking approach is not only for the benefit of patients with tSCI, it also provides a better insight into the complete rehabilitation process for health care professionals. For optimal applicability of the process maps, it is important to determine the severity and level of the injury accurately prior to the start of the rehabilitation program.
Although a broad range of functional outcomes are of interest in the tSCI population, the prognostication of ambulation outcomes have been studied most intensively.9 The severity of the injury is the principal prognostic factor for the prediction of ambulation outcomes after tSCI. In clinical practice, the distinction between “complete” and “incomplete” SCI is commonly made to express the injury’s severity. However, van Middendorp et al recently demonstrated that this distinction results in a suboptimal prediction for ambulation outcomes after tSCI.4 A more nuanced method for the prediction of ambulation outcomes can be achieved with use of the ASIA/ISCoS neurological standard scale (see Table 2 ). With use of the AIS grades, more accurate predictions can be made than with distinction between a “complete” and an “incomplete” injury ( Table 3 ).4 30 As can be discerned from Table 3 , patients with AIS grades A and D have the smallest (8.3%) and biggest (97.3%) probability of being able to walk independently 1 year after the injury, respectively. On the contrary, the variability of the probable ambulation outcomes in patients with AIS grades B and C remains relatively high.31
Providing a solution to the suboptimal accuracy of the two mentioned approaches, a novel, simple, and highly accurate prediction rule for independent ambulation outcomes after tSCI was published in 2011.32 The prediction rule consists of five prognostic parameters: age (<65 versus ≥65 years of age); motor scores of the quadriceps femoris (myotome L3) and gastroc-soleus (myotome S1) muscles; and light touch sensation of dermatomes L3 and S1 ( Table 4 ). Considering the best score of each pair of myotomes and dermatomes, this novel prediction rule showed excellent discrimination in distinguishing independent walkers from dependent walkers and nonwalkers (area under the curve: 0.956, p < 0.001, 95% confidence interval: 0.936 to 0.976; Fig. 3 ).32 Further studies are needed to introduce prediction rules not only for ambulation outcomes but also for autonomic functions such as bladder, bowel, cardiorespiratory, and reproductive functions.
Table 4. The Five Predictors of a Novel Clinical Prediction Rule for Independent Ambulation Outcomes After Traumatic Spinal Cord Injury32 .
| Variablea | Range of Test Scores | Weighted Coefficient | Minimum Score | Maximum Score |
|---|---|---|---|---|
| Age ≥ 65 y | 0–1b | −10 | −10 | 0 |
| Motor score, myotome L3 | 0–5c | 2 | 0 | 10 |
| Motor score, myotome S1 | 0–5c | 2 | 0 | 10 |
| Light touch score, dermatome L3 | 0–2d | 5 | 0 | 10 |
| Light touch score, dermatome S1 | 0–2d | 5 | 0 | 10 |
| Total | −10 | 40 |
aOnly the best score of each myotome or dermatome (i.e., right or left) should be applied for the prediction rule (see text).
b0 = no, 1 = yes.
cGraded on a 5-point scale adapted from the “Medical Research Council” scale.
d0 = absent, 1 = impaired, 2 = normal.12
Figure 3.
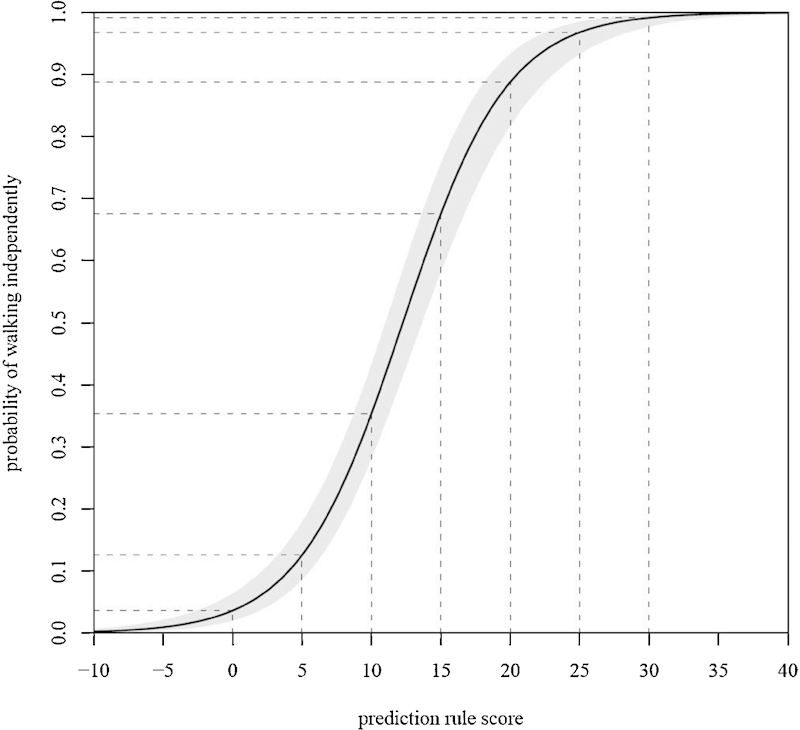
Graphic representation of the predicted probability of independent ambulation 1 year postinjury based on the prediction rule score.32 The prediction rule score (x-axis, see Table 4 ) is plotted out against the probability of walking independently 1 year postinjury (y-axis). The light gray area around the curve represents the 95% confidence interval of the prediction rule based on the regression model. The dashed lines are a visual aid to determine the probability of walking independently.
Future Perspectives
The International Standards are currently the reference standards for the assessment of the severity and level of the injury. Although minor improvements in the neurological diagnostics are to be expected, the principal clinical scientific challenge for the next decade will be to improve the accuracy of the prognostication of functional outcomes after tSCI.
The diagnostic and prognostic value of new imaging techniques in the field of tSCI is also being investigated. Diffusion-weighted imaging and diffusion tensor imaging are promising techniques that may provide a more detailed visualization the injury than conventional MRI.33–35 A relatively new approach for evaluating the extent of the spinal cord damage is the assessment of biomarker concentrations in the cerebrospinal fluid.36 Kwon et al showed several biomarkers to be significantly correlated to the severity of neurological deficits as measured with the International Standards in patients with tSCI.37 Moreover, the authors stated that the biomarker concentrations have a stronger relation to neurological outcomes when compared with the initial AIS scores.
Despite these promising diagnostic advances, the initial neurological examination according to the International Standards will most likely remain the reference standard for the diagnosis of tSCI for the next decade. Nonetheless, new imaging techniques and biomarkers do have the potential to become incorporated into the standard diagnostic workup for patients with tSCI who are unable to participate in a reliable neurological examination.
References
- 1.National Spinal Cord Injury Statistical Center (SCISC). The 2008 annual statistical report for the spinal cord injury model systems. Available at: https://www.nscisc.uab.edu/public_content/pdf/2008%20NSCISC%20Annual%20Statistical%20Report%20-%20Complete%20Public%20Version.pdf. Accessed February 2010
- 2.Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 3.Norton L. Spinal Cord Injury, Australia 2007–08. Injury Research and Statistics Series No. 52. Cat No. INJCAT 128. Canberra, Australia: AIHW; 2010 [Google Scholar]
- 4.van Middendorp JJ Hosman AJ Pouw MH Van de Meent H; EM-SCI Study Group. Is determination between complete and incomplete traumatic spinal cord injury clinically relevant? Validation of the ASIA sacral sparing criteria in a prospective cohort of 432 patients Spinal Cord 200947809–816. [DOI] [PubMed] [Google Scholar]
- 5.Lopez AD Mathers CD Ezzati M Jamison DT Murray CJL, eds. Global Burden of Disease and Risk Factors. New York: Oxford University Press; 2006
- 6.Tator CH Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations Neurosurgery 200659957–982.; discussion 982–987 [DOI] [PubMed] [Google Scholar]
- 7.van Middendorp JJ, Sanchez GM, Burridge AL. The Edwin Smith papyrus: a clinical reappraisal of the oldest known document on spinal injuries. Eur Spine J. 2010;19:1815–1823. doi: 10.1007/s00586-010-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhard M, Gries A, Kremer P, Böttiger BW. Spinal cord injury (SCI)—prehospital management. Resuscitation. 2005;66:127–139. doi: 10.1016/j.resuscitation.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Burns AS Ditunno JF Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management Spine 20012624, Suppl):S137–S145. [DOI] [PubMed] [Google Scholar]
- 10.Frankel HL, Hancock DO, Hyslop G. et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 11.Furlan JC, Fehlings MG, Tator CH, Davis AM. Motor and sensory assessment of patients in clinical trials for pharmacological therapy of acute spinal cord injury: psychometric properties of the ASIA Standards. J Neurotrauma. 2008;25:1273–1301. doi: 10.1089/neu.2008.0617. [DOI] [PubMed] [Google Scholar]
- 12.American Spinal Injury Association/International Medical Society of Paraplegia (ASIA/IMSOP). International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients, 1992 Revision. Chicago, IL: American Spinal Injury Association; 1992 [Google Scholar]
- 13.American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury, 2002 Revision. Chicago, IL: American Spinal Injury Association; 2002 [Google Scholar]
- 14.Holtz A Levi R. Spinal Cord Injury. New York: Oxford University Press; 2010 [Google Scholar]
- 15.Furlan JC Noonan V Singh A Fehlings MG Assessment of impairment in patients with acute traumatic spinal cord injury: a systematic review of the literature J Neurotrauma 2011281445–1477.; April 6 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ditunno JF, Little JW, Tessler A, Burns AS. Spinal shock revisited: a four-phase model. Spinal Cord. 2004;42:383–395. doi: 10.1038/sj.sc.3101603. [DOI] [PubMed] [Google Scholar]
- 17.Flanders AE Schwartz ED. In: Atlas SW, ed. Spinal Trauma in Magnetic Resonance Imaging of the Brain and Spine. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2009
- 18.Demaerel P. Magnetic resonance imaging of spinal cord trauma: a pictorial essay. Neuroradiology. 2006;48:223–232. doi: 10.1007/s00234-005-0039-y. [DOI] [PubMed] [Google Scholar]
- 19.Parizel PM van der Zijden T Gaudino S et al. Trauma of the spine and spinal cord: imaging strategies Eur Spine J 201019( Suppl 01S8–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozzo A, Marcoux J, Radhakrishna M, Pelletier J, Goulet B. The role of magnetic resonance imaging in the management of acute spinal cord injury. J Neurotrauma. 2011;28:1401–1411. doi: 10.1089/neu.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selden NR Quint DJ Patel N d’Arcy HS Papadopoulos SM Emergency magnetic resonance imaging of cervical spinal cord injuries: clinical correlation and prognosis Neurosurgery 199944785–792.; discussion 792–793 [DOI] [PubMed] [Google Scholar]
- 22.Mahmood NS, Kadavigere R, Avinash KR, Rao VR. Magnetic resonance imaging in acute cervical spinal cord injury: a correlative study on spinal cord changes and 1 month motor recovery. Spinal Cord. 2008;46:791–797. doi: 10.1038/sc.2008.55. [DOI] [PubMed] [Google Scholar]
- 23.van Middendorp JJ. On the Injuries of the Vertebrae and Spinal Marrow: Prognostic Factors and Classifications. Nijmegen, The Netherlands: Department of Orthopaedics, Radboud University Nijmegen Medical Centre; 2010 [Google Scholar]
- 24.Miyanji F, Furlan JC, Aarabi B, Arnold PM, Fehlings MG. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome—prospective study with 100 consecutive patients. Radiology. 2007;243:820–827. doi: 10.1148/radiol.2433060583. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs SR, Yeaney NK, Herbison GJ, Ditunno JF Jr. Future ambulation prognosis as predicted by somatosensory evoked potentials in motor complete and incomplete quadriplegia. Arch Phys Med Rehabil. 1995;76:635–641. doi: 10.1016/s0003-9993(95)80632-6. [DOI] [PubMed] [Google Scholar]
- 26.Curt A, Keck ME, Dietz V. Functional outcome following spinal cord injury: significance of motor-evoked potentials and ASIA scores. Arch Phys Med Rehabil. 1998;79:81–86. doi: 10.1016/s0003-9993(98)90213-1. [DOI] [PubMed] [Google Scholar]
- 27.Curt A, Dietz V. Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord. 1999;37:157–165. doi: 10.1038/sj.sc.3100809. [DOI] [PubMed] [Google Scholar]
- 28.Ditunno PL, Patrick M, Stineman M, Ditunno JF. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord. 2008;46:500–506. doi: 10.1038/sj.sc.3102172. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin-Wilson C, Watkins M, Gardner-Elahi C. Developing evidence-based process maps for spinal cord injury rehabilitation. Spinal Cord. 2010;48:122–127. doi: 10.1038/sc.2009.94. [DOI] [PubMed] [Google Scholar]
- 30.Curt A, Dietz V. Ambulatory capacity in spinal cord injury: significance of somatosensory evoked potentials and ASIA protocol in predicting outcome. Arch Phys Med Rehabil. 1997;78:39–43. doi: 10.1016/s0003-9993(97)90007-1. [DOI] [PubMed] [Google Scholar]
- 31.van Middendorp JJ Hosman AJ Pouw MH Van de Meent H; EM-SCI Study Group. ASIA impairment scale conversion in traumatic SCI: is it related with the ability to walk? A descriptive comparison with functional ambulation outcome measures in 273 patients Spinal Cord 200947555–560. [DOI] [PubMed] [Google Scholar]
- 32.van Middendorp JJ, Hosman AJ, Donders AR. et al. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet. 2011;377:1004–1010. doi: 10.1016/S0140-6736(10)62276-3. [DOI] [PubMed] [Google Scholar]
- 33.Tsuchiya K, Fujikawa A, Honya K, Tateishi H, Nitatori T. Value of diffusion-weighted MR imaging in acute cervical cord injury as a predictor of outcome. Neuroradiology. 2006;48:803–808. doi: 10.1007/s00234-006-0133-9. [DOI] [PubMed] [Google Scholar]
- 34.Lammertse D, Dungan D, Dreisbach J. et al. Neuroimaging in traumatic spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med. 2007;30:205–214. doi: 10.1080/10790268.2007.11753928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas MI, Delavelle J, Jlassi H. et al. Clinical applications of diffusion tensor tractography of the spinal cord. Neuroradiology. 2008;50:25–29. doi: 10.1007/s00234-007-0309-y. [DOI] [PubMed] [Google Scholar]
- 36.Pouw MH, Hosman AJ, van Middendorp JJ, Verbeek MM, Vos PE, van de Meent H. Biomarkers in spinal cord injury. Spinal Cord. 2009;47:519–525. doi: 10.1038/sc.2008.176. [DOI] [PubMed] [Google Scholar]
- 37.Kwon BK, Stammers AM, Belanger LM. et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma. 2010;27:669–682. doi: 10.1089/neu.2009.1080. [DOI] [PubMed] [Google Scholar]


